Abstract
Soybean root rot, a soil-borne fungal disease, is caused by multiple pathogens that seriously affect soybean production. During spring 2021, 92 pathogenic fungal strains were isolated from soybean plants with root rot in Hailun City, Heilongjiang Province, China. Through morphological and molecular identification, these strains were identified as Fusarium oxysporum (39.1%), F. asiaticum (30.4%), F. graminearum (13.0%), Pythium macrosporum (8.7%), and Rhizoctonia solani (8.7%). Among them, F. oxysporum was the dominant species, and F. asiaticum, not previously reported as a soybean root rot pathogen in Northeast China. Approximately 50% of the F. asiaticum isolates were moderately pathogenic. In addition, F. asiaticum had a wide host range, infecting black soybean, French bean, white hyacinth bean, mung bean, and adzuki bean but not corn, peanut, rice, and oat roots. Regarding field management, fludioxonil and pyraclostrobin had the best control effects of 73.8% and 69.4%, with EC50 values of 0.0029–0.0071 μg·mL−1 and 0.0045–0.0076 μg·mL−1, respectively. The study reported that F. asiaticum is a pathogen causing soybean root rot in northeast China. The application of chemical fungicides and non-host crop rotation can effectively control the disease caused by F. asiaticum.
1. Introduction
Soybeans [Glycine max (Linn.) Merr.] hold the highest economic value among food and oil crops worldwide. They are abundant in protein, oil, vitamins, and various mineral nutrients. Moreover, they can be easily adapted to different environments and are widely cultivated globally for human consumption, animal feed, and biodiesel production [1]. With the adjustment of the supply side structure in China, soybean planting area in this country is increasing every year [2]. Soybean planting area will increase by 667,000 hectares to 45,700,000 hectares from 2021 to 2022 in Heilongjiang Province, accounting for nearly 50% of China’s total soybean planting area. Located in one of the three major black soil zones in the world, Heilongjiang has fertile soil, suitable climatic conditions, and a high-quality ecological environment, providing a suitable production environment for soybeans. However, soybean root rot has become a major obstacle to soybean production [3]. Pathogenic fungi can infect root cells, causing serious damage to soybean roots. Infected plants often exhibit growth retardation due to the weakened ability of their roots to absorb water and nutrients [4]. In severe cases, it can lead to plant death, significantly reducing the yield and quality of soybeans [5]. The economic loss caused by the impact of various pathogenic fungi on soybeans has increased in recent years [6].
The community structure of fungal pathogens that cause soybean root rot is complex. At present, 64 fungal pathogens causing soybean root rot have been reported internationally, including species from the genus Fusarium such as Fusarium pseudograminearum, F. proliferatum, F. sporotrichioides, F. fujikuroi, F. graminearum, F. armeniacum, F. commune, F. tricinctum, and F. asiaticum [7,8,9,10,11,12,13,14,15]; species from the genus Pythium, including P. oopapillum, P. macrosporum, P. aphanidermatum, and P. deliense [4,16,17,18]; Rhizoctonia solani; Helicobasidium mompa; Thielaviopsis basicola; Stachybotrys chartarum; Sclerotium rolfsii; Mycoleptodiscus terrestris; and Phymatotrichopsis omnivora [19,20,21,22,23,24,25]. Thirty species have been reported domestically, including F. oxysporum var. rendolens, F. oxysporum, F. graminearum, F. chlamydosporum, F. merismoidescorda, F. episphaeria, F. camptoceras, ‘Candidatus Pythium huanghuaiense’, Phytophthora sojae, Phytophthora sansomeana, Rhizoctonia solani, Phomopsis longicolla, and Pratylenchus coffeae [26,27,28,29,30,31,32,33,34,35,36]. Sixteen species have been reported in Heilongjiang Province, e.g., Fusarium graminearum, Phytophthora sojae [28,37]. In addition, Rhizoctonia solani, Phomopsis longicolla have also been reported. The above results indicate that the species diversity of fungal pathogens that cause soybean root rot in the Heilongjiang Province, the main soybean production area, is potentially very complex. However, F. asiaticum, as a pathogenic fungus of soybean root rot, has not been reported in Heilongjiang province.
Soybean root rot caused by Fusarium spp. is a major disease, mainly transmitted in the soil [38,39]. Fusarium root rot of soybeans can endanger any stage of soybean development, resulting in water-soaked decay after sowing and before germination, which affects the germination rate of seeds after infection. Seedling infection leads to the decay of the root epidermis, browning of vascular bundles, withered yellow leaves, and plant death in serious cases, along with shriveled grains and serious economic costs [40].
Reducing the impact of diseases during soybean cultivation is crucial for increasing yield [41,42]. Currently, the most cost-effective way to control soybean root rot is to cultivate disease-resistant soybean varieties [43]. However, there are few specially bred Fusarium-resistant cultivars [44]. Therefore, fungicide treatment is one of the most effective disease management strategies for controlling soybean root rot [45]. The fungicides commonly used to control Fusarium root rot are pyraclostrobin, fludioxonil, and azoxystrobin [46,47]. However, F. asiaticum has unique genetic variations that may make it resistant to commonly used fungicides, and existing control methods for other Fusarium species may no longer be effective [48]. Therefore, it is necessary to screen fungicides and determine the sensitivity of target pathogens causing soybean root rot in the field.
In 2020 and 2021, soybean root rot occurred in Hailun city, Heilongjiang Province, with a diseased seedling rate of 20–30% in general plots and over 60% in severely infected plots. The objectives of this study were to identify the pathogenic microorganisms causing soybean root rot, analyze their pathogenicity, and determine the sensitivity and efficacy of common fungicides against these pathogens, providing a basis for formulating control strategies.
2. Materials and Methods
2.1. Pathogen Isolation and Assessment of Their Pathogenicity
Field investigation of soybean root rot at the seedling stage was conducted at 3 sites (5 hectares per field) in an important soybean planting area in Hailun city. The local soil, mainly black sandy clay with 4–5% organic matter, had a disease incidence of 10–20% in the surveyed fields (about 5 ha each). Soybean plants (n = 182) with root rot symptoms were collected using five-spot sampling (Figure 1). The roots were thoroughly rinsed under running tap water for 10 min to remove soil and debris. Pathogens were isolated from symptomatic root tissues following a published method and cultured on potato dextrose agar (PDA) at 28 °C in the dark [49]. After three days, hyphal tips were transferred to isolate and purify the fungal cultures. One diseased tissue sample from each infected seedling was selected and isolated. The number of isolated and purified pathogens was recorded and the percentage isolated to each species was calculated.

Figure 1.
Soybean plants with root rot symptoms in the field.
The pathogenicity of isolated and purified strains was evaluated according to the Koch hypothesis [50]. A method of inoculating soybeans with fungus by embedding roots of sorghum grain [51]. All strains were re-isolated from diseased soybean seedlings and observed. The specific method was as follows: A total of 1/3 of the volume of the sterile soil was inserted into a flowerpot (diameter of 15 cm), and 20 g of sorghum grains that are already overgrown with pathogenic fungi were evenly sprinkled. Then, 12 soybean seeds (the variety used was HeNong 511) were evenly placed on the surface, covered with a layer of culture soil (1 cm), and after the seedlings emerged, 10 were kept in each flowerpot [2]. Seedlings cultured without isolation strains were used as the control group. Each treatment had three replicates, and the experiment was conducted twice. After 20 days, the incidence of root rot was investigated, and the infected plants were re-isolated and morphologically identified according to Koch’s postulates; the disease index was calculated according to the classification standard of root rot by Wang et al. [52]. Ten highly pathogenic strains were selected for subsequent experiments.
Disease severity was visually scored on a scale of 0–7 based on the growth status of soybean seedlings: 0 = no symptoms; 1 = the taproot was basically unchanged or slightly browned, the fibrous root was not long, the growth point was browned, and the plant growth was normal; 3 = the taproot turned black but continued to grow through the infection point, the fibrous root tip turned black, and the plant grew normally; 5 = the taproot was seriously blackened and could not continue growing through the infection point, the fibrous root was obviously reduced or absent, the aboveground growth was poor, and the plant growth was short; and 7 = root rot, failure of normal growth or emergence, partial cotyledon rot, or plant death [52].
The percentage of disease index (PDI) for soybean root rot was calculated using the following formula: PDI = ∑(the number of diseased plants at each level × the corresponding relative ratings)/(the total number of surveyed plants × the highest disease level rating) × 100.
The pathogenicity of the isolates was classified based on the average disease index of the two repeated experiments. Isolates with a disease index less than 50 were classified as having weak pathogenicity (designated as W), those with a disease index between 50 (inclusive) and 60 (exclusive) were classified as having moderate pathogenicity (designated as M), and those with a disease index of 60 or greater were classified as having high pathogenicity (designated as H).
2.2. Identification of the Pathogen
Isolates responsible for soybean root rot were identified through their morphological characteristics and molecular methods [53]. For molecular identification, genomic DNA was extracted from the mycelia of representative isolates using a Tiangen Genome Extraction Kit (Tiangen Biotech, Beijing, China). The internal transcribed spacer region (ITS), translation elongation factor 1-α (Tef1), and β-tubulin (Tub2) genes were amplified and sequenced using primers ITS1/ITS4, EF1-728F/EF1-986R, and Bt2a/Bt2b, respectively [54,55,56]. Subsequently, the obtained sequences were submitted to the GenBank database (Table A1). Polymerase chain reaction (PCR) was carried out in a 50 µL reaction system containing 10 µM of each primer, 2 × Taq Master Mix, and 10 ng of template DNA. The PCR conditions were as follows: initial denaturation at 94 °C for 5 min, followed by 35 cycles, each cycle including 1 min denaturation at 94 °C, 1 min annealing at 52 °C, 1.5 min extension at 72 °C, and finally a 10 min final extension at 72 °C. The PCR products were purified and sequenced by GENEWIZ (Azenta Life Sciences, Suzhou, China).
Phylogenetic trees of representative isolates were constructed using PhyloSuite v1.2.2 (http://phylosuite.jushengwu.com/, accessed on 2 June 2023) following the MrBayes method and were further edited in FigTree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 2 June 2023) [57,58].
2.3. Biological Characteristics of Fusarium asiaticum
To determine the optimum pH and temperature for the isolates, the mycelium growth rate method was employed [59,60]. Ten isolates were cultured on PDA at different pH levels (5.0, 6.0, 7.0, 8.0, 9.0, 10.0 and 11.0) and temperatures (10 °C, 20 °C, 25 °C, 28 °C, 30 °C, and 35 °C). A 0.5 cm diameter mycelial plug from a 96 h PDA-grown isolate was transferred to different treated PDA plates and incubated under the corresponding conditions. Each treatment had three replicates, and the experiment was conducted twice. The colony diameters of the isolates were measured after 72 h.
2.4. Host Range Determination of Fusarium asiaticum
The F. asiaticum isolates causing soybean root rot were inoculated on crops commonly grown in Heilongjiang, which were black soybean (Glycine max Linn.), French bean (Phaseolus vulgaris Linn.), white hyacinth bean (Dolicho Lablab Linn.), mung bean (P. radiatus Linn.), corn (Zea mays Linn.), peanut (Arachis hypogaea Linn.), rice (Oryza sativa Linn.), adzuki bean (Vigna umbellata Thunb.), and oat (Avena sativa Linn.) seedlings. The inoculation method was consistent with that used to determine the pathogenicity of the above isolates. Each treatment was replicated thrice. Approximately 20 days after inoculation, the pathogenicity of the isolate in each crop was investigated and evaluated. The pathogen was re-isolated and identified from the inoculated seedlings to complete Koch’s postulates. All experiments were performed twice.
2.5. Sensitivity of Fusarium asiaticum to Fungicides
The mycelial growth rate method was employed to evaluate the sensitivity of the isolates to the following fungicides that are commonly utilized for controlling Fusarium root rot [46,47]: pyraclostrobin [25% flowable concentrate (Jiangsu Tuoqiu Agrochemicals Co., Ltd., Yancheng, China)], prochloraz (450 g·L−1 EW) [Shanghai Hulian Biopharmaceutical (Xiayi) Co., Ltd., Shanghai, China], fludioxonil (25% FSB) [Syngenta (Nantong) Crop Protection Co., Ltd., Nantong, China], and a mixture of 11.7% propiconazole + 7% azoxystrobin [18.7% suspoemulsion (Syngenta Nantong Crop Protection Co., Ltd., Nantong, China)] [61].
The fungicides were dissolved in 1000 mL of sterile distilled water. Stock solutions of the four fungicides were then added to PDA at concentrations of 0 (control), 0.001, 0.002, 0.005, 0.01, and 0.02 μg·mL−1. The PDA plates were incubated at 26 °C for five days. Subsequently, a 0.7 cm diameter mycelial plug of the isolate was placed at the center of each fungicide-amended PDA plate and incubated in the dark at 26 °C for seven days. Each treatment was replicated three times, and the entire experiment was conducted twice. After the incubation period, the colony diameters were measured. The effective concentration resulting in 50% mycelial growth reduction (EC50) of the four fungicides was calculated according to the method described by Lehner et al. [62]. Data from the two replicate experiments were pooled, and EC50 values were calculated. The inhibitory effect was expressed as a percentage relative to the control, using the formula: 1 − [(diameter of treated colony − 0.5)/(diameter of control colony − 0.5) × 100] [63].
2.6. Efficacy of Pyraclostrobine and Fludioxonil Against Soybean Root Rot Caused by Fusarium asiaticum
Pot experiments were conducted in 20 cm diameter plastic pots in a greenhouse at the experimental station of Northeast Agricultural University, Harbin, China. The greenhouse conditions were set at 25 ± 3 °C with a 12 h/12 h (light/dark) photoperiod. The specific treatments applied were as follows: (1) pyraclostrobine at effective doses of 62.5, 125, and 250 µg·mL−1; (2) fludioxonil at effective doses of 62.5, 125, and 250 µg·mL−1; (3) controls treated with sterile water. The sorghum seed inoculation method was once again applied for pathogen inoculation to determine pathogenicity. Ten soybean seedlings were kept in each bowl, with three replicates for each treatment. The experiment was carried out by soaking seeds, first washing the seeds with sterile water, and then soaking with different concentrations of fungicide liquids configured for 20–30 min. Control seeds are soaked in equal amounts of sterile distilled water. After 20 days, the disease severity was determined using the same method as the pathogenicity determination of the isolates. The disease index was calculated as described above. Seedling height, mass, and root length were measured using a graduated ruler (1 mm) and balance (1 mg). The control efficacy was calculated using the following formula:
The control efficacy was calculated using the formula: Control efficacy = (Disease index of the control group − Disease index of the fungicide − treatment group)/Disease index of the control group × 100%.
2.7. Data Analysis
Analysis of variance (ANOVA) was conducted using SPSS Statistics 17.0 (IBM/SPSS, Armonk, NY, USA). The treatment means were compared and separated by applying the least significant difference (LSD) test at a significance level of p = 0.05. The EC50 values were estimated with the assistance of GraphPad Prism 8 (GraphPad Software Inc., USA).
3. Results
3.1. Disease Symptoms and Identification of Causal Organisms
In May 2021, diseased soybean seedlings were detected in Hailun City, Heilongjiang Province, China (47.46093° N, 126.9682° E). Lesion plaques were evident at the base of the stem and were initially reddish-brown and then gradually enlarged, followed by blackening of the cortex, decay, and necrosis. The above-ground parts of infected plants were dwarfed by healthy plants, with the green leaves fading. According to the Koch postulates, 92 pathogenic isolates were isolated from 182 symptomatic seedlings. Based on morphological and molecular identification, these isolates were classified into five species (Table 1, Figure 2): F. oxysporum (39.1%), Fusarium asiaticum (30.4%), F. graminearum (13.0%), Pythium macrosporum (8.7%), and Rhizoctonia solani (8.7%). In addition to F. asiaticum, other pathogenic fungi that cause soybean root rot have been reported in China. Therefore, further systematic identification of F. asiaticum was performed in this study. Twenty-eight isolates from infected soybean roots (Figure 3B,C) were white in color, flocculent, luxuriant, and dense with a rose red pigment (Figure 3A). The average growth rate of mycelium was 20.7 mm·d−1 on PDA at 28 °C. The macroconidia were thick, with curved apical and basal cells, usually having 4–6 septa, and measuring 44.9 to 44.2 × 3.4 to 5.4 μm on carnation leaf agar. The apical cells were beak-shaped and slightly curved, and the podocydia were not obvious (Figure 3D–F). The chlamydospores were globose to subglobose. Based on these characteristics, the isolates were identified as F. asiaticum [64,65,66].

Table 1.
Frequency of pathogens isolated from soybean root rot samples in Hailun, Heilongjiang province, China.
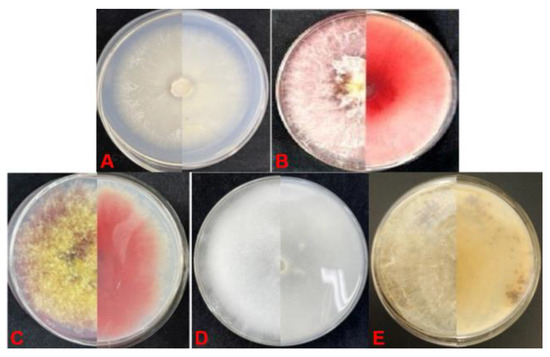
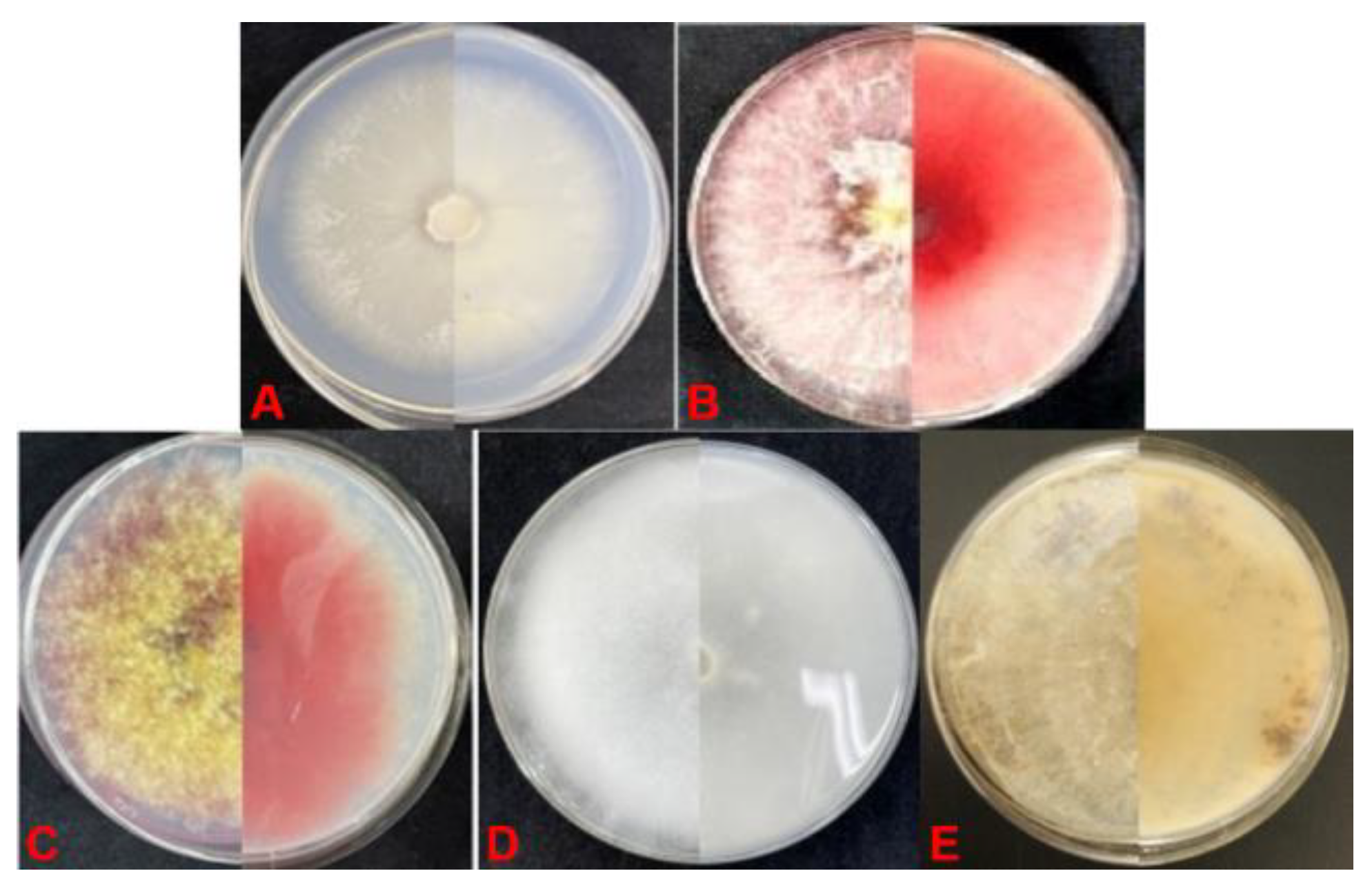
Figure 2.
Colony of pathogenic fungi causing soybean root rot on PDA. (A) Fusarium oxysporum; (B) F. asiaticum; (C) F. graminearum; (D) Pythium spp.; (E) Rhizoctonia solani.
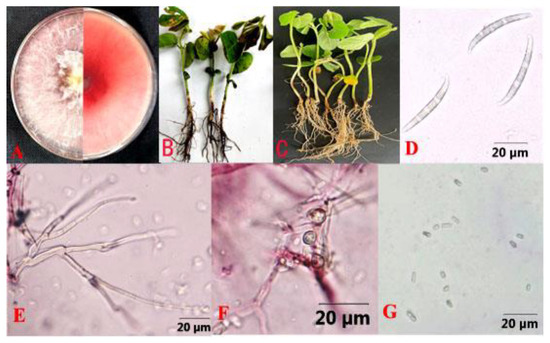
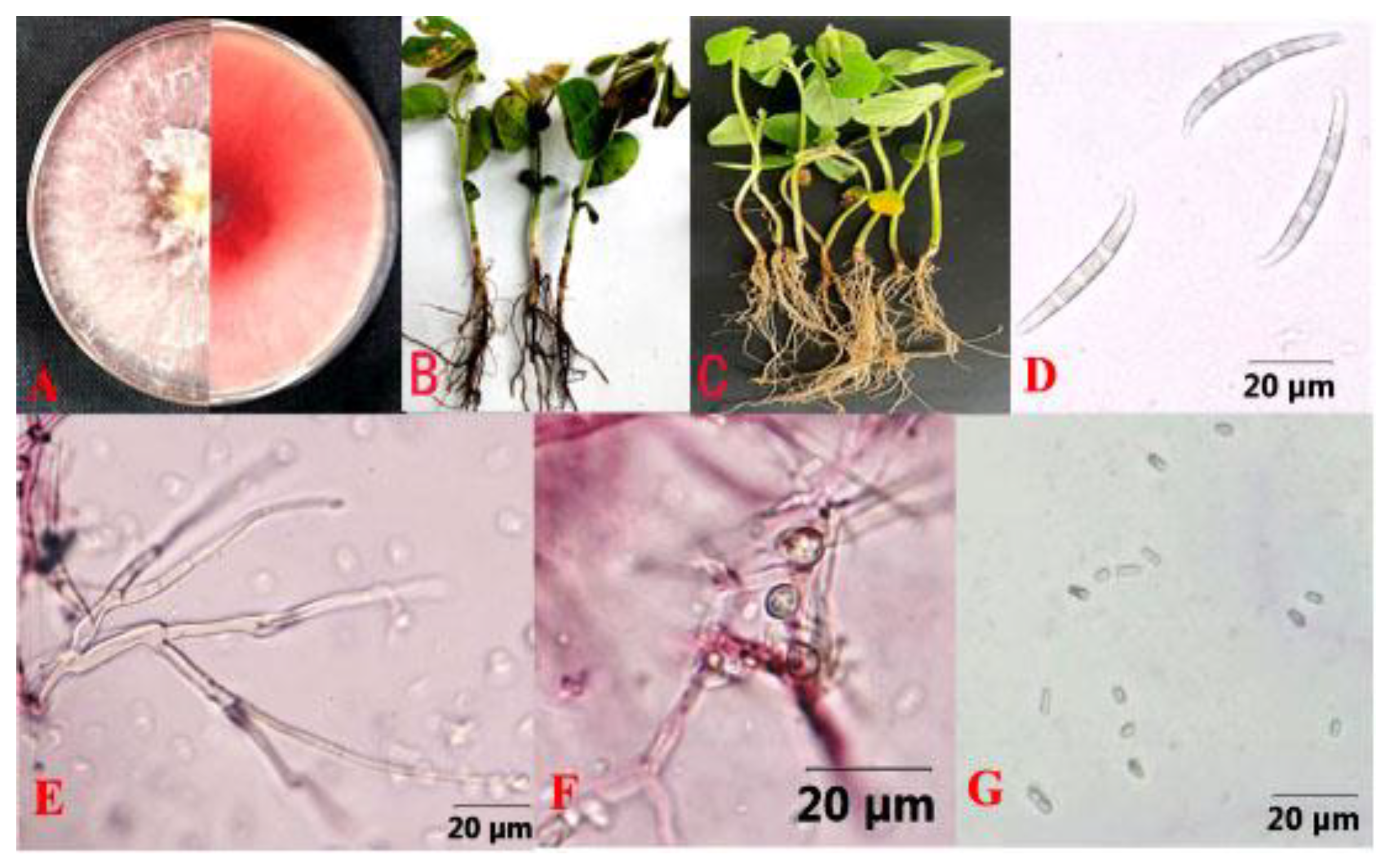
Figure 3.
Fusarium asiaticum causing soybean root rot. (A) Colony of F. asiaticum isolate HL35 on PDA; (B) Field symptoms of soybean root rot caused by F. asiaticum; (C) Indoor potting symptoms of soybean root rot caused by F. asiaticum; (D) Macroconidia; (E) Conidiophores; (F) Chlamydospore; (G) Microconidia.
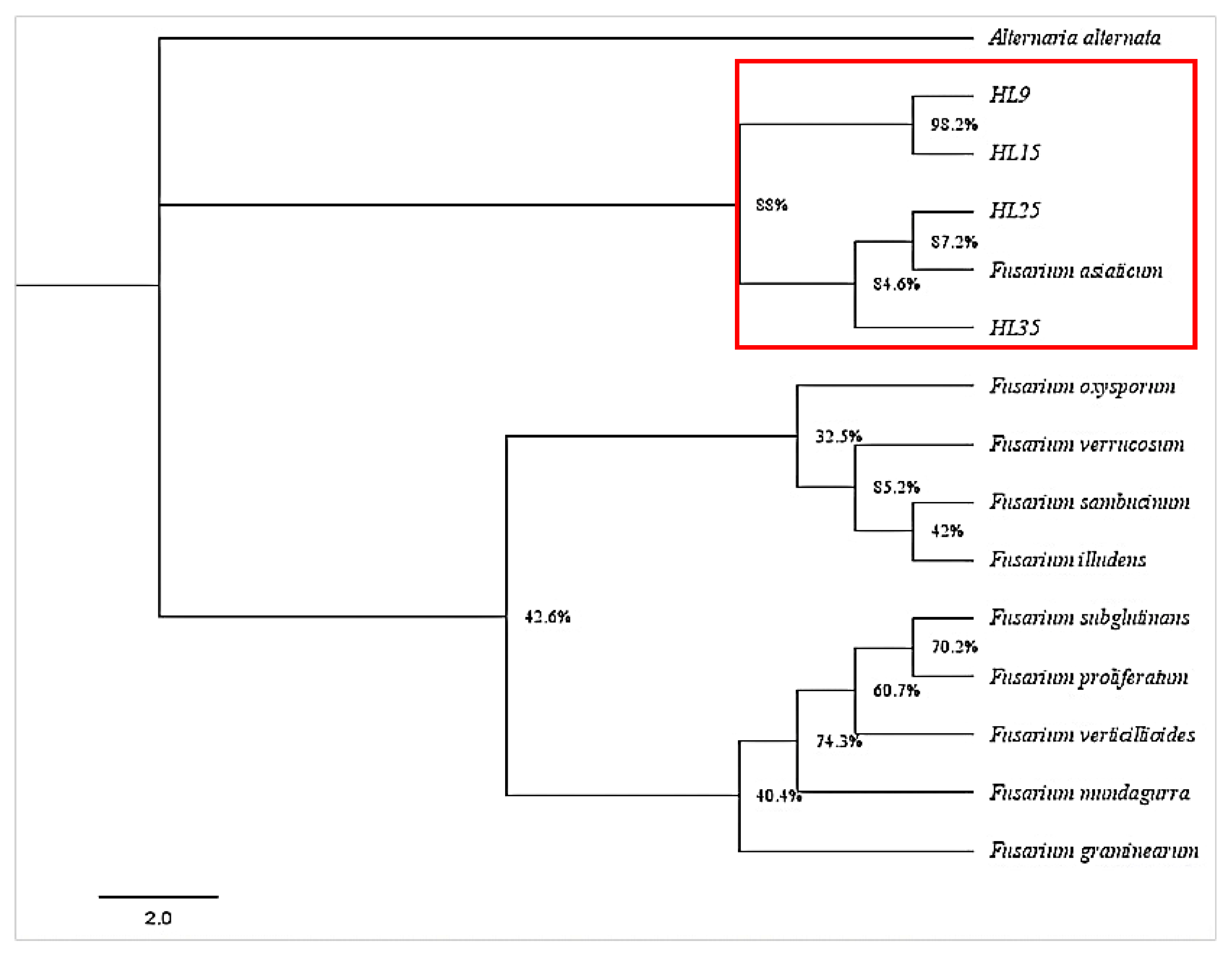
Genomic DNA from four single-conidium cultures (HL9, HL15, HL25, and HL35) was extracted and amplified using fungal universal primers ITS, Tef1, and Tub2. The obtained sequences were deposited in the GenBank (accession numbers are shown in Table A1). BLAST analysis revealed that the ITS1/4, EF1-728F/986R, and Bt2a/Bt2b sequence amplicons of HL9, HL15, HL25, and HL35 shared high similarity with those of F. asiaticum strain MTLYB02 (OM100564.1), strain RTH17 (LC500693.1), and strain HBTS484 (KM062027.1), respectively. In addition, the phylogenetic analysis showed that isolates HL9, HL15, HL25, and HL35 belonged to the same evolutionary branch as F. asiaticum, with high similarity (Figure 4). The combination of molecular and morphological methods confirmed the twenty-eight isolates were F. asiaticum.
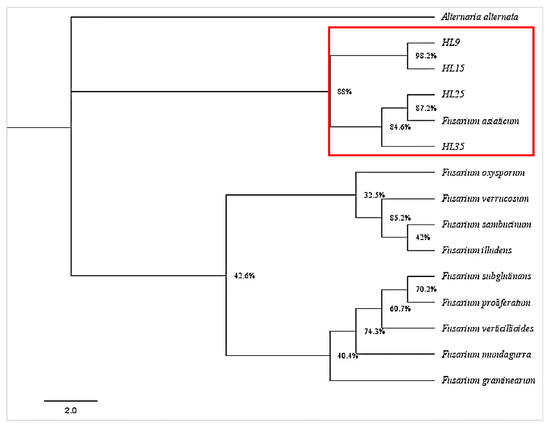
Figure 4.
A phylogenetic tree of Fusarium asiaticum isolates HL9, HL15, HL25, and HL35, along with members of Fusarium spp., was constructed based on Bayesian inference. The analysis was performed on the combined dataset of internal transcribed spacer region (ITS), translation elongation factor 1-α (Tef1), and β-tubulin (Tub2) gene sequences. The tree-sampling frequency was set at 1000 generations. Branches with Bayesian posterior probabilities of 0.997 were considered significantly supported. F. asiaticum was designated as the outgroup.
3.2. Pathogenicity of Fusarium asiaticum on Soybean Roots
Differences in pathogenicity were detected among the 28 isolates of F. asiaticum. Among these were four highly pathogenic isolates, 10 moderately pathogenic isolates, and 14 weakly pathogenic isolates (Table 2). The four highly pathogenic isolates and six moderately pathogenic isolates were selected for subsequent tests.

Table 2.
Disease index and pathogenicity of representative Fusarium asiaticum isolates isolated from soybean root rot samples in Hailun, Heilongjiang province, China.
3.3. Biological Characteristics of Fusarium asiaticum
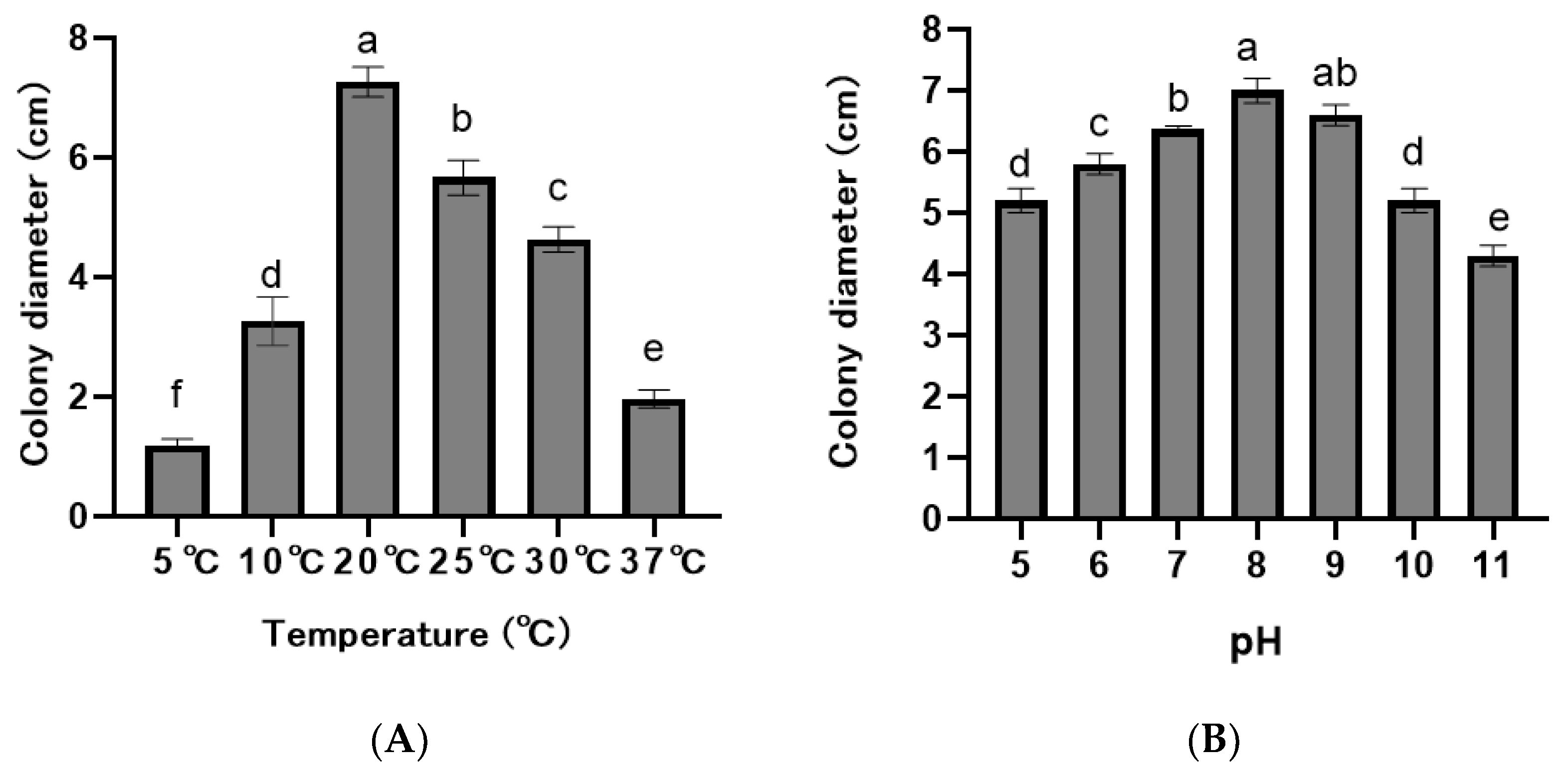
The F. asiaticum isolates grew in the pH range 5.0–11.0, but mycelial growth varied significantly at different pH values (p < 0.05), with an optimal pH of 8.0 (Figure 5A). The ten isolates could grow in the temperatures of 10–30 °C and did not grow at 5 °C and 37 °C, with an optimal temperature of 20 °C (Figure 5B).
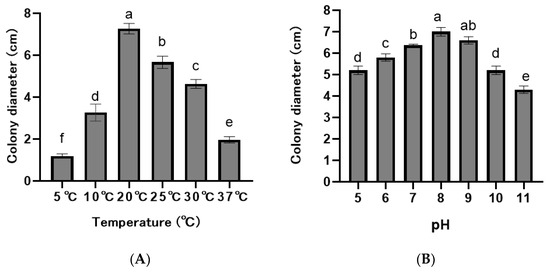
Figure 5.
Colony diameters of Fusarium asiaticum isolates at different pHs and temperatures. (A) Temperature. (B) pH. According to the least significant difference test (p = 0.05), the different letters above the bar indicate significant differences for each isolate.
3.4. Host Range Determination of Fusarium asiaticum
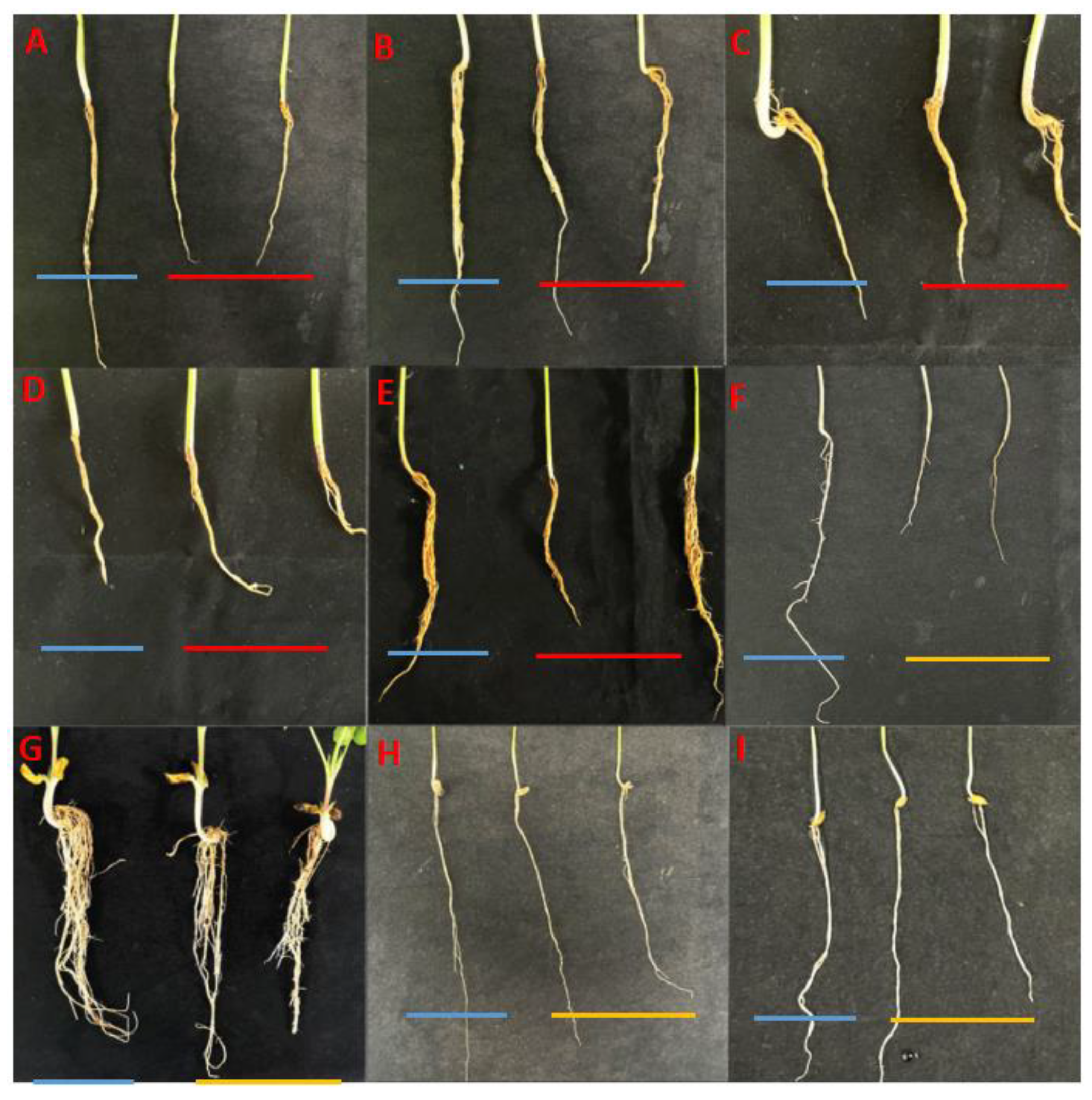
The pathogenicity tests of ten F. asiaticum isolates on different crops showed that they were pathogenic to the roots of black soybean, French bean, white hyacinth bean, mung bean, and adzuki bean roots, but not peanut, corn, adzuki bean, and oat roots (Figure 6). No symptoms were observed in the control seedlings of each crop that were treated with sterile water. Fusarium asiaticum isolates inoculated on seedlings of different crops were successfully re-isolated from the diseased parts of inoculated black soybean, French bean, white hyacinth bean, mung bean, and adzuki bean roots but could not be isolated from the inoculated parts of corn, peanut, rice, and oat roots.
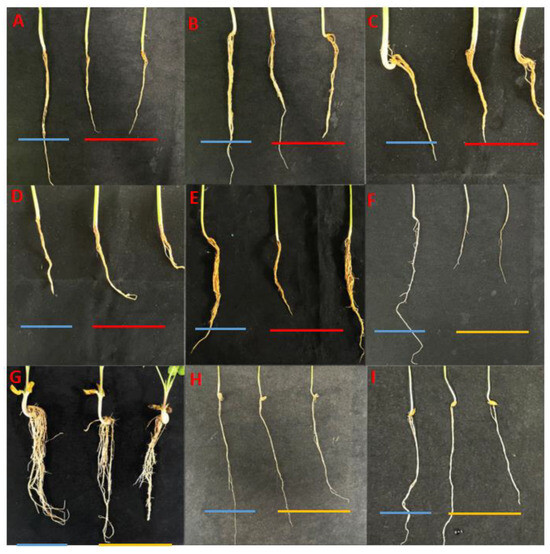
Figure 6.
Host range of Fusarium asiaticum determined. (A) black soybean (Glycine max L.) roots. (B) French bean (Phaseolus vulgaris L.) roots. (C) white hyacinth bean (Dolicho Lablab L.) roots. (D) mung bean (P. radiatus L.) roots. (E) adzuki bean (Vigna umbellata T.) roots. (F) rice (Oryza sativa L.) roots. (G) peanut (Arachis hypogaea L.) roots. (H) corn (Zea mays L.) roots. (I) oats (Avena sativa L.) roots. In each picture, the blue line represents the control plant; the red line represents the plants inoculated with F. asiaticum, whose roots showed significant underdevelopment and even decay compared to the control; the yellow line represents the plants inoculated with F. asiaticum but showed no significant difference in root morphology compared to the control.
3.5. Sensitivity to Fungicides
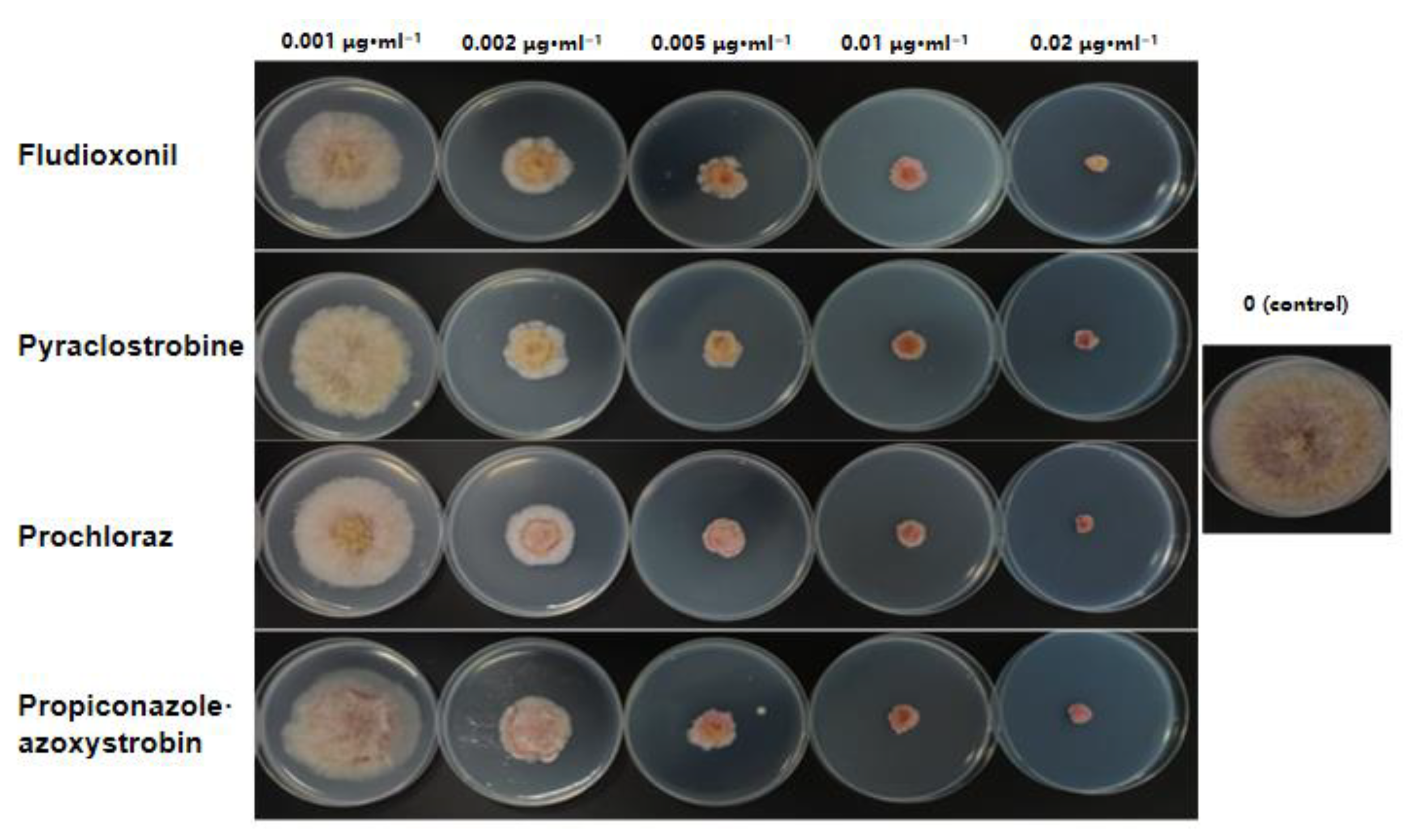
The ten tested F. asiaticum isolates showed consistent sensitivity to pyraclostrobine, prochloraz, fludioxonil, and propiconazole·azoxystrobin. Fludioxoni had the strongest inhibitory effect on F. asiaticum, with an EC50 value of 0.0029–0.0071 μg·mL−1, followed by pyraclostrobine and prochloraz, with EC50 values of 0.0045–0.0076 and 0.0059–0.0126 μg·mL−1, respectively. In addition, propiconazole·azoxystrobin had the weakest inhibitory effect, with an EC50 value of 0.0101–0.0187 μg·mL−1 (Table 3, Figure 7).

Table 3.
Sensitivity of the ten tested Fusarium asiaticum isolates to frequently used fungicides for the control of soybean root rot in northeast China.
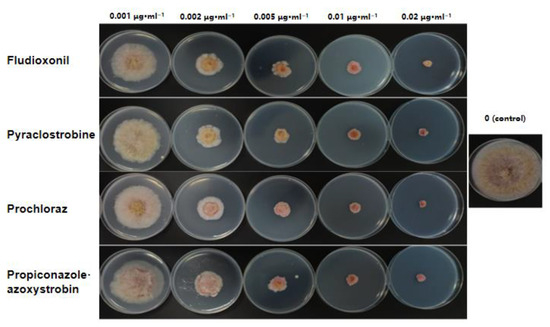
Figure 7.
Sensitivity of Fusarium asiaticum isolate HL35 to fungicides.
3.6. Efficacy of Fungicides on Soybean Root Rot Caused by Fusarium asiaticum
Based on the result of the sensitivity of F. asiaticum to fungicides, fludioxonil and pyraclostrobin were selected as field control agents. In the tested doses, the higher the effective dose of fludioxonil and pyraclostrobin, the better the pot control effects (Table 4). As shown in Table 4, fludioxonil at 250 μg·mL−1 markedly reduced the severity of soybean root rot caused by F. asiaticum and had the best control efficacy of 73.8%. Pyraclostrobin at 250 μg·mL−1 also had good control efficacy of 69.4%. Overall, fludioxonil at 250 μg·mL−1 was the most effective dose for controlling soybean root rot. Moreover, the average plant height, root length, and fresh weight of all treated plants were significantly greater than those of the control group (p < 0.05).

Table 4.
Control effect of fludioxonil and pyraclostrobin on soybean root rot through pot experiments in a greenhouse.
4. Discussion
Soybean is a pivotal food crop and oil crop in China, of which Heilongjiang Province is the main soybean-producing area [67]. Soybean root rot, a soil-borne ailment, affects the entire soybean production lifecycle and severely curtails soybean yields globally [68]. However, distinguishing F. asiaticum from other Fusarium species using traditional morphological inspections or molecular analysis relying on rDNA-ITS sequencing proves challenging [69]. To ensure the accuracy of identification, the translation elongation factor 1-α (Tef1) and β-tubulin (Tub2) genes of representative isolates can be amplified and sequenced, as was performed in the current study. Fusarium asiaticum has been reported to infect soybeans and cause root rot in southwest China [15], but it is the first identified pathogen of soybean root rot in northeastern China. There are significant differences in climate and soil between the two places. Thus, characterizing F. asiaticum is crucial for understanding the etiology of the disease, including its occurrence and prevalence, as well as developing more scientific and appropriate prevention strategies.
There are many inoculation methods for determining the pathogenicity of Fusarium spp., including the root injury inoculation method, root-dipping inoculation method, and root-burying method using sorghum grains with fungal hyphae [70,71,72]. The inoculation amount, investigation time, and investigation standards also differ among these methods. Because the root embedding method is simple, fast, and more closely reflects natural disease infection, this inoculation method was selected in the current study.
A previous study showed that the optimum temperature and pH for F. asiaticum were 20–25 °C and 7.0–9.0, respectively. Soybean root rot occurred during the entire soybean growth period. The optimum growth conditions for F. asiaticum causing soybean root rot were temperatures of 20–25 °C and pH between 7.0 and 9.0, which was consistent with temperature and soil alkalinity conditions in Northeast China. Our results differed from other studies to some extent, which may be due to the different environmental conditions in which the disease occurs. Therefore, this may be one of the reasons why these isolates may seriously harm soybean production in Northeast China.
In the host range determination, F. asiaticum isolates infected black soybean, French bean, white hyacinth bean, mung bean, and adzuki bean roots, but did not infect rice, peanut, corn, and oat roots. It has been reported in China that F. asiaticum infection caused panicle rot in foxtail millet [Setaria italica (L.) P. Beauv.], stem rot in Ligusticum chuanxiong, seedling blight in maize, fruit rot in melon (Cucumis melo L.), and Fusarium head blight (FHB) in wheat (Triticum aestivum L.) [73,74,75,76]. Fusarium asiaticum has a relatively wide geographical distribution and host range, which can lead to significant yield losses. The non-host crops can disrupt the disease cycle by reducing the pathogen’s population in the soil. By alternating soybean cultivation with non-host crops, the accumulation of F. asiaticum in the soil can be minimized, thus reducing the risk of root rot in subsequent soybean crops. However, the selection of non-host crops depends on various factors, such as soil type, climate, and local agricultural practices. Further research is still being conducted to optimize the combination of non-host crops in different regions to achieve the best disease-control and yield-improvement effects.
Currently, the most efficient way to mitigate soybean root rot caused by Fusarium spp. is soybean seed coating with appropriate fungicides [77]. In this study, four chemical fungicides—pyraclostrobin, prochloraz, fludioxonil, and pyraclostrobine—were selected. Results indicated that fludioxonil exerted the strongest inhibitory effect on the growth of F. asiaticum, followed by pyraclostrobin, based on their sensitivities to the selected fungi. In the greenhouse pot experiment, 250 μg·mL−1 fludioxonil reduced disease incidence by 73.8% and improved soybean-seedling quality. However, Qiu et al. (2018) found that four field strains of F. asiaticum were highly resistant to fludioxonil, the EC50 values ranging from 80 to > 400 μg·mL−1 [78]. In the present study, F. asiaticum isolates from diseased soybean roots showed high sensitivity to fludioxonil. F. asiaticum is a newly emerged pathogen causing soybean root rot in northeast China; the isolates have not developed resistance to fludioxonil. Thus, fludioxonil holds potential for controlling soybean root rot caused by F. asiaticum in northeast China. Nevertheless, further research is advisable to precisely determine the appropriate application method and timing.
5. Conclusions
To the best of our knowledge, this study is the first to delve into the effects of Fusarium asiaticum on soybean root rot in northeast China. Our results show that F. asiaticum has a broad host range and can cause root rot, thus posing a potential risk to regional crop production. Employing intercropping with non-host plants, combined with the application of fludioxonil, can effectively control the soybean root rot caused by F. asiaticum. Therefore, when devising advanced strategies for soybean disease management, it is crucial to conduct in-depth investigations into the occurrence of this disease. This not only aids in better understanding the disease mechanism but also facilitates the development of more targeted and effective control measures. By comprehensively examining disease occurrence and considering factors such as soil conditions, climate, and crop growth cycles, we can optimize the application of control methods like intercropping and fungicide use. This comprehensive approach will contribute to the sustainable development of soybean production in northeast China, ensuring both high yields and good quality.
Author Contributions
Conceptualization, Y.L. and J.L.; methodology, Y.L. and J.D.; software, J.L., W.C., Q.Z., Z.R., L.L., and L.S.; validation, J.L., W.C., Q.Z., Z.R., L.L., and L.S.; formal analysis, Y.L. and J.D.; investigation, J.L., W.C., Q.Z., Z.R., L.L., and L.S.; resources, Y.L. and J.D.; data curation, J.L., W.C., Q.Z., Z.R., L.L., and L.S.; writing—original draft preparation, J.L., W.C., and Q.Z.; writing—review and editing, Y.L. and J.L.; funding acquisition, Y.L. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Project of the Heilongjiang Provincial Natural Science Foundation (ZD2023C001), the National Natural Science Foundation of China (32372048), the China Agriculture Research System of MOF and MARA (CARS04), the Heilongjiang Province Seed Industry Innovation and Development Project, the Heilongjiang Collaborative Innovation and Extension System of Modern Agricultural Industry Technology of Forage and Feed, and the Talent Introduction Project of Northeast Agricultural University (54960312).
Data Availability Statement
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Sequences used for concatenated alignment.
Table A1.
Sequences used for concatenated alignment.
| Strains | ITS Regions | TEF Gene | GPD Gene |
|---|---|---|---|
| Fusarium asiaticum HL9 | OQ061210.1 | OQ378361.1 | OQ378358.1 |
| F. asiaticum HL15 | OQ061466.1 | OQ378360.1 | OQ378363.1 |
| F. asiaticum HL25 | OQ061472.1 | OQ378362.1 | OQ378359.1 |
| F. asiaticum HL35 | OM967192.1 | ON011079.1 | ON011080.1 |
| F. asiaticum | OM100564.1 | LC500693.1 | KM062027.1 |
| F. oxysporum | MH221085.1 | KY123890.1 | LC592361.1 |
| F. subglutinans | KY318486.1 | KF467375.1 | OK000516.1 |
| F. verticillioides | KX385055.1 | KF467376.1 | OK000520.1 |
| F. mundagurra | MZ379241.1 | MZ399212.1 | MZ399215.1 |
| F. verrucosum | KM231812.1 | KM231940.1 | KM232077.1 |
| F. proliferatum | GU074010.1 | KF467371.1 | GU338455.1 |
| F. sambucinum | DQ132833.1 | KM231941.1 | KF896804.1 |
| F. illudens | KM231806.1 | KM231934.1 | KM232068.1 |
| F. graminearum | JX162395.1 | MW620072.1 | OM048104.1 |
| Alternaria alternata | MK351431.1 | MT178330.1 | MN607983.1 |
References
- Patil, G.; Vuong, T.D.; Kale, S.; Valliyodan, B.; Deshmukh, R.; Zhu, C.S.; Wu, X.L.; Bai, Y.H.; Yungbluth, D.; Lu, F.; et al. Dissecting genomic hotspots underlying seed protein, oil, and sucrose content in an interspecific mapping population of soybean using high-density linkage mapping. Plant Biotechnol. J. 2018, 16, 1939–1953. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Kang, Z.; Huang, H.N.; Chen, H.B.; Gong, G.S.; Yong, T.W.; Yang, W.; Chang, X.L. Development of 8% uniconzole·propiconazole·abamectin suspension seed coating agent and its control effect against Fusarium root rot of soybean. J. Nucl. Agric. 2020, 34, 954–962. [Google Scholar]
- Li, Y.G.; Zhao, T.X.; Khuong Gia, H.H.; Xu, L.K.; Liu, J.X.; Li, S.X.; Huang, H.W.; Ji, P.S. Pathogenicity and genetic diversity of Fusarium oxysporum causing soybean root rot in northeast China. J. Agric. Sci. 2018, 10, 13–24. [Google Scholar] [CrossRef][Green Version]
- Li, N.N.; Zhou, Q.X.; Chang, K.F.; Yu, H.T.; Hwang, S.F.; Conner, R.L.; Strelkov, S.E.; McLaren, D.L.; Turnbull, G.D. Occurrence, pathogenicity and species identification of Pythium causing root rot of soybean in Alberta and Manitoba, Canada. Crop Prot. 2019, 118, 36–43. [Google Scholar] [CrossRef]
- Roth, M.G.; Webster, R.W.; Mueller, D.S.; Chilvers, M.I.; Faske, T.R.; Mathew, F.M.; Bradley, C.A.; Damicone, J.P.; Kabbage, M.; Smith, D.L. Integrated management of important soybean pathogens of the United States in changing climate. J. Integr. Pest. Manag. 2020, 11, 17. [Google Scholar] [CrossRef]
- Han, S.Y.; Chen, J.X.; Zhao, Y.J.; Cai, H.S.; Guo, C.H. Bacillus subtilis HSY21 can reduce soybean root rot and inhibit the expression of genes related to the pathogenicity of Fusarium oxysporum. Pestic. Biochem. Phys. 2021, 178, 104916. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.G.; Cober, E.; Voldeng, H.D.; Cober, E.; Voldeng, H.D.; Babcock, C.; Clear, R.M. Different aggressiveness in isolates of Fusarium graminearum and Fusarium pseudograminearum causing root rot of soybean. Can. J. Plant Pathol. 2006, 28, 369. [Google Scholar]
- Chang, K.F.; Hwang, S.F.; Conner, R.L.; Ahmed, H.U.; Zhou, Q.; Turnbull, G.D.; Strelkov, S.E.; McLaren, D.L.; Gossen, B.D. First report of Fusarium proliferatum causing root rot in soybean (Glycine max L.) in Canada. Crop Prot. 2015, 67, 52–58. [Google Scholar] [CrossRef]
- Abdelmagid, A.; Hafez, M.; Soliman, A.; Adam, L.R.; Daayfe, F. First report of Fusarium sporotrichioides causing root rot of soybean in Canada and detection of the pathogen in host tissues by PCR. Can. J. Plant Pathol. 2021, 43, 527–536. [Google Scholar] [CrossRef]
- Detranaltes, C.; Jones, C.R.; Cai, G. First report of Fusarium fujikuroi causing root rot and seedling elongation of soybean in Indiana. Plant Dis. 2021, 105, 3762. [Google Scholar] [CrossRef]
- Naeem, M.; Munir, M.; Li, H.J.; Ali Raza, M.; Song, C.; Wu, X.L.; Irshad, G.; Bin Khalid, M.H.; Yang, W.Y.; Chang, X.L. Transcriptiona1 responses of Fusarium graminearum interacted with soybean to cause root rot. J. Fungi. 2021, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Yi, H.; Maharjan, R.; Jeong, M.; Yoon, Y. First report of root rot caused by Fusarium armeniacum on soybean in Korea. Plant Dis. 2022, 106, 1306. [Google Scholar] [CrossRef] [PubMed]
- Detranaltes, C.; Saldanha, M.; Scofield Steven, R.; Cai, G.H. First report of Fusarium commune causing root rot of soybean seedlings in Indiana. Plant Dis. 2022, 106, 3216. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Nelson Jr, B. Effects of soil type, temperature and moisture on development of Fusarium root rot of soybean by Fusarium solani (FSSC 11) and Fusarium tricinctum. Plant Dis. 2022, 106, 2974–2983. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.L.; Yan, L.; Naeem, M.; Khaskheli, M.I.; Zhang, H.; Gong, G.S.; Zhang, M.; Song, C.; Yang, W.Y.; Liu, T.G.; et al. Maize/Soybean relay strip intercropping reduces the occurrence of Fusarium root rot and changes the diversity of the pathogenic Fusarium species. Pathogens 2020, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Lerch, E.; Arritt, N.; Dorrance, A.E.; Robertson, A.E. Quantitative Trait Loci (QTL) conferring resistance in soybean to root rot caused by Pythium oopapillum. In Proceedings of the 2017 APS Annual Meeting, San Antonio, TX, USA, 5–9 August 2017. [Google Scholar]
- Grijalba, P.E.; Ridao, A.D.; Steciow, M. Damping off on soybean (Glycine max) caused by Pythium aphanidermatum in Buenos Aires Province (Argentina). Rev. Fac. Cienc. Agrar. 2020, 52, 282–288. [Google Scholar]
- Kumar, S.; Rajput, L.S.; Ramteke, R.; Nataraj, V.; Ratnaparkhe, M.B.; Maheshwari, H.S.; Shivakumar, M. First report of root rot and damping-off disease in soybean (Glycine max) caused by Pythium deliense in India. Plant Dis. 2021, 105, 2022. [Google Scholar] [CrossRef]
- Liu, B.; Shen, W.S.; Wei, H.; Smith, H.; Louws, F.J.; Steadman, J.R.; Correl, J.C. Rhizoctonia communities in soybean fields and their relation with other microbes and nematode communities. Eur. J. Plant Pathol. 2016, 144, 671–686. [Google Scholar] [CrossRef]
- Shehata, M.A.; Pflege, F.L.; Davis, D.W. Response of susceptible and moderately resistant pea genotypes to interaction between rhizoctonia and three other stem and root rot pathogens. Plant Dis. 1983, 67, 1146–1148. [Google Scholar] [CrossRef]
- Anderson, T.R. Fungi isolated from stems and roots of soybean in Ontario. Can. Plant Dis. Sur. 1987, 67, 3–5. [Google Scholar]
- Li, S.; Hartman, G.L. First report of Stachybotrys chartarum causing soybean root rot. Plant Dis. 2000, 84, 100. [Google Scholar] [CrossRef] [PubMed]
- Susilowati, A.; Wahyudi, A.T.; Lestari, Y.; Suwanto, A.; Wiyono, S. Potential Pseudomonas isolated from soybean rhizosphere as biocontrol against soilborne phytopathogenic fungi. Hayati J. Biosci. 2011, 18, 51–56. [Google Scholar] [CrossRef]
- Detranaltes, C.; Cai, G. First report of Mycoleptodiscus terrestris causing root rot of soybean in Indiana. Plant Dis. 2020, 105, 1194. [Google Scholar] [CrossRef] [PubMed]
- Mattupalli, C.; Cuenca, F.P.; Shiller, J.B.; Watkins, T.; Hansen, K.; Garzon, C.D.; Marek, S.M.; Young, C.A. Genetic diversity of Phymatotrichopsis omnivora based on mating type and microsatellite markers reveal heterothallic mating system. Plant Dis. 2022, 106, 2105–2116. [Google Scholar] [CrossRef]
- Xin, H.P.; Ma, H.Q.; Liu, J.R.; Zhang, Y.P.; Liu, Y.C.; Zhang, X.D. A preliminary study on epidemiology and control of disease. Soybean Sci. 1987, 189–196. [Google Scholar]
- Li, B.Y.; Ma, S.M. Pathogen species and antigen screening of soybean root rot. J. Plant Prot. 2000, 27, 91–92. [Google Scholar]
- Xing, A.; Wen, J.Z.; Lv, G.Z.; Sun, X.D. Isolation and identification of Fusarium spp. on soybean root rot plants in Heilongjiang Province. J. Northeast Agri. Univ. 2009, 40, 5–9. [Google Scholar]
- Wang, X.Y.; Wen, J.Z. Analysis on species and pathogenicity of Fusarium sojae root rot in three northeastern provinces. Chin. J. Oil Crop 2011, 33, 391–395. [Google Scholar]
- Bai, L.Y.; Zhang, Q.D.; Li, B.; Guo, Q.Y. Identification and pathogenicity determination of the pathogenic Fusarium of soybean root rot in the altay region of Xinjiang. Xinjiang Agric. Sci. 2009, 46, 543–548. [Google Scholar]
- Geng, X.B.; Wang, C.L.; Huang, M.H.; Li, Y.G. Identification of soybean stem rot pathogen causing soybean seedling root rot. Plant Prot. 2015, 41, 127–129. [Google Scholar]
- Yang, X.H.; Gu, X.; Zhao, H.H.; Yao, L.L.; Liu, W.; Shen, H.B.; Zhang, Y.; Liu, L.J.; Ding, J.J. Investigation report on soybean root rot in Sanjiang Plain Area. Chin. Agron. Bull. 2015, 31, 113–116. [Google Scholar]
- Sugimoto, T.; Watanabe, K.; Yoshida, S.; Aino, M.; Matsuyama, M.; Maekawa, K.; Irie, K. The effects of inorganic elements on the reduction of Phytophthora stem rot disease of soybean, the growth rate and zoospore release of Phytophthora sojae. J. Phytopathol. 2007, 155, 97–107. [Google Scholar] [CrossRef]
- Lin, F.; Li, W.; Mccoy, A.G.; Gao, X.; Collins, P.J.; Zhang, N.; Wen, Z.X.; Cao, S.Z.; Wani, S.H.; Gu, C.H.; et al. Molecular mapping of quantitative disease resistance loci for soybean partial resistance to Phytophthora sansomeana. Theor. Appl. Genet. 2021, 134, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.M. Pathogenic pathogen categories distribution and germplasm resistance identification of soybean root rot in Heilongjiang Province. Bull. Chin. Agric. Sci. 2012, 28, 230–235. [Google Scholar]
- Wang, K.; Liu, Y.; Hao, P.H.; Xia, Y.H.; Sun, B.J.; Li, H.L.; Li, Y.U. Occurrence of Pratylenchus coffeae causing root rot of soybean in Shandong Province of China. Plant Dis. 2021, 105, 1227. [Google Scholar] [CrossRef]
- Shen, C.Y.; Su, Y.C. Discovery and preliminary study of Phytophthora soybean in China. Acta Phytopathol. Sin. 1991, 4, 60. [Google Scholar]
- Zhang, L.; Geng, X.B.; Wang, C.L.; Li, Y.G. Identification and virulence of Fusarium spp. causing soybean root rot in Heilongjiang Province. Plant Prot. 2014, 40, 165–168. [Google Scholar]
- Du, Y.X.; Shi, N.N.; Ruan, H.C.; Lian, J.F.; Gan, L.; Chen, F.R. Study on pathogenic fungi causing soybean root rot in Yinchuan and field disease control efficiency of seed coating. Chin. Agric. Sci. Bull. 2021, 37, 103–109. [Google Scholar]
- Broders, K.D.; Lipps, P.E.; Paul, P.A.; Dorrance, A.E. Evaluation of Fusarium graminearum associated with corn and soybean seed and seedling disease in Ohio. Plant Dis. 2007, 91, 1155–1160. [Google Scholar] [CrossRef]
- Dorrance, A.; McClure, S.; Martin, S. Effect of partial resistance on phytophthora stem rot incidence and yield of soybean in Ohio. Plant Dis. 2003, 87, 308–312. [Google Scholar] [CrossRef]
- Xi, X.D.; Fan, J.L.; Yang, X.Y.; Liang, Y.; Zhao, X.L.; Wu, Y.H. Evaluation of the anti-oomycete bioactivity of rhizosphere soil-borne isolates and the biocontrol of soybean root rot caused by Phytophthora sojae. Biol. Control 2022, 166, 104818. [Google Scholar] [CrossRef]
- Zhang, C.J.; Liao, S.Q.; Song, H.; Zhao, X.; Han, Y.P.; Liu, Q.; Li, W.B.; Wu, X.X. Identification for resistance to root rot caused by Fusarium Oxysporum in soybean germplasm and physiological analysis. Soybean Sci. 2017, 36, 441–446. [Google Scholar]
- Wang, C.L. The Analysis and Genetic Diversity of the 48 Soybean Varities Against Three Species of Fusaium Causing Soybean Root Rot. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2016. [Google Scholar]
- Díaz Arias, M.M.; Leandro, L.F.; Munkvold, G.P. Aggressiveness of Fusarium Species and impact of root infection on growth and yield of soybeans. Phytopathology 2013, 103, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Hegde, N.P. Evaluating Chemical Seed Treatments for Fusarium Root Rot Control in Dry Beans and Field Peas. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2014. [Google Scholar]
- Degani, O.; Kalman, B. Assessment of commercial fungicides against onion (Allium cepa) basal rot disease caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum. J. Fungi 2021, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Z.; Man, J.; Xu, D.; Wen, L.; Yang, C.; Xu, Q.; Jiang, Q.T.; Chen, G.Y.; Deng, M.; et al. Genetic diversity of field Fusarium asiaticum and Fusarium graminearum isolates increases the risk of fungicide resistance. Phytopathol. Res. 2023, 5, 51. [Google Scholar] [CrossRef]
- Li, W.Q.; Huang, W.; Zhou, J.; Wang, J.; Liu, J.; Li, Y. Evaluation and control of Alternaria alternata causing leaf spot in soybean in Northeast China. J. Appl. Microbiol. 2023, 134, lxad004. [Google Scholar] [CrossRef]
- Shi, J.L.; Li, Y.Q.; Hu, K.M.; Ren, J.G.; Liu, H.M. Isolation and identification of pathogens from rotted root of Pinellia ternata in Guizhou Province. J. Microbiol. Chin. 2015, 42, 289–299. [Google Scholar]
- Chang, X.L.; Dai, H.; Wang, D.P.; Zhou, H.H.; He, W.Q.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.S.; Shang, J.; et al. Identification of Fusarium species associated with soybean root rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Wang, C.L.; Geng, X.B.; Huang, M.H.; Sun, L.P.; Li, Y.G. A Method to determine pathogenicity of Fusarium oxysporum Causing root rot of soybean at seedling stage. Jilin Agr. Sci. 2015, 40, 69–72. [Google Scholar]
- Wei, J.C. Handbook of Fungus Identification; Shanghai Scientific: Shanghai, China, 1979. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W.; Innis, M.A.; Gelfand, D.H.; Sninsky, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pcr Protocols: A Guide to Methods and Application. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.L.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Mark, P.; van der Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Vasi, T.; Vojinovi, U.; Ujovi, S.; Krnjaja, V.; Stevi, M. In vitro toxicity of fungicides with different modes of action to alfalfa anthracnose fungus, Colletotrichum destructivum. J. Environ. Sci. Health A 2019, 54, 1–8. [Google Scholar]
- Zhang, C.Q.; Liu, Y.H.; Wu, H.M.; Xu, B.C.; Sun, P.L.; Xu, Z.H. Baseline sensitivity of Pestalotiopsis microspora, which causes black spot disease on chinese hickory (Carya cathayensis), to pyraclostrobin. Crop Prot. 2012, 42, 256–259. [Google Scholar] [CrossRef]
- Jiang, N.; Hu, F.Y.; Ye, Y.F. Pathogen identification of a new disease in Siraitia grosvenorii and screening of effective fungicides. Plant Prot. 2015, 41, 173–177. [Google Scholar]
- Lehner, M.S.; Paula Júnior, T.J.; Silva, R.A.; Vieira, R.F.; Carneiro, J.E.S.; Schnabel, G.; Mizubuti, E.S.G. Fungicide sensitivity of Sclerotinia sclerotiorum: A thorough assessment using discriminatory dose, EC50, high-resolution melting analysis, and description of new point mutation associated with thiophanate-methyl resistance. Plant Dis. 2015, 99, 1537–1543. [Google Scholar] [CrossRef]
- Ji, X.; Li, J.; Meng, Z.; Zhang, S.; Dong, B.; Qiao, K. Synergistic effect of combined application of a new fungicide fluopimomide with a biocontrol agent Bacillus methylotrophicus TA-1 for management of gray mold in tomato. Plant Dis. 2019, 103, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Todd, J.W.; David, M.G.; Kistler, H.C.; Aoki, T. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet. Biol. 2004, 41, 600–623. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A.; Bullock, S. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006; p. 176. [Google Scholar]
- Xing, H.Q.; Wang, C.M.; Jin, S.L.; Zhou, T.W.; Guo, C. Isolation and identification of Fusarium asiaticum causing Lathyrus sativus root rot. Acta Agrestia Sin. 2021, 29, 1350–1356. [Google Scholar]
- Xiao, J.L.; Wang, G.J.; Ming, Z.; Jing, Y.; Wen, L.; Bi, Y.; Wang, L.; Lai, Y.C.; Shu, X.T.; Wang, Z. Effect of cultivation pattern on the light radiation of group canopy and yield of spring soybean (Glycine Max L. Merrill). American J. Plant Sci. 2013, 4, 1204–1211. [Google Scholar] [CrossRef][Green Version]
- Yang, X.B.; Ruff, R.L.; Meng, X.Q. Race of Phytophthora sojae in Iowa sobean fields. Plant Dis. 1996, 80, 1418–1420. [Google Scholar] [CrossRef]
- Wu, W.X.; Liu, Y.; Huang, X.Q.; Zhang, L.; Zhou, X.Q.; Liu, H.Y. Establishment and application of rapid molecular detection for Fusarium oxysporum. Acta Pratacul. Sin. 2016, 25, 109–115. [Google Scholar]
- Yin, Y.; Liu, X.; Li, B.; Ma, Z. Characterization of sterol demethylation inhibitor-resistant isolates of Fusarium asiaticum and F. graminearum collected from wheat in China. Phytopathology 2009, 99, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; Lee, T.; Chen, W.Q.; Xu, J.; Xu, J.S.; Yang, L.; Yu, D.; Waalwijk, C.; Feng, J. Population genetic analyses of Fusarium asiaticum populations from barley suggest a recent shift favoring 3ADON producers in southern China. Phytopathology. 2010, 100, 328–336. [Google Scholar] [CrossRef]
- Chang, X.L.; Naeem, M.; Li, H.J.; Yan, L.; Liu, T.G.; Liu, B.; Zhang, H.; Khaskheli, M.I.; Gong, G.S.; Zhang, M.; et al. First report of Fusarium asiaticum as a causal agent for seed decay of soybean (Glycine max) in Sichuan, China. Plant Dis. 2020, 104, 1542. [Google Scholar] [CrossRef]
- Li, B.J.; Chen, Q.H.; Lan, C.Z.; Wang, N.N.; Wang, Y.C.; Weng, Q.Y. Indenfication and pathogenicity test of the pathogens causing soybean root in Fujian. Fujian J. Agricl. Sci. 2011, 26, 798–803. [Google Scholar]
- Yang, S.; Wang, J.S.; Ma, Z.C.; Wang, Y.C.; Wang, K.R. Isolation and identification of Fusarium proliferatum causing soybean root rot and its biological characterization. J. Plant Prot. 2012, 39, 187–188. [Google Scholar]
- Li, Y.G.; Zhang, X.; Zhang, R.; Liu, J.X.; Ali, E.; Ji, P.; Pan, H.Y. Occurrence of seedling blight caused by Fusarium tricinctum on rice in China. Plant Dis. 2019, 103, 1789. [Google Scholar] [CrossRef]
- Kong, F.X.; Zhang, H.J.; Liu, Z.; Chen, G.Q.; Xu, J. First report of panicle rot caused by Fusarium asiaticum on Foxtail millet in China. Plant Dis. 2022, 106, 1062. [Google Scholar] [CrossRef] [PubMed]
- Nyandoro, R.; Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Turnbull, G.D.; Strelkov, S.E. Management of root rot of soybean in Alberta with fungicide seed treatments and genetic resistance. Can. J. Plant Sci. 2019, 99, 499–509. [Google Scholar] [CrossRef]
- Qiu, J.B.; Yu, M.Z.; Yin, Q.; Xu, J.H.; Shi, J.R. Molecular characterization, fitness, and mycotoxin production of Fusarium asiaticum strains resistant to fludioxonil. Plant Dis. 2018, 102, 1759–1765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).