Slope Position Modulates Soil Chemical Properties and Microbial Dynamics in Tea Plantation Ecosystems
Abstract
1. Introduction
2. Materials and Methods
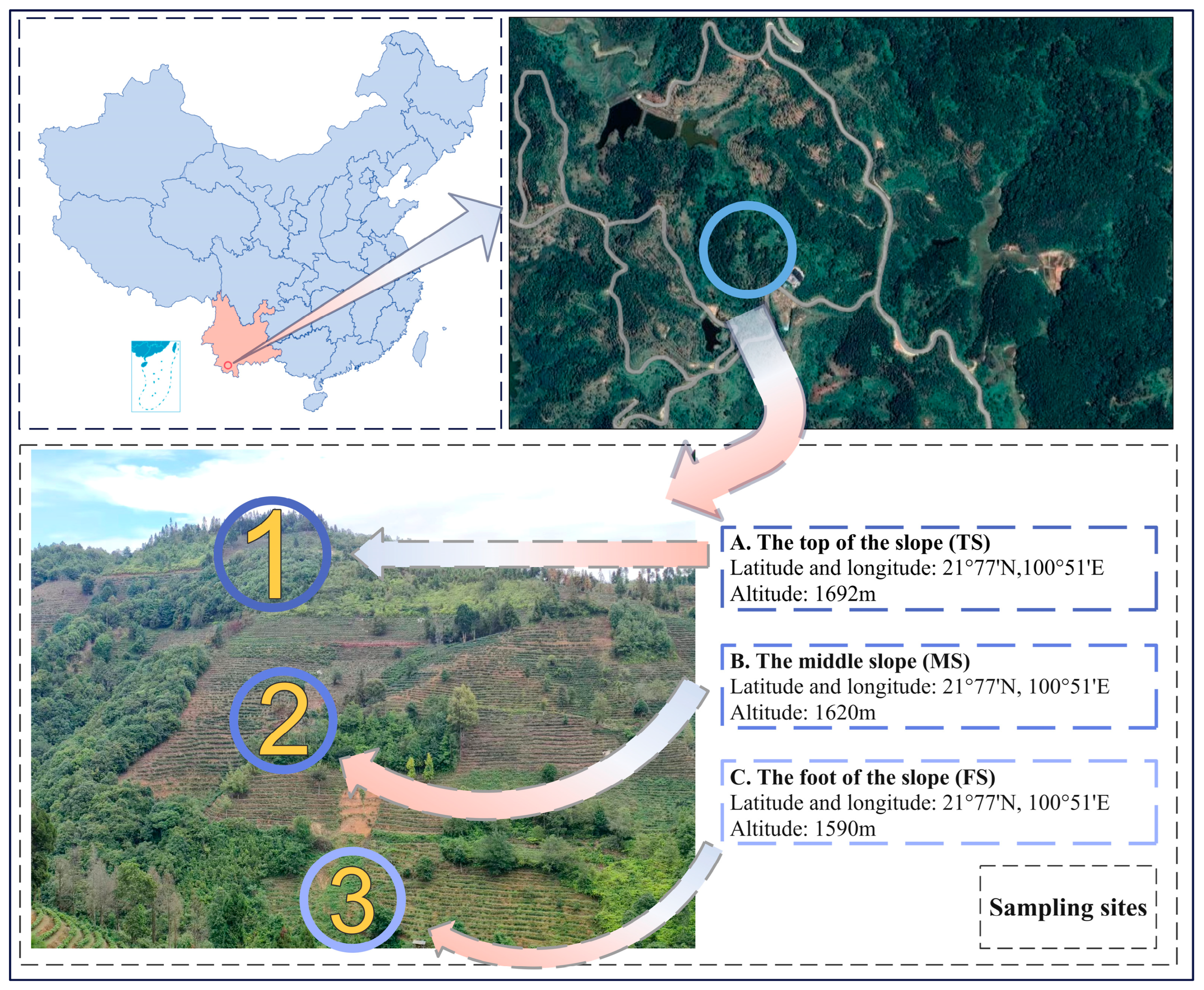
2.1. Overview of the Study Region
2.2. Research Methodology
2.2.1. Experimental Materials
2.2.2. Analysis of Soil Chemical Properties
2.2.3. Sequencing of Microbial Amplicons
2.3. Statistical Analysis
3. Results
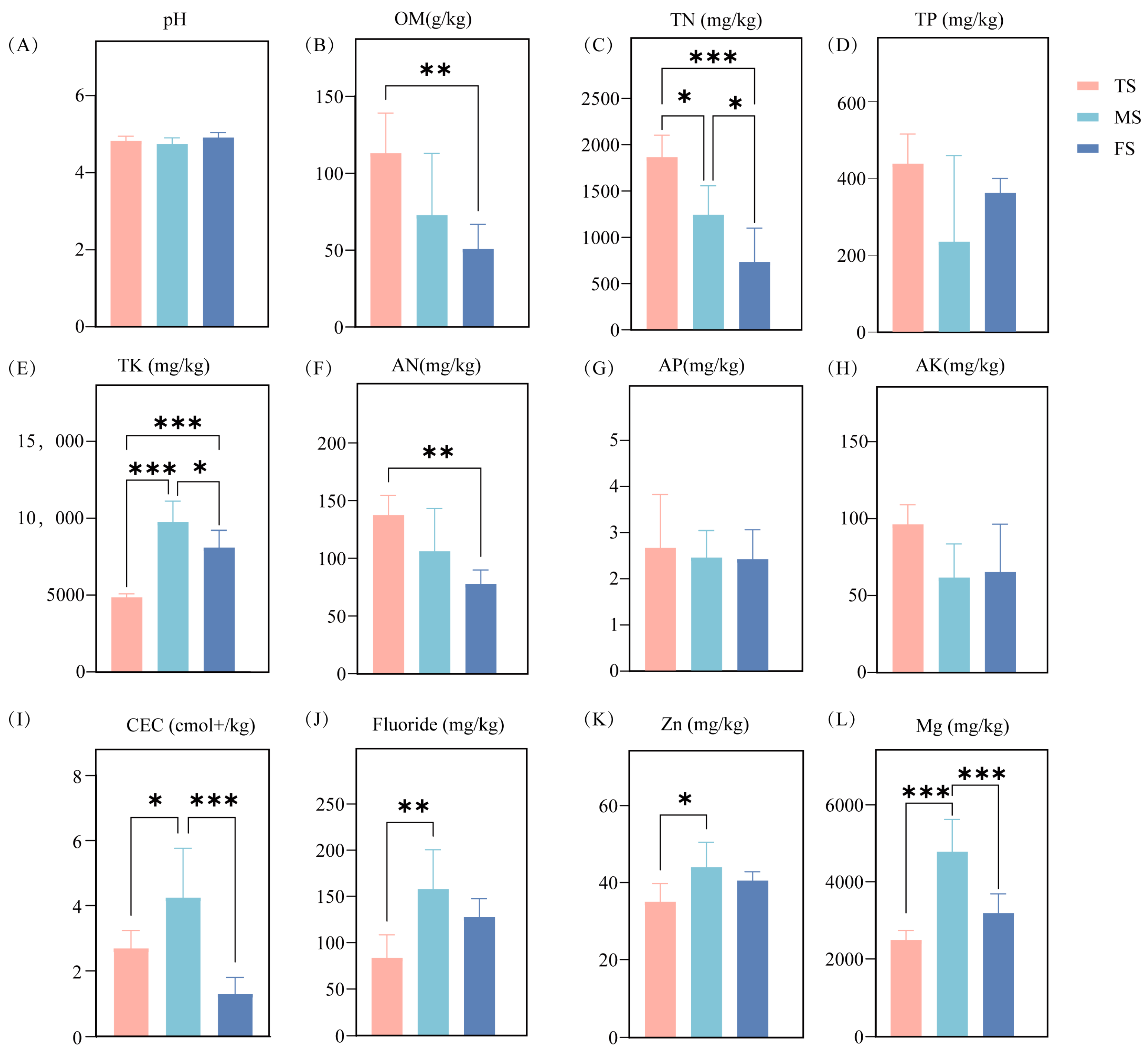
3.1. Soil Chemistry Across Slopes
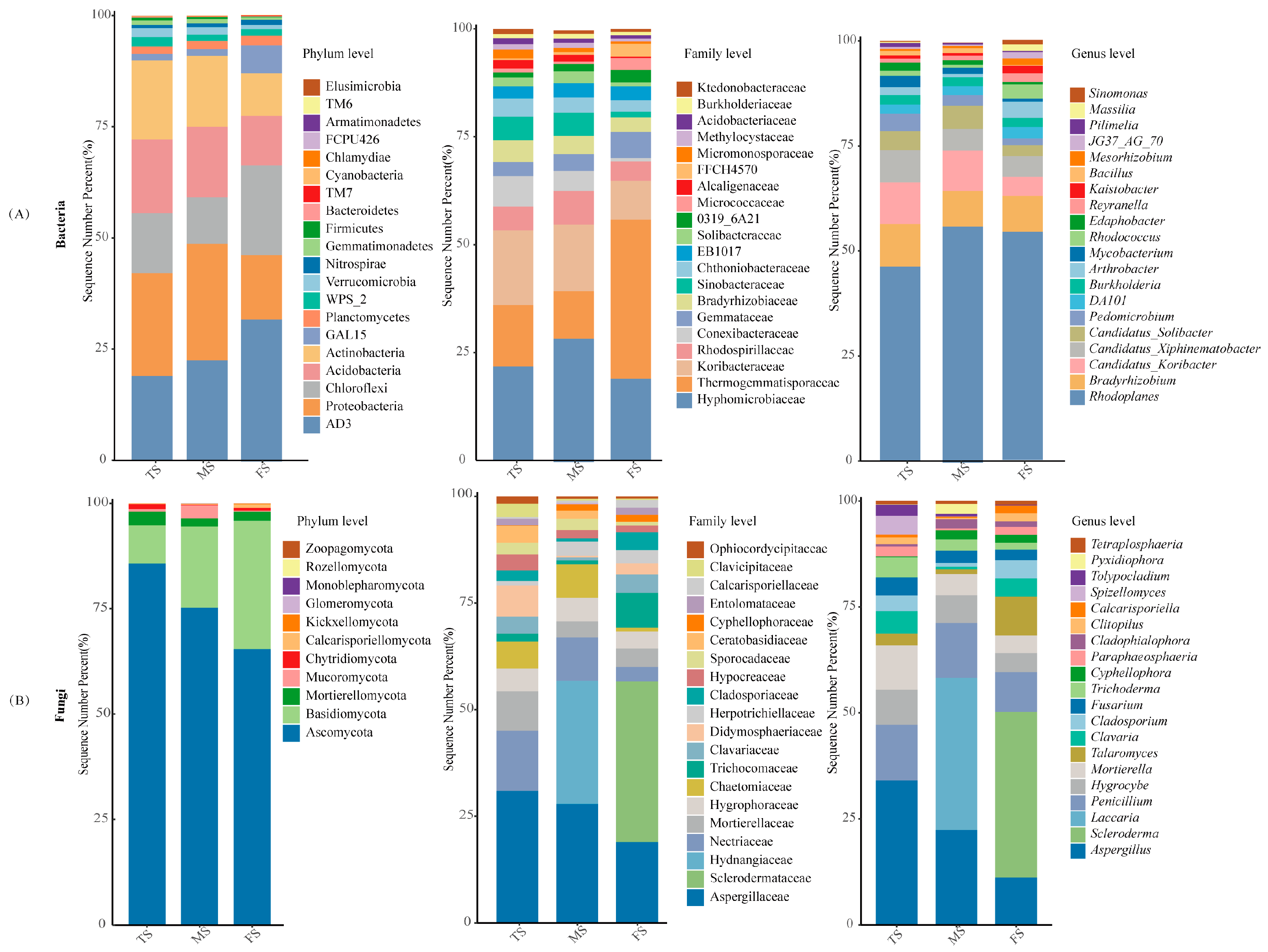
3.2. Microbial Community Composition Across Regions
3.3. Microbial Diversity Analysis
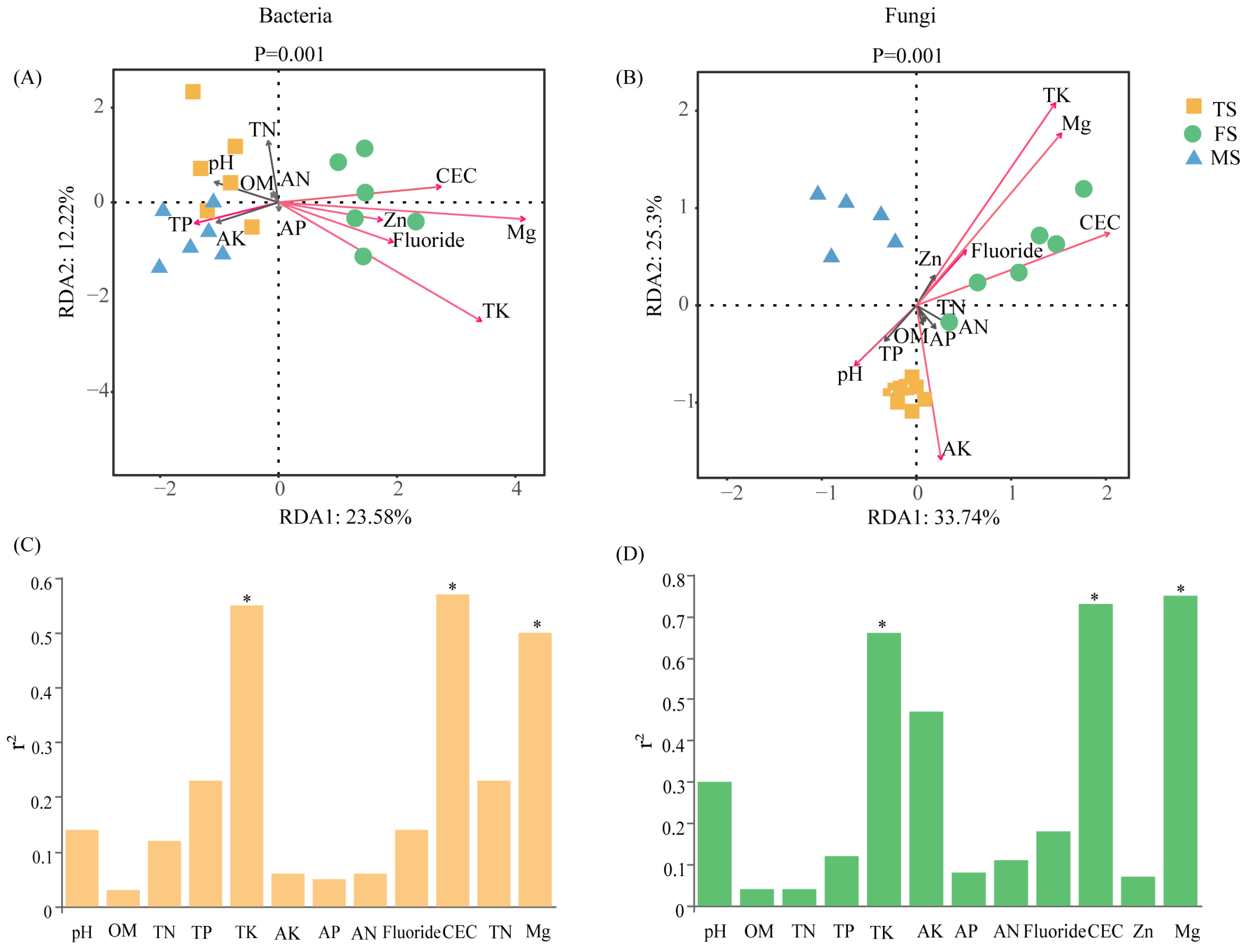
3.4. Soil Composition’s Contribution to Microorganisms
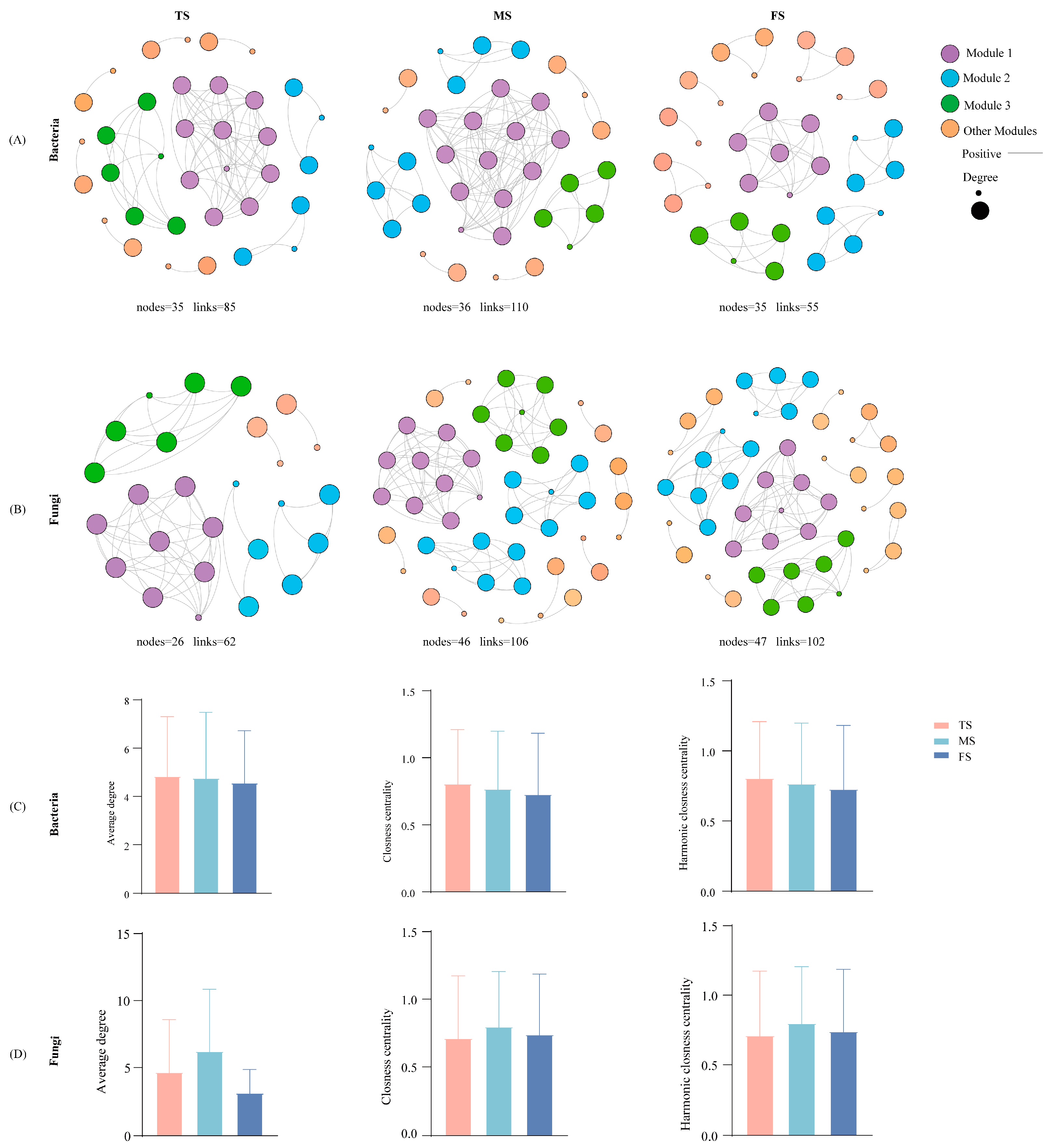
3.5. Slope Difference Drove Differences in Microbial Community Network Structure
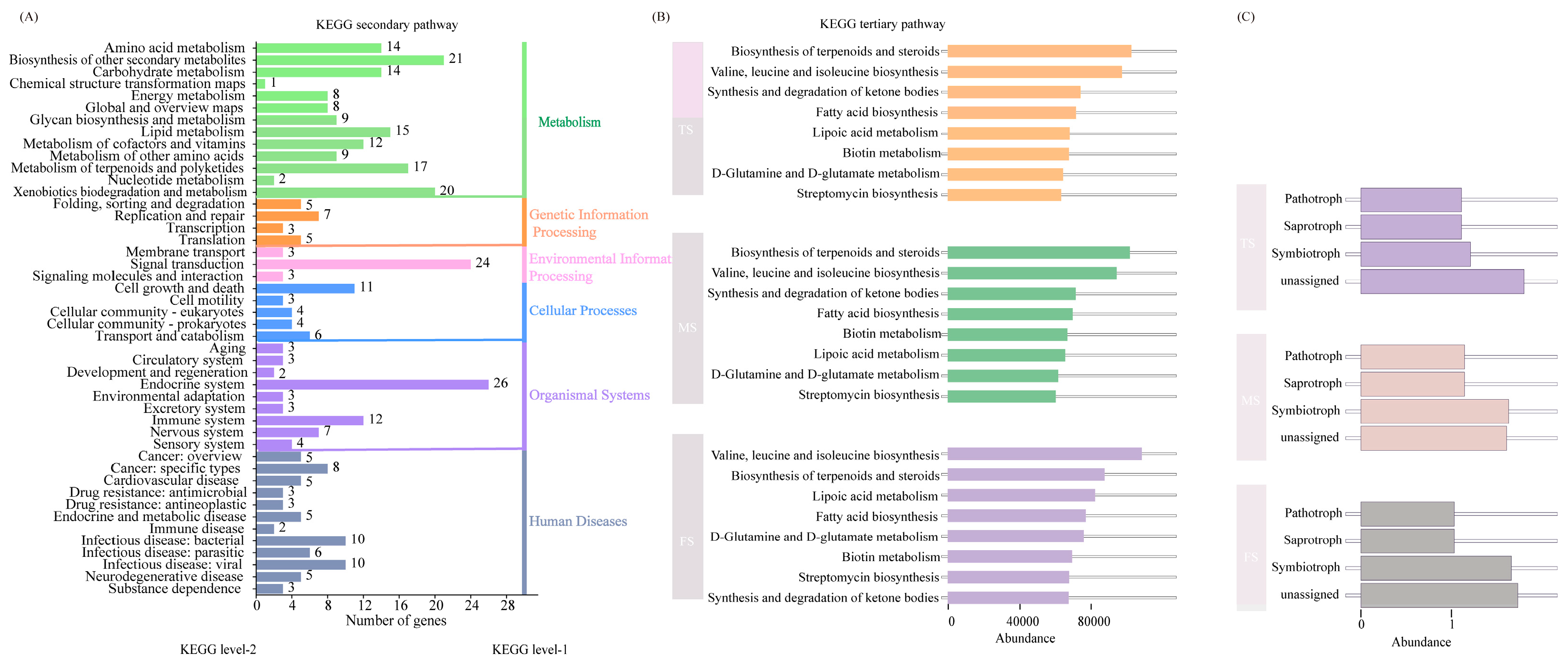
3.6. Microbiological Predictive Functional Analysis
4. Discussion
4.1. Slope Variation in Soil Chemical Properties of Tea Plantations
4.2. Slope Differences in Soil Microbial Community Composition
4.3. Slope Variations in Soil Microbial Co-Occurrence Networks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Wang, Q.; Wang, W.; Ma, R.; Ding, C.; Wei, K.; Wang, L.; Ge, S.; Shi, Y.; Li, X. Transcriptomic and metabolomic insights into temperature-dependent changes in catechin and anthocyanin accumulation in tea plants with different leaf colors. Plant Stress 2024, 14, 100705. [Google Scholar] [CrossRef]
- Karlsson, B.G. The imperial weight of tea: On the politics of plants, plantations and science. Geoforum 2022, 130, 105–114. [Google Scholar] [CrossRef]
- Dai, Y. The overlap of suitable tea plant habitat with Asian elephant (Elephus maximus) distribution in southwestern China and its potential impact on species conservation and local economy. Environ. Sci. Pollut. Res. 2021, 29, 5960–5970. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Q.; Liu, Z.; Chen, W.; Zhao, X.; Zhang, J.; Bao, L.; Zhang, N. Distribution and soil threshold of selenium in the cropland of southwest mountainous areas in China. Sci. Rep. 2024, 14, 16923. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, B.; Li, P.; Chen, A.; Cui, J.; Chen, Y.; Gao, W. Organic management promotes nitrogen transformation in tea plantations soil: A case study from southwestern China. Appl. Soil Ecol. 2025, 206, 105878. [Google Scholar] [CrossRef]
- Yang, H.; Suntornvongsagul, K.; Wang, J.; Wang, Q.; Zhang, W. Indigenous knowledge in the sustainable management of a biodiversity-based millennium-old tea forest in the highlands of Yunnan Province, southwestern China. Biodiversity 2024, 25, 142–152. [Google Scholar] [CrossRef]
- Pennino, A.; Strahm, B.D.; McGuire, K.J.; Bower, J.A.; Bailey, S.W.; Schreiber, M.E.; Ross, D.S.; Duston, S.A.; Benton, J.R. Forest catchment structure mediates shallow subsurface flow and soil base cation fluxes. Geoderma 2024, 450, 117045. [Google Scholar] [CrossRef]
- Xu, L. The Relationship Between Vegetation and Terrain Factor Based on Topographic Correction. Acad. J. Sci. Technol. 2023, 6, 162–167. [Google Scholar] [CrossRef]
- Hancock, G.R.; Murphy, D.; Evans, K.G. Hillslope and catchment scale soil organic carbon concentration: An assessment of the role of geomorphology and soil erosion in an undisturbed environment. Geoderma 2010, 155, 36–45. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, J.; Fu, F.; Zhang, B.; Zhu, S.; Liu, Y.; Li, J.; Chen, G.; Zhang, Y. Correlations and stoichiometric characteristics of C, N, and P contents in Juniperus saltuaria plants, litter, and soil in the Sygera Mountains, southeastern Xizang, China. J. Mt. Sci. 2024, 21, 4254–4268. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Z.; Zhao, W.; Huang, G.; Liu, G.; Li, X.; Wu, J. Effect of slope position on leaf and fine root C, N and P stoichiometry and rhizosphere soil properties in Tectona grandis plantations. J. For. Res. 2023, 34, 1997–2009. [Google Scholar] [CrossRef]
- Cheraghi, J.; Heydari, M.; Rostaminia, M.; Rostaminia, M.; Omidipour, R.; Kooch, Y.; Dey, D.C. Spatial Variability of Soil Properties and Quality along Different Hillslope Sequences in a Protected Semiarid Oak Forest: The Role of Landscape Position and Shape. J. Earth Sci. 2023. [Google Scholar] [CrossRef]
- Sigua, G.C.; Coleman, S.W. Spatial distribution of soil carbon in pastures with cow-calf operation: Effects of slope aspect and slope position. J. Soils Sediments 2009, 10, 240–247. [Google Scholar] [CrossRef]
- Huang, A.; Wang, Z.; Yang, D.; Yang, S.; Bai, W.; Wu, N.; Lu, X.; Liu, Z. Effects of tea oil camellia (Camellia oleifera Abel.) shell-based organic fertilizers on the physicochemical property and microbial community structure of the rhizosphere soil. Front. Microbiol. 2023, 14, 1231978. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Shen, C.; Zou, Z.; Fu, J.; Li, X.; Zhang, L.; Zhang, L.; Han, W.; Fan, L. Biochar stimulates tea growth by improving nutrients in acidic soil. Sci. Hortic. 2021, 283, 110078. [Google Scholar] [CrossRef]
- Miao, P.; Pang, X.; Zhang, M.; Cheng, W.; Zhou, Z.; Li, Y.; Wang, H.; Jia, X.; Ye, J.; Zhang, Q. Enhancing Soil Health and Tea Plant Quality Through Integrated Organic and Chemical Fertilization Strategies. Horticulturae 2024, 10, 1311. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, W.; Wang, Q.; Xia, Y.; Chun, W.; Li, H.; Peng, G.; Huang, W.; Wang, B. Comprehensive Assessment of the Correlation Between Ancient Tea Garden Soil Chemical Properties and Tea Quality. Horticulturae 2024, 10, 1207. [Google Scholar] [CrossRef]
- Singh, A.K.; Chauhan, R.K.; Kumar, C.; Bera, B. Sustaining Darjeeling Organic Tea (Camellia sinensis L) Production and Tea Quality Through Organic Inputs in the Himalayan Foothills. J. Soil Sci. Plant Nutr. 2023, 23, 1774–1796. [Google Scholar] [CrossRef]
- Pradhan, D.; Bharose, R.; Thomas, T. Assessment of Physico-Chemical Properties of Tea Garden Soils of Darjeeling, West Bengal, India. J. Adv. Biol. Biotechnol. 2024, 27, 920–928. [Google Scholar] [CrossRef]
- Sarkar, I.; Kar, P.; Sen, G.; Chhetri, S.; Bhattacharya, M.; Bhattacharyya, S.; Sen, A. Metagenomic outlooks of microbial dynamics influenced by organic manure in tea garden soils of North Bengal, India. Arch. Microbiol. 2022, 204, 33. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Li, L.; Liu, H.; Yao, J.; Ma, C.; Pu, L.; Lei, Z. Biochar, Organic Fertilizer, and Bio-Organic Fertilizer Improve Soil Fertility and Tea Quality. Agronomy 2024, 14, 2339. [Google Scholar] [CrossRef]
- Cai, J.; Qiu, Z.; Liao, J.; Li, A.; Chen, J.; Wu, Z.; Khan, W.; Sun, B.; Liu, S.; Zheng, P. Comprehensive Analysis of the Yield and Leaf Quality of Fresh Tea (Camellia sinensis cv. Jin Xuan) under Different Nitrogen Fertilization Levels. Foods 2024, 13, 2091. [Google Scholar] [CrossRef]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Sitienei, K.; Home, P.G.; Kamau, D.M.; Wanyoko, J.K. Nitrogen and Potassium Dynamics in Tea Cultivation as Influenced by Fertilizer Type and Application Rates. Am. J. Plant Sci. 2013, 04, 59–65. [Google Scholar] [CrossRef]
- Bai, R.; Liu, H.; Liu, Y.; Yong, J.W.H. Effects of Foliar Application of Magnesium Fertilizer on Photosynthesis and Growth in Grapes. Agronomy 2024, 14, 2659. [Google Scholar] [CrossRef]
- Xian, X.; Sun, W.; Zhang, Z.; Gao, Y.; Li, C.; Ding, L.; Wang, Y. Effects of combined application of phosphorus and zinc on growth and physiological characteristics of apple rootstock M9-T337 seedlings (Malus domestica Borkh.). BMC Plant Biol. 2024, 24, 998. [Google Scholar] [CrossRef]
- Trukhachev, V.; Velikii, A.V.; Skuratov, A.; Shitikova, A.; Migunov, R.; Abbas, R.Z. The effectiveness of the combined use of zinc, boron, and magnesium on tea plantations in the humid subtropics of Russia. BIO Web Conf. 2024, 139, 04001. [Google Scholar] [CrossRef]
- Anh-Dao, L.-T.; Minh-Huy, D.; Thu-Huong, N.; Tuong-Thu, V.-T.; Kim-Sinh, N.-T.; Thanh-Nho, N.; Cong-Hau, N. An optimized smartphone-based colorimetric detection method for evaluating the fluoride released in tea extracts from different brewing conditions. J. Iran. Chem. Soc. 2024, 21, 2137–2146. [Google Scholar] [CrossRef]
- Jibola-Shittu, M.Y.; Heng, Z.; Keyhani, N.O.; Dang, Y.; Chen, R.; Liu, S.; Lin, Y.; Lai, P.; Chen, J.; Yang, C.; et al. Understanding and exploring the diversity of soil microorganisms in tea (Camellia sinensis) gardens: Toward sustainable tea production. Front. Microbiol. 2024, 15, 1379879. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Banik, A. Exploring tea (Camellia sinensis) microbiome: Insights into the functional characteristics and their impact on tea growth promotion. Microbiol. Res. 2022, 254, 126890. [Google Scholar] [CrossRef]
- Bora, S.S.; Hazarika, D.J.; Gogoi, R.; Dullah, S.; Gogoi, M.; Barooah, M. Long-term pruning modulates microbial community structure and their functional potential in Tea (Camellia sinensis L.) soils. Appl. Soil Ecol. 2022, 176, 104483. [Google Scholar] [CrossRef]
- Bora, P.; Chandra Bora, L.; Bhuyan, R.P.; Hashem, A.; Fathi Abd-Allah, E. Bioagent consortia assisted suppression in grey blight disease with enhanced leaf nutrients and biochemical properties of tea (Camellia sinensis). Biol. Control 2022, 170, 104907. [Google Scholar] [CrossRef]
- Długosz, J.; Dębska, B.; Piotrowska-Długosz, A. The Effect of Soil Tillage Systems on the Soil Microbial and Enzymatic Properties Under Soybean (Glycine max L. Merrill) Cultivation—Implications for Sustainable Soil Management. Sustainability 2024, 16, 11140. [Google Scholar] [CrossRef]
- Lei, Y.; Ding, D.; Duan, J.; Luo, Y.; Huang, F.; Kang, Y.; Chen, Y.; Li, S. Soil Microbial Community Characteristics and Their Effect on Tea Quality under Different Fertilization Treatments in Two Tea Plantations. Genes 2024, 15, 610. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Niu, Z.; Cao, S.; Chen, S.; Zhao, S.; Zi, Q.; Yu, W.; Xu, H.; Zhao, D.; Qing, M. Effects of pea-tea intercropping on rhizosphere soil microbial communities. Plant Soil 2023, 506, 125–135. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Wang, H.; Li, L.; Wang, X.; Wu, T.; Wang, C.; Qian, Y.; Wang, X.; Xia, Y.; et al. LCLN-CA: A Survival Regression Analysis-Based Prediction Method for Catechin Content in Yunnan Sun-Dried Tea. Horticulturae 2024, 10, 1321. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Luo, W.; Liu, S.; Chen, W.; Chen, C.; Jiao, S.; Wei, G. Soil potassium is correlated with root secondary metabolites and root-associated core bacteria in licorice of different ages. Plant Soil 2020, 456, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, K.; Zhang, Q.; Wang, W. Changes in the composition of rhizosphere bacterial communities in response to soil types and acid rain. J. Environ. Manag. 2023, 325, 116493. [Google Scholar] [CrossRef] [PubMed]
- Zebec, V.; Rastija, D.; Lončarić, Z.; Bensa, A.; Popović, B.; Ivezić, V. Comparison of Chemical Extraction Methods for Determination of Soil Potassium in Different Soil Types. Eurasian Soil Sci. 2018, 50, 1420–1427. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Loftus, S.; Sauer, A.M.; Schneider, E.M.; Erugoti, L.K.; Tharanya, M.; Rötter, R.P.; Kholová, J.; Ahmed, M.A.; Dippold, M.A. Distinct water and phosphorus extraction patterns are key to maintaining the productivity of sorghum under drought and limited soil resources. Sci. Rep. 2025, 15, 4949. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Abbas, Z.; Bilal, M.; Habib, F.; Iqbal, J.; Bashir, F.; Noor, S.; Qazi, M.A.; Niaz, A.; Baig, K.S.; et al. Method development and validation for the determination of potassium (K2O) in fertilizer samples by flame photometry technique. J. King Saud Univ. Sci. 2022, 34, 102070. [Google Scholar] [CrossRef]
- Goda, D.A.; El-Gamal, E.H.; Rashad, M.; Abdel-Fattah, Y.R. The optimization of calcareous soil cation exchange capacity via the feather hydrolysate and N-P fertilizers integration. Sci. Rep. 2025, 15, 4676. [Google Scholar] [CrossRef]
- GB/T 22104-2008; Soil Quality—Analysis of Fluoride—Ion Selective Electrometry. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008.
- Fierer, N.; Jackson, R.B. Robert.The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wu, Y.; Liu, X.; Wu, J.; Wu, P.; Wu, W. Microplastics abundance associated with farmland use types and the impact on soil microbial communities: A case study in Southern China. J. Hazard. Mater. 2024, 480, 136477. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Abbott, S.L. 16S rRNA Gene Sequencing for Bacterial Identification in the Diagnostic Laboratory: Pluses, Perils, and Pitfalls. J. Clin. Microbiol. 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Neilan, B.; Op De Beeck, M.; Lievens, B.; Busschaert, P.; Declerck, S.; Vangronsveld, J.; Colpaert, J.V. Comparison and Validation of Some ITS Primer Pairs Useful for Fungal Metabarcoding Studies. PLoS ONE 2014, 9, e97629. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, W.; Zong, L.; Yang, J.; Jiao, S.; Lin, Y.; Wang, E.; Wei, G. Two cultivated legume plants reveal the enrichment process of the microbiome in the rhizocompartments. Mol. Ecol. 2017, 26, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhao, P.; Wang, C.; Huasai, S.; Chen, H.; Chen, A. Differences in the intestinal microbiota and association of host metabolism with hair coat status in cattle. Front. Microbiol. 2024, 15, 1296602. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, C.; Simujide, H.; Liu, B.; Chen, Z.; Zhao, P.; Huangfu, M.; Liu, J.; Gao, X.; Wu, Y.; et al. Reproductive Hormones Mediate Intestinal Microbiota Shifts during Estrus Synchronization in Grazing Simmental Cows. Animals 2022, 12, 1751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, N.; Chen, J.; Ran, W.; Zhao, Z.; Song, Y. Interpretation of bacterial composition patterns and community assembly processes in the rhizosphere soil of tea trees in karst areas. BMC Microbiol. 2024, 24, 492. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yang, K.; Tang, Z.; Wang, Y. Relationships between Soil Crust Development and Soil Properties in the Desert Region of North China. Sustainability 2017, 9, 725. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, Y.; Xu, A.; Lin, C.; Wang, H.; Yu, J.; Ding, H.; Zhang, Y. Response of Soil Microbial Communities and Functions to Long-Term Tea (Camellia sinensis L.) Planting in a Subtropical Region. Forests 2023, 14, 1288. [Google Scholar] [CrossRef]
- Lv, X.; Kong, H.; Luo, Y.; Dong, D.; Liu, W.; Wu, D.; Ye, Z.; Ma, J.; Liu, D. The Impact of Magnesium on the Growth, Physiology and Quality of Tea (Camellia sinensis L.) Plants under Acid Stress. Agronomy 2024, 14, 767. [Google Scholar] [CrossRef]
- Li, J.; Wu, B.; Zhang, D.; Cheng, X. Elevational variation in soil phosphorus pools and controlling factors in alpine areas of Southwest China. Geoderma 2023, 431, 116361. [Google Scholar] [CrossRef]
- Dessalegn, D.; Beyene, S.; Ram, N.; Walley, F.; Gala, T.S. Effects of topography and land use on soil characteristics along the toposequence of Ele watershed in southern Ethiopia. Catena 2014, 115, 47–54. [Google Scholar] [CrossRef]
- Miheretu, B.A.; Yimer, A.A. Spatial variability of selected soil properties in relation to land use and slope position in Gelana sub-watershed, Northern highlands of Ethiopia. Phys. Geogr. 2017, 39, 230–245. [Google Scholar] [CrossRef]
- Moges, A.; Holden, N.M. Soil Fertility in Relation to Slope Position and Agricultural Land Use: A Case Study of Umbulo Catchment in Southern Ethiopia. Environ. Manag. 2008, 42, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cao, X.; Zhou, Y.; Li, Z.; Yang, Y.; Zhao, D.; Li, Y.; Xu, Z.; Zhang, C.-S. Effect of planting salt-tolerant legumes on coastal saline soil nutrient availability and microbial communities. J. Environ. Manag. 2023, 345, 118574. [Google Scholar] [CrossRef] [PubMed]
- Krause, H.-M.; Mueller, R.C.; Lori, M.; Mayer, J.; Mäder, P.; Hartmann, M. Organic cropping systems alter metabolic potential and carbon, nitrogen and phosphorus cycling capacity of soil microbial communities. Soil Biol. Biochem. 2025, 203, 109737. [Google Scholar] [CrossRef]
- Macdonald, C.A.; Clark, I.M.; Zhao, F.-J.; Hirsch, P.R.; Singh, B.K.; McGrath, S.P. Long-term impacts of zinc and copper enriched sewage sludge additions on bacterial, archaeal and fungal communities in arable and grassland soils. Soil Biol. Biochem. 2011, 43, 932–941. [Google Scholar] [CrossRef]
- Gou, X.; Kong, W.; Sadowsky, M.J.; Chang, X.; Qiu, L.; Liu, W.; Shao, M.; Wei, X. Global responses of soil bacteria and fungi to inoculation with arbuscular mycorrhizal fungi. Catena 2024, 237, 107817. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, J.; Wu, H.; Zhu, J.; Zong, N.; An, H.; Wang, C.; Zuo, X.; Wei, C.; Zhang, F.; et al. Factors controlling the contributions of bacterial and fungal residue carbon to soil organic carbon in grassland ecosystems. Catena 2024, 241, 108006. [Google Scholar] [CrossRef]
- Silar, P. Phenotypic instability in fungi. Adv. Appl. Microbiol. 2019, 107, 141–187. [Google Scholar] [PubMed]
- Duan, Y.; Lian, J.; Wang, L.; Wang, X.; Luo, Y.; Wang, W.; Wu, F.; Zhao, J.; Ding, Y.; Ma, J.; et al. Variation in Soil Microbial Communities Along an Elevational Gradient in Alpine Meadows of the Qilian Mountains, China. Front. Microbiol. 2021, 12, 684386. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fan, K.; Li, Y.; Yang, T.; He, J.S.; Chu, H. Archaea Enhance the Robustness of Microbial Co-occurrence Networks in Tibetan Plateau Soils. Soil Sci. Soc. Am. J. 2019, 83, 1093–1099. [Google Scholar] [CrossRef]
- Bittleston, L.S. Connecting microbial community assembly and function. Curr. Opin. Microbiol. 2024, 80, 102512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tian, X.; Xiang, Q.; Penttinen, P.; Gu, Y. Response of soil microbial community structure and function to different altitudes in arid valley in Panzhihua, China. BMC Microbiol. 2022, 22, 86. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, L.; Li, H.; Xia, Y.; Wang, H.; Wang, Q.; Yuan, W.; Zhou, M.; Tian, J.; Wang, B. Slope Position Modulates Soil Chemical Properties and Microbial Dynamics in Tea Plantation Ecosystems. Agronomy 2025, 15, 538. https://doi.org/10.3390/agronomy15030538
Li L, Chen L, Li H, Xia Y, Wang H, Wang Q, Yuan W, Zhou M, Tian J, Wang B. Slope Position Modulates Soil Chemical Properties and Microbial Dynamics in Tea Plantation Ecosystems. Agronomy. 2025; 15(3):538. https://doi.org/10.3390/agronomy15030538
Chicago/Turabian StyleLi, Limei, Lijiao Chen, Hongxu Li, Yuxin Xia, Houqiao Wang, Qiaomei Wang, Wenxia Yuan, Miao Zhou, Juan Tian, and Baijuan Wang. 2025. "Slope Position Modulates Soil Chemical Properties and Microbial Dynamics in Tea Plantation Ecosystems" Agronomy 15, no. 3: 538. https://doi.org/10.3390/agronomy15030538
APA StyleLi, L., Chen, L., Li, H., Xia, Y., Wang, H., Wang, Q., Yuan, W., Zhou, M., Tian, J., & Wang, B. (2025). Slope Position Modulates Soil Chemical Properties and Microbial Dynamics in Tea Plantation Ecosystems. Agronomy, 15(3), 538. https://doi.org/10.3390/agronomy15030538





