Boosting Aeroponic System Development with Plasma and High-Efficiency Tools: AI and IoT—A Review
Abstract
1. Introduction
2. Review Methodology
- What developments in plasma-assisted aeroponic systems have occurred recently?
- How is aeroponic farming enhanced by the integration of AI and IoT?
- How do plant development and nutrient uptake become affected by plasma-activated water (PAW) and plasma-activated mist (PAM)?
- What scaling constraints and problems arise when artificial intelligence, the Internet of Things, and plasma are combined in aeroponics?
- How may new technologies enhance the sustainability and efficiency of aeroponic systems?
3. Results and Discussion
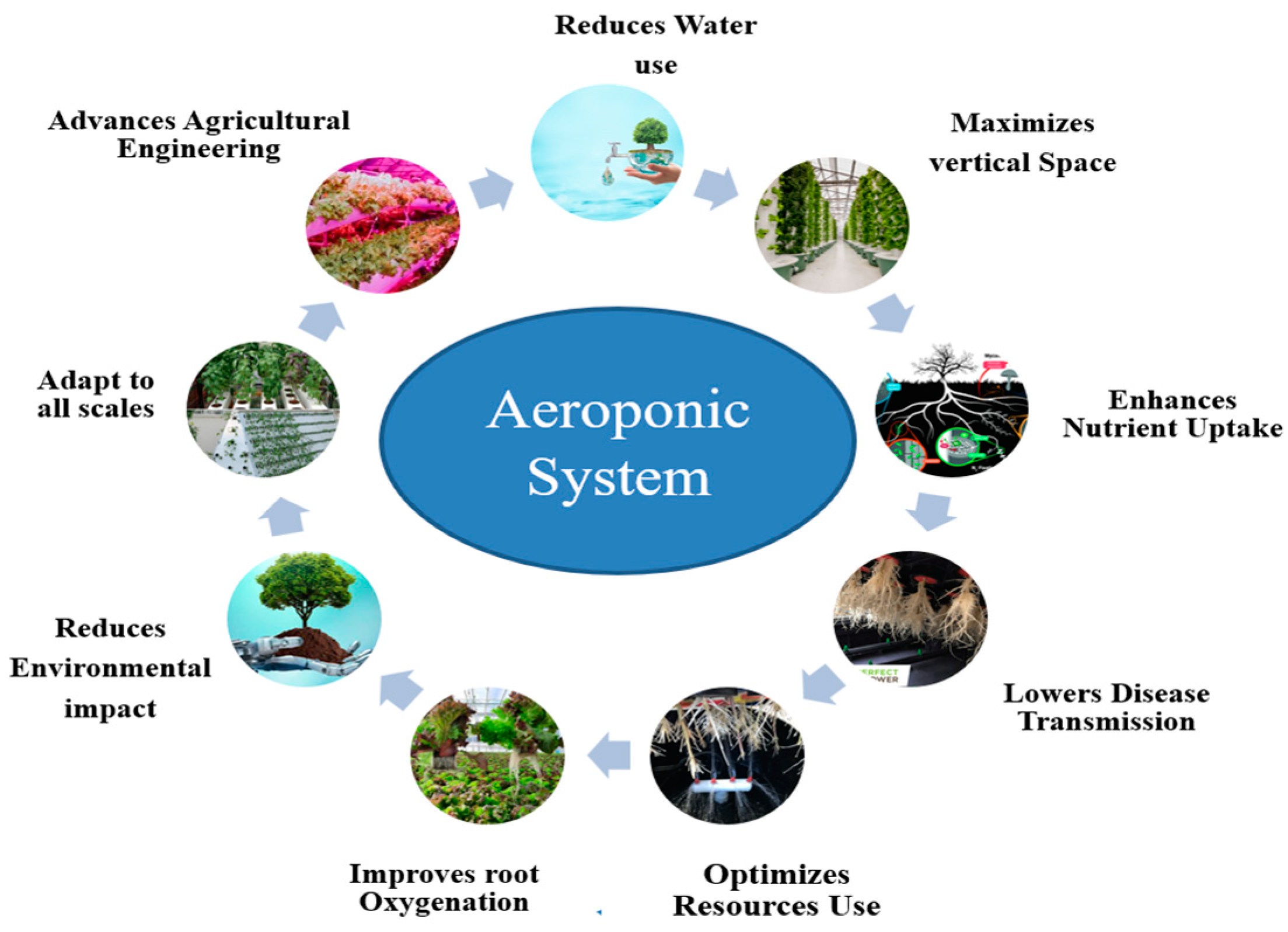
3.1. Aeroponics
3.1.1. Impact of Droplet Size in Low-Pressure and High-Pressure Aeroponic Misting Systems
3.1.2. Comparative Analysis of Aeroponic Systems with Soil and Soilless Systems
3.1.3. Scalability and Resource Efficiency of Aeroponic Systems in Modern Agriculture
3.2. Plasma-Activated Water (PAW) Characteristics
3.3. Plasma-Activated Water (PAW) in Soil-Based and Soilless Cultivation
3.3.1. Effects of Plasma-Activated Water (PAW) in Soil-Based Systems
3.3.2. Effects of Plasma-Activated Water (PAW) in Soilless Cultivation Systems
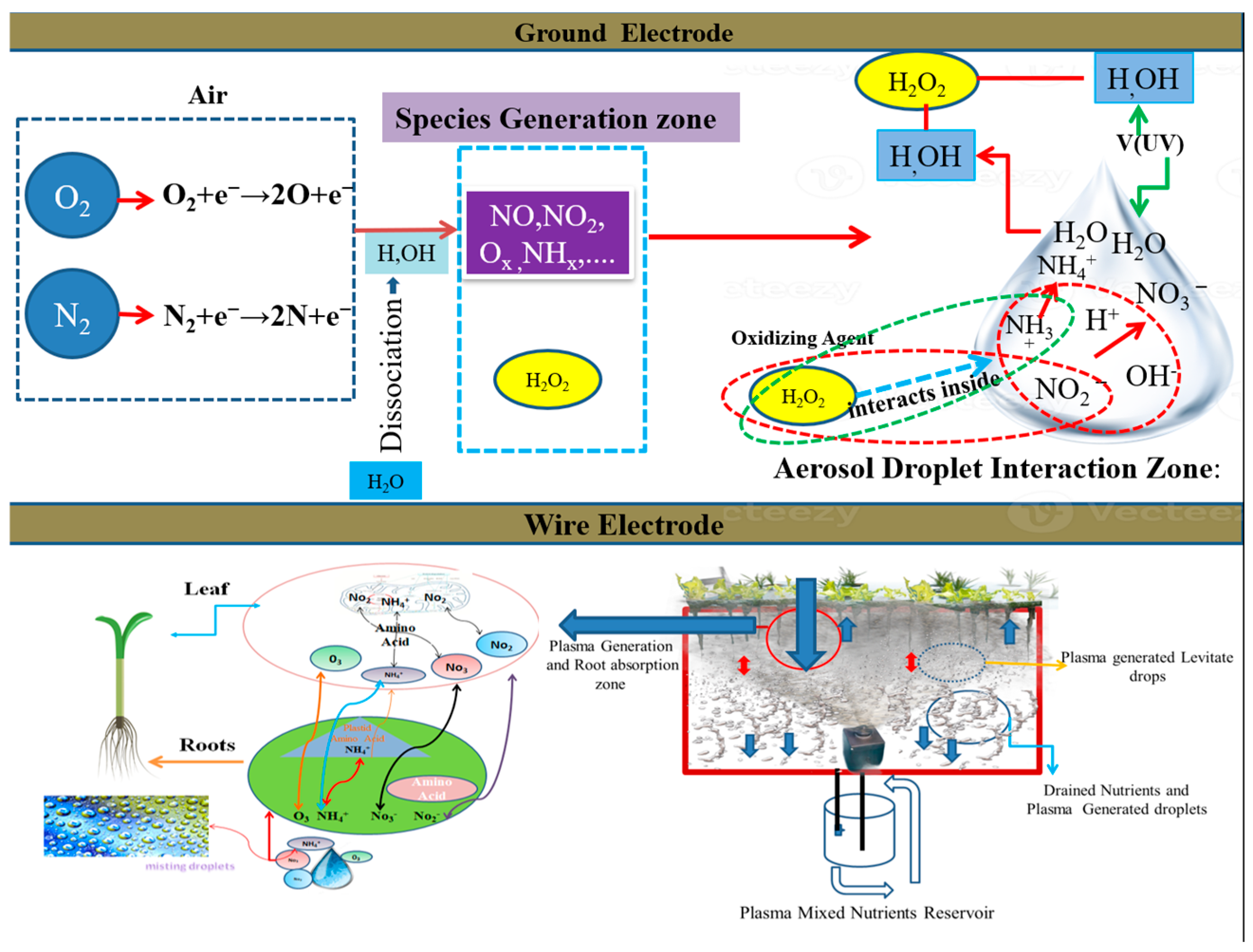
3.4. PAM Generation in Aeroponics Systems
O2+e−→2O+e−
NO+O→NO2
HNO3→H++NO3−
3.4.1. PAM in Aeroponics
3.4.2. Direct Application of PAM
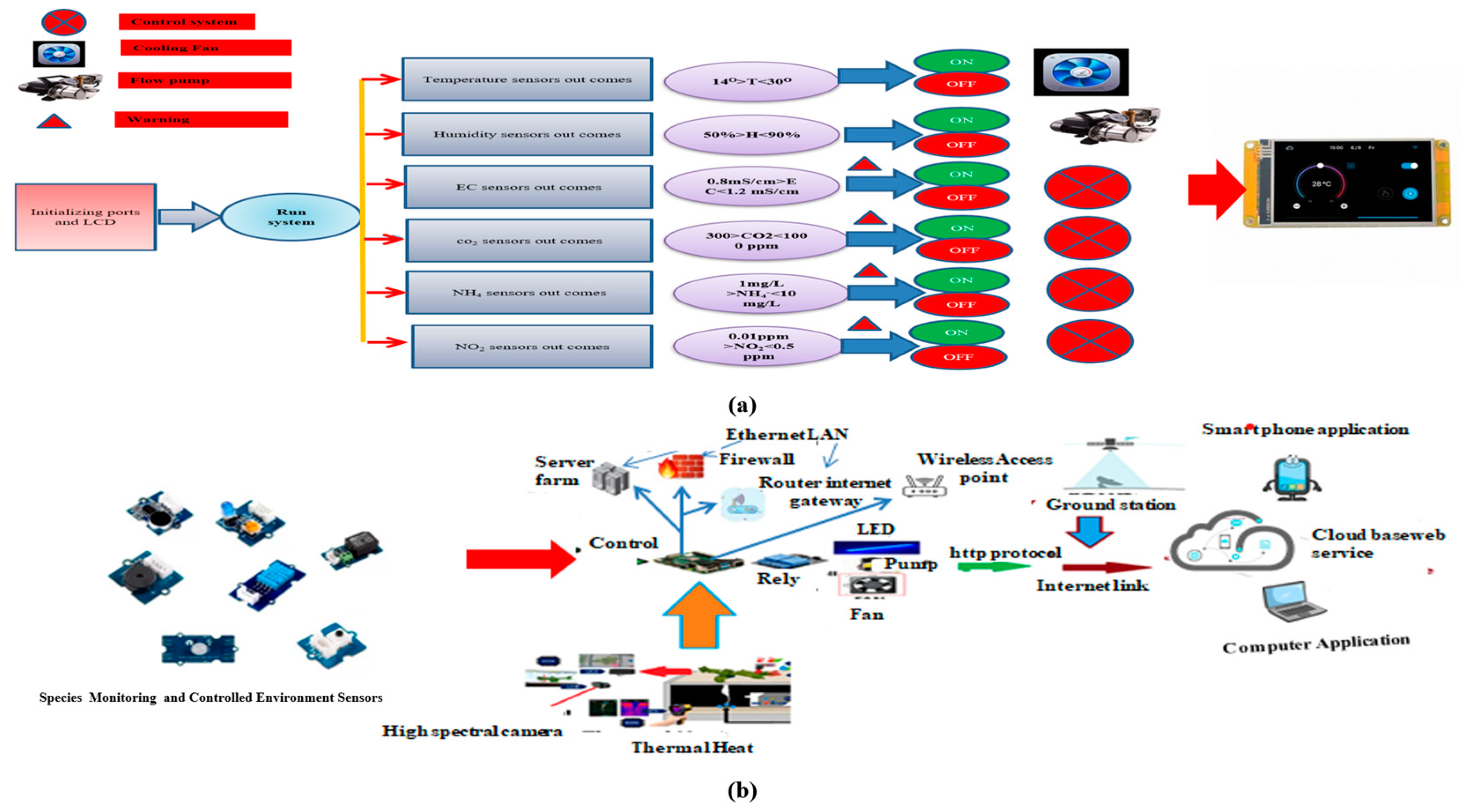
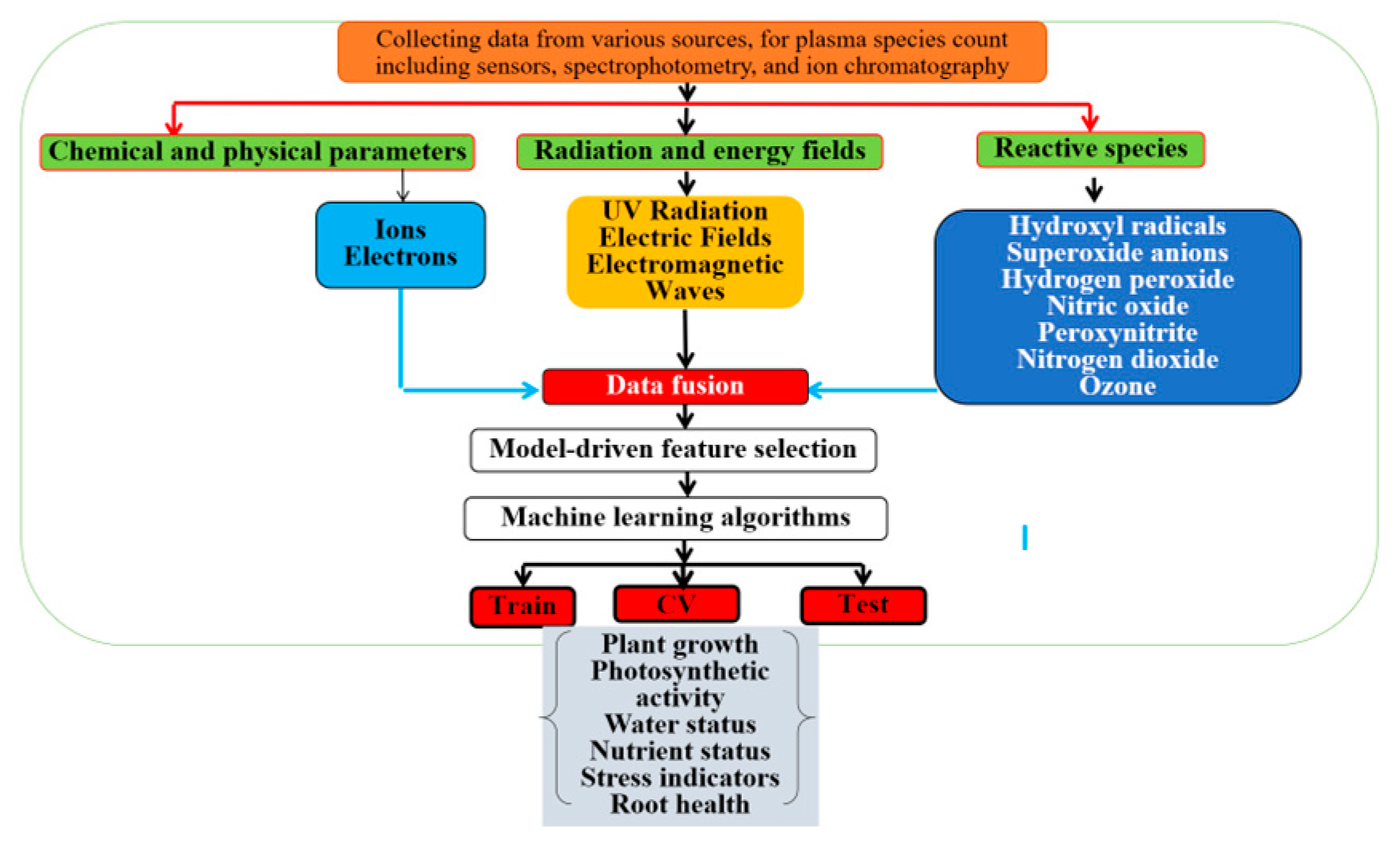
3.5. Impact of Control Systems, AI, and IoT in Aeroponic Systems
Future Prospects of AI and IoT-Driven Plasma Control in Aeroponics
3.6. Challenges and Optimization of Plasma in Aeroponics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lal, R. Feeding 11 Billion on 0.5 Billion Hectare of Area under Cereal Crops. Food Energy Secur. 2016, 5, 239–251. [Google Scholar] [CrossRef]
- Rhodes, C. Soil Erosion, Climate Change and Global Food Security: Challenges and Strategies. Sci. Prog. 2014, 97, 97–153. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, M.; Mattson, N.; Grab, H. Evaluating Propagation Techniques for Cannabis sativa L. Cultivation: A Comparative Analysis of Soilless Methods and Aeroponic Parameters. Plants 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization). Building a Common Vision for Sustainable Food and Agriculture: Principles and Approaches; FAO: Rome, Italy, 2014. [Google Scholar]
- Garzón, J.; Montes, L.; Garzón González, J.; Lampropoulos, G. Systematic Review of Technology in Aeroponics: Introducing the Technology Adoption and Integration in Sustainable Agriculture Model. Agronomy 2023, 13, 2517. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture—Trends and and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Kumar, S.; Fandan, R.; Sachin, P. Hydroponics and Aeroponics: Advancement in Soilless Cultivation; Integrated Publications: Delhi, India, 2023; pp. 99–112. ISBN 978-93-5834-887-3. [Google Scholar]
- Balasundram, S.; Shamshiri, R.; Sridhara, S.; Rizan, N. The Role of Digital Agriculture in Mitigating Climate Change and Ensuring Food Security: An Overview. Sustainability 2023, 15, 5325. [Google Scholar] [CrossRef]
- Min, A.; Nguyen, N.; Howatt, L.; Tavares, M.; Seo, J. Aeroponic Systems Design: Considerations and Challenges. J. Agric. Eng. 2022, 54. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-Activation of Tap Water Using DBD for Agronomy Applications: Identification and Quantification of Long Lifetime Chemical Species and Production/Consumption Mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef]
- Wu, X.; Wu, C.; Bian, Z.; Ye, Z.; Meng, L.; Xia, L.; Bao, E.; Cao, K. Abscisic Acid and Reactive Oxygen Species Were Involved in Slightly Acidic Electrolyzed Water-Promoted Seed Germination in Watermelon. Sci. Hortic. 2022, 291, 110581. [Google Scholar] [CrossRef]
- Ruamrungsri, S.; Sawangrat, C.; Panjama, K.; Sojithamporn, P.; Jaipinta, S.; Srisuwan, W.; Intanoo, M.; Inkham, C.; Thanapornpoonpong, S. Effects of Using Plasma-Activated Water as a Nitrate Source on the Growth and Nutritional Quality of Hydroponically Grown Green Oak Lettuces. Horticulturae 2023, 9, 248. [Google Scholar] [CrossRef]
- Date, M.B. Design and Development of Laboratory Scale Hydroponic System for Growing Sweet Basil Using Plasma Activated Nutrient Solution. Master’s Thesis, Rutgers The State University of New Jersey, School of Graduate Studies, New Brunswick, NJ, USA, 2020. [Google Scholar]
- Gao, H.; Wang, G.; Huang, Z.; Nie, L.; Liu, D.; Lu, X.; He, G.; Ostrikov, K.K. Plasma-Activated Mist: Continuous-Flow, Scalable Nitrogen Fixation, and Aeroponics. ACS Sustain. Chem. Eng. 2023, 11, 4420–4429. [Google Scholar] [CrossRef]
- Han, Z.; Ahmad, W.; Rong, Y.; Chen, X.; Zhao, S.; Yu, J.; Zheng, P.; Huang, C.; Li, H. A Gas Sensors Detection System for Real-Time Monitoring of Changes in Volatile Organic Compounds during Oolong Tea Processing. Foods 2024, 13, 1721. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, M.; Xu, B.; Sun, J.; Mujumdar, A.S. Artificial Intelligence Assisted Technologies for Controlling the Drying of Fruits and Vegetables Using Physical Fields: A Review. Trends Food Sci. Technol. 2020, 105, 251–260. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Gao, J.; Ma, M.; Guo, Y.; Tunio, M.H.; Mosha, A.H. Advancing Lettuce Physiological State Recognition in IoT Aeroponic Systems: A Meta-Learning-Driven Data Fusion Approach. Eur. J. Agron. 2024, 161, 127387. [Google Scholar] [CrossRef]
- Lin, L.; Yan, D.; Lee, T.; Keidar, M. Self-adaptive Plasma Chemistry and Intelligent Plasma Medicine. Adv. Intell. Syst. 2022, 4, 2100112. [Google Scholar] [CrossRef]
- Petric, M.; Dodigović, F.; Grčić, I.; Markužić, P.; Radetić, L.; Topić, M. Ammonia Concentration Monitoring Using Arduino Platform. Environ. Eng.-Inženjerstvo Okoliša 2019, 6, 21–26. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Y.; Jiang, P. Sustainable Soilless Cultivation Mode: Cultivation Study on Droplet Settlement of Plant Roots Under Ultrasonic Aeroponic Cultivation. Sustainability 2022, 14, 13705. [Google Scholar] [CrossRef]
- Islam, M.R.; Oliullah, K.; Kabir, M.M.; Alom, M.; Mridha, M.F. Machine Learning Enabled IoT System for Soil Nutrients Monitoring and Crop Recommendation. J. Agric. Food Res. 2023, 14, 100880. [Google Scholar] [CrossRef]
- Jain, S.; Srivastava, A.; Khadke, L.; Chatterjee, U.; Elbeltagi, A. Global-Scale Water Security and Desertification Management amidst Climate Change. Environ. Sci. Pollut. Res. 2024, 31, 58720–58744. [Google Scholar] [CrossRef] [PubMed]
- Barak, P.; Smith, J.; Krueger, A.; Peterson, L. Measurement of Short-term Nutrient Uptake Rates in Cranberry by Aeroponics. Plant Cell Environ. 1996, 19, 237–242. [Google Scholar] [CrossRef]
- Clawson, J.; Hoehn, A.; Stodieck, L.; Todd, P.; Stoner, R. Re-Examining Aeroponics for Spaceflight Plant Growth; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2000. [Google Scholar] [CrossRef]
- Wainwright, R.E.; Bissonnette, W.M.; Stoner, I.R.J. Low Pressure Aeroponic Growing Apparatus. U.S. Patent US 6,807,770, 26 October 2004. [Google Scholar]
- Martin-Laurent, F.; Tham, F.-Y.; Lee, S.-K.; He, J.; Diem, H.G. Field Assessment of Aeroponically Grown and Nodulated Acacia mangium. Aust. J. Bot. 2000, 48, 109–114. [Google Scholar] [CrossRef]
- Gonyer, D.; Jones, S. Modular Automated Aeroponic Growth System. U.S. Patent US 9,516,822, 13 December 2016. [Google Scholar]
- Kumari, R.; Kumar, R. Aeroponics: A Review on Modern Agriculture Technology. Indian Farmer 2019, 6, 286–292. [Google Scholar]
- Choudhury, M.R.; Dutta, A. Aeroponics. SSRN Electron J. 2022. [Google Scholar] [CrossRef]
- Weathers, P.J.; Wyslouzil, B.E. Bioreactors, Mist. In Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology; Wiley: Hoboken, NJ, USA, 2009; pp. 1–7. [Google Scholar]
- Ramakrishnan, D.; Curtis, W.R. Trickle-bed Root Culture Bioreactor Design and Scale-up: Growth, Fluid-dynamics, and Oxygen Mass Transfer. Biotechnol. Bioeng. 2004, 88, 248–260. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Hung, C.-H.; Chou, S.-N. Innovative Strategies for Operation of Mist Trickling Reactors for Enhanced Hairy Root Proliferation and Secondary Metabolite Productivity. Enzym. Microb. Technol. 2004, 35, 22–32. [Google Scholar] [CrossRef]
- DiIorio, A.A.; Cheetham, R.D.; Weathers, P.J. Carbon Dioxide Improves the Growth of Hairy Roots Cultured on Solid Medium and in Nutrient Mists. Appl. Microbiol. Biotechnol. 1992, 37, 463–467. [Google Scholar] [CrossRef]
- Kim, Y.J.; Weathers, P.J.; Wyslouzil, B.E. Growth of Artemisia annua Hairy Roots in Liquid-and Gas-phase Reactors. Biotechnol. Bioeng. 2002, 80, 454–464. [Google Scholar] [CrossRef]
- Kim, Y.; Wyslouzil, B.E.; Weathers, P.J. Secondary Metabolism of Hairy Root Cultures in Bioreactors. Vitr. Cell. Dev. Biol. Plant 2002, 38, 1–10. [Google Scholar] [CrossRef]
- Williams, G.R.; Doran, P.M. Hairy Root Culture in a Liquid-dispersed Bioreactor: Characterization of Spatial Heterogeneity. Biotechnol. Prog. 2000, 16, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Tunio, M.; Gao, J.; Mohamed, T.; Ahmad, F.; Abbas, I.; Shaikh, S. Comparison of Nutrient Use Efficiency, Antioxidant Assay, and Nutritional Quality of Butter-Head Lettuce (Lactuca sativa L.) in Five Cultivation Systems. Int. J. Agric. Biol. Eng. 2023, 16, 95. [Google Scholar] [CrossRef]
- Mateus-Rodriguez, J.R.; de Haan, S.; Andrade-Piedra, J.L.; Maldonado, L.; Hareau, G.; Barker, I.; Chuquillanqui, C.; Otazú, V.; Frisancho, R.; Bastos, C.; et al. Technical and Economic Analysis of Aeroponics and Other Systems for Potato Mini-Tuber Production in Latin America. Am. J. Potato Res. 2013, 90, 357–368. [Google Scholar] [CrossRef]
- Movahedi, Z.; Moieni, A.; Soroushzadeh, A. Comparison of Aeroponics and Conventional Soil Systems for Potato Minitubers Production and Evaluation of Their Quality Characters. J. Plant Physiol. Breed. 2012, 2, 13–21. [Google Scholar]
- Brocic, Z.; Mилинкoвић, M.; Momčilović, I.; Oljaca, J.; Veljkovic, B.; Milošević, D.; Poštić, D. Comparison of Aeroponics and Conventional Production System of Virus-Free Potato Mini Tubers in Serbia. Agroznanje Agro-Knowl. J. 2019, 20, 95–105. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef]
- Wimmerova, L.; Keken, Z.; Solcova, O.; Bartos, L.; Spacilova, M. A Comparative LCA of Aeroponic, Hydroponic, and Soil Cultivations of Bioactive Substance Producing Plants. Sustainability 2022, 14, 2421. [Google Scholar] [CrossRef]
- AlShrouf, A. Hydroponics, Aeroponic and Aquaponic as Compared with Conventional Farming. Am. Sci. Res. J. Eng. Technol. Sci 2017, 27, 247–255. [Google Scholar]
- Khater, E.; Ali, S.A. Effect of Flow Rate and Length of Gully on Lettuce Plants in Aquaponic and Hydroponic Systems. J. Aquac. Res. Dev. 2015, 6. [Google Scholar] [CrossRef]
- Spinoff, N. Innovative Partnership Program; Publications and Graphics Department, NASA Center for Aerospace Information (CASI): Hanover, MD, USA, 2006.
- Park, Y.; Williams, K.A. Organic Hydroponics: A Review. Sci. Hortic. 2024, 324, 112604. [Google Scholar] [CrossRef]
- Eldridge, B.; Manzoni, L.; Graham, C.; Rodgers, B.; Farmer, J.; Dodd, A. Getting to the Roots of Aeroponic Indoor Farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- NASA. Experiments Advance Gardening at Home and in Space; NASA: Washington, DC, USA, 1997.
- Despommier, D. The Vertical Farm: Feeding the World in the 21st Century; Macmillan: London, UK, 2010; ISBN 1-4299-4604-0. [Google Scholar]
- Parkes, M.; Azevedo, D.; Domingos, T.; Teixeira, R. Narratives and Benefits of Agricultural Technology in Urban Buildings: A Review. Atmosphere 2022, 13, 1250. [Google Scholar] [CrossRef]
- Ampim, P.A.Y.; Obeng, E.; Olvera-Gonzalez, E. Indoor Vegetable Production: An Alternative Approach to Increasing Cultivation. Plants 2022, 11, 2843. [Google Scholar] [CrossRef]
- Méndez-Guzmán, H.A.; Padilla-Medina, J.A.; Martínez-Nolasco, C.; Martinez-Nolasco, J.J.; Barranco-Gutiérrez, A.I.; Contreras-Medina, L.M.; Leon-Rodriguez, M. IoT-Based Monitoring System Applied to Aeroponics Greenhouse. Sensors 2022, 22, 5646. [Google Scholar] [CrossRef]
- Panotra, N.; Belagalla, N.; Mohanty, L.K.; Ramesha, N.; Tiwari, A.K.; Abhishek, G.; Gulaiya, S.; Yadav, K.; Pandey, S.K. Vertical Farming: Addressing the Challenges of 21st Century Agriculture Through Innovation. Int. J. Environ. Clim. Change 2024, 14, 664–691. [Google Scholar] [CrossRef]
- de Sousa, R.; Bragança, L.; da Silva, M.V.; Oliveira, R.S. Challenges and Solutions for Sustainable Food Systems: The Potential of Home Hydroponics. Sustainability 2024, 16, 817. [Google Scholar] [CrossRef]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.-N.; Fridman, A.; Choi, E.H. Generation Mechanism of Hydroxyl Radical Species and Its Lifetime Prediction during the Plasma-Initiated Ultraviolet (UV) Photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef] [PubMed]
- Chirokov, A.; Gutsol, A.; Fridman, A. Atmospheric Pressure Plasma of Dielectric Barrier Discharges. Pure Appl. Chem. 2005, 77, 487–495. [Google Scholar] [CrossRef]
- Deng, S.; Cheng, C.; Ni, G.; Meng, Y.; Chen, H. Bacillus Subtilis Devitalization Mechanism of Atmosphere Pressure Plasma Jet. Curr. Appl. Phys. 2010, 10, 1164–1168. [Google Scholar] [CrossRef]
- Huang, Z.; Xiao, A.; Liu, D.; Lu, X.; Ostrikov, K. Plasma-water-based Nitrogen Fixation: Status, Mechanisms, and Opportunities. Plasma Process. Polym. 2022, 19, 2100198. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of Short-Lived Reactive Species in Plasma–Air–Water Systems: The Dos and the Do Nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef]
- Pipliya, S.; Kumar, S.; Babar, N.; Srivastav, P.P. Recent Trends in Non-Thermal Plasma and Plasma Activated Water: Effect on Quality Attributes, Mechanism of Interaction and Potential Application in Food & Agriculture. Food Chem. Adv. 2023, 2, 100249. [Google Scholar] [CrossRef]
- Woo, R. RF Voltage Breakdown and the Paschen Curve. Proc. IEEE 1974, 62, 521. [Google Scholar] [CrossRef]
- Chen, T.; Qi, X.; Lu, D.; Chen, B. Gas Chromatography-Ion Mobility Spectrometric Classification of Vegetable Oils Based on Digital Image Processing. J. Food Meas. Charact. 2019, 13, 1973–1979. [Google Scholar] [CrossRef]
- Arslan, M.; Zareef, M.; Tahir, H.E.; Guo, Z.; Rakha, A.; Xuetao, H.; Shi, J.; Zhihua, L.; Xiaobo, Z.; Khan, M.R. Discrimination of Rice Varieties Using Smartphone-Based Colorimetric Sensor Arrays and Gas Chromatography Techniques. Food Chem. 2022, 368, 130783. [Google Scholar] [CrossRef]
- Do, J.-S.; Chang, W.-B. Amperometric Nitrogen Dioxide Gas Sensor Based on PAn/Au/Nafion® Prepared by Constant Current and Cyclic Voltammetry Methods. Sens. Actuators B Chem. 2004, 101, 97–106. [Google Scholar] [CrossRef]
- Kumi, F.; Mao, H.; Li, Q.; Luhua, H. Assessment of Tomato Seedling Substrate-Root Quality Using X-Ray Computed Tomography and Scanning Electron Microscopy. Appl. Eng. Agric. 2016, 32, 417–427. [Google Scholar] [CrossRef]
- Julák, J.; Hujacová, A.; Scholtz, V.; Khun, J.; Holada, K. Contribution to the Chemistry of Plasma-Activated Water. Plasma Phys. Rep. 2018, 44, 125–136. [Google Scholar] [CrossRef]
- Koppenol, W.H. The Basic Chemistry of Nitrogen Monoxide and Peroxynitrite. Free. Radic. Biol. Medicine 1998, 25, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Mok, Y.S.; Kim, S.-J.; Kim, Y.J.; Lee, J.H.; Kim, J.H.; Heo, I. Non-Thermal Plasma in Honeycomb Catalyst for the High-Throughput Removal of Dilute Styrene from Air. J. Environ. Chem. Eng. 2021, 9, 105780. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of Plasma Activated Water on the Postharvest Quality of Button Mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Ma, R.; Feng, H.; Li, F.; Liang, Y.; Zhang, Q.; Zhu, W.; Zhang, J.; Becker, K.H.; Fang, J. An Evaluation of Anti-Oxidative Protection for Cells against Atmospheric Pressure Cold Plasma Treatment. Appl. Phys. Lett. 2012, 100, 123701. [Google Scholar] [CrossRef]
- Kurake, N.; Tanaka, H.; Ishikawa, K.; Takeda, K.; Hashizume, H.; Nakamura, K.; Kajiyama, H.; Kondo, T.; Kikkawa, F.; Mizuno, M. Effects of •OH and •NO Radicals in the Aqueous Phase on H2O2 and NO2− Generated in Plasma-Activated Medium. J. Phys. D Appl. Phys. 2017, 50, 155202. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Alam, D.; Zhou, R.; Zhang, T.; Ostrikov, K.; Cullen, P.J. Microbial Decontamination of Chicken Using Atmospheric Plasma Bubbles. Plasma Process. Polym. 2020, 18, 2000052. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.; Ostrikov, K.K.; Bazaka, K. Plasma-Activated Water: Generation, Origin of Reactive Species and Biological Applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on Formation of Cold Plasma Activated Water (PAW) and the Applications in Food and Agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold Atmospheric Plasma Delivery for Biomedical Applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Si, F.; Zhang, X.; Yan, K. The Quantitative Detection of HO˙ Generated in a High Temperature H2O2 Bleaching System with a Novel Fluorescent Probe Benzenepentacarboxylic Acid. RSC Adv. 2014, 4, 5860. [Google Scholar] [CrossRef]
- Hirano, Y.; Hayashi, M.; Tamura, M.; Yoshino, F.; Yoshida, A.; Masubuchi, M.; Imai, K.; Ogiso, B. Singlet Oxygen Generated by a New Nonthermal Atmospheric Pressure Air Plasma Device Exerts a Bactericidal Effect on Oral Pathogens. J. Oral Sci. 2019, 61, 521–525. [Google Scholar] [CrossRef]
- Alugoju, P.; Jestadi, D.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2014, 30, 11–26. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E. Superoxide and Hydroxyl Radical Chemistry in Aqueous Solution. In Active Oxygen in Chemistry; Foote, C.S., Valentine, J.S., Greenberg, A., Liebman, J.F., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 66–104. ISBN 978-94-007-0874-7. [Google Scholar]
- Shih, K.-Y.; Locke, B.R. Chemical and Physical Characteristics of Pulsed Electrical Discharge Within Gas Bubbles in Aqueous Solutions. Plasma Chem. Plasma Process. 2010, 30, 1–20. [Google Scholar] [CrossRef]
- Patange, A.; Lu, P.; Boehm, D.; Cullen, P.J.; Bourke, P. Efficacy of Cold Plasma Functionalised Water for Improving Microbiological Safety of Fresh Produce and Wash Water Recycling. Food Microbiol. 2019, 84, 103226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhang, T.; Zhou, R.; Mai-Prochnow, A.; Ponraj, S.B.; Fang, Z.; Masood, H.; Kananagh, J.; McClure, D.; Alam, D.; et al. Underwater Microplasma Bubbles for Efficient and Simultaneous Degradation of Mixed Dye Pollutants. Sci. Total Environ. 2021, 750, 142295. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S. Aureus and Physicochemical Properties of Plasma Activated Water Stored at Different Temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Agrawal, A.; Taylor, R. Clean Combustion of Different Liquid Fuels Using a Novel Injector. Exp. Therm. Fluid Sci. 2014, 57, 275–284. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I. Aqueous-Phase Chemistry and Bactericidal Effects from an Air Discharge Plasma in Contact with Water: Evidence for the Formation of Peroxynitrite through a Pseudo-Second-Order Post-Discharge Reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Krakkó, D.; Illés, Á.; Licul-Kucera, V.; Dávid, B.; Dobosy, P.; Pogonyi, A.; Demeter, A.; Mihucz, V.; Dóbé, S. Application of (V)UV/O3 Technology for Post-Treatment of Biologically Treated Wastewater: A Pilot-Scale Study. Chemosphere 2021, 275, 130080. [Google Scholar] [CrossRef] [PubMed]
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of Plasma-Activated Water on Physical and Physical–Chemical Soil Properties. Water 2020, 12, 2357. [Google Scholar] [CrossRef]
- Sasi, S.; Prasad, K.; Weerasinghe, J.; Bazaka, O.; Ivanova, E.P.; Levchenko, I.; Bazaka, K. Plasma for Aquaponics. Trends Biotechnol. 2023, 41, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cai, L.; Zhang, A.; Li, G.; Zhang, Y.; Filatova, I.; Liu, Y. Simultaneous Remediation of Diesel-Polluted Soil and Promoted Ryegrass Growth by Non-Thermal Plasma Pretreatment. Sci. Total Environ. 2024, 912, 169295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Hong, Y.; Cai, Y.; Dong, F.; Song, J. The Removal of CH4 and NOx from Marine LNG Engine Exhaust by NTP Combined with Catalyst: A Review. Materials 2023, 16, 4969. [Google Scholar] [CrossRef] [PubMed]
- Machado-Moreira, B.; Tiwari, B.; Richards, K.; Abram, F.; Burgess, C. Application of Plasma Activated Water for Decontamination of Alfalfa and Mung Bean Seeds. Food Microbiol. 2020, 96, 103708. [Google Scholar] [CrossRef]
- Škarpa, P.; Klofáč, D.; Krčma, F.; Šimečková, J.; Kozáková, Z. Effect of Plasma Activated Water Foliar Application on Selected Growth Parameters of Maize (Zea mays L.). Water 2020, 12, 3545. [Google Scholar] [CrossRef]
- Puač, N.; Živković, S.; Selaković, N.; Milutinović, M.; Boljević, J.; Malović, G.; Petrović, Z.L. Long and Short Term Effects of Plasma Treatment on Meristematic Plant Cells. Appl. Phys. Lett. 2014, 104, 214106. [Google Scholar] [CrossRef]
- Patil, B.S.; Cherkasov, N.; Lang, J.; Ibhadon, A.O.; Hessel, V.; Wang, Q. Low Temperature Plasma-Catalytic NOx Synthesis in a Packed DBD Reactor: Effect of Support Materials and Supported Active Metal Oxides. Appl. Catal. B Environ. 2016, 194, 123. [Google Scholar] [CrossRef]
- Gogoi, K.; Gogoi, H.; Borgohain, M.; Saikia, R.; Chikkaputtaiah, C.; Hiremath, S.; Basu, U. The Molecular Dynamics between Reactive Oxygen Species (ROS), Reactive Nitrogen Species (RNS) and Phytohormones in Plant’s Response to Biotic Stress. Plant Cell Rep. 2024, 43, 263. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K. Oxidative Stress in Crop Plants. In Agronomic Crops: Volume 3: Stress Responses and Tolerance; Hasanuzzaman, M., Ed.; Springer Singapore: Singapore, 2020; pp. 349–380. ISBN 978-981-15-0025-1. [Google Scholar]
- Rashid, M.M.; Rashid, M.; Hasan, M.M.; Talukder, M.R. Rice Plant Growth and Yield: Foliar Application of Plasma Activated Water. Plasma Sci. Technol. 2021, 23, 075503. [Google Scholar] [CrossRef]
- Karimi, J.; Bansal, S.A.; Kumar, V.; Pasalari, H.; Badr, A.A.; Nejad, Z.J. Effect of Cold Plasma on Plant Physiological and Biochemical Processes: A Review. Biologia 2024, 79, 3475–3487. [Google Scholar] [CrossRef]
- Rizwan, M.; Tanveer, H.; Ali, M.H.; Sanaullah, M.; Wakeel, A. Role of Reactive Nitrogen Species in Changing Climate and Future Concerns of Environmental Sustainability. Environ. Sci. Pollut. Res. 2024, 31, 51147–51163. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, K.; Wang, X.; Lyu, C.; Yang, N.; Li, Y.; Wang, J. Inactivation of Yeast on Grapes by Plasma-Activated Water and Its Effects on Quality Attributes. J. Food Prot. 2017, 80, 225–230. [Google Scholar] [CrossRef]
- Khan, M.; Kim, Y.-J. Inactivation Mechanism of Salmonella Typhimurium on the Surface of Lettuce and Physicochemical Quality Assessment of Samples Treated by Micro-Plasma Discharged Water. Innov. Food Sci. Emerg. Technol. 2018, 52, 17–24. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, H.W.; Kim, S.B.; Ryu, S.; Lim, J.; Hong, E.J.; Byeon, Y.S.; Chun, H.H. Sequential Application of Plasma-Activated Water and Mild Heating Improves Microbiological Quality of Ready-to-Use Shredded Salted Kimchi Cabbage (Brassica pekinensis L.). Food Control 2019, 98, 501–509. [Google Scholar] [CrossRef]
- Vaka, M.; Sone, I.; Garcia Alvarez, R.; Walsh, J.; Prabhu, L.; Sivertsvik, M.; Noriega Fernández, E. Towards the Next-Generation Disinfectant: Composition, Storability and Preservation Potential of Plasma Activated Water on Baby Spinach Leaves. Foods 2019, 8, 692. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, S.; Xiang, Q.; Lyu, Y.; Shen, R. Effect of Plasma-Activated Water on the Microbial Decontamination and Food Quality of Thin Sheets of Bean Curd. Appl. Sci. 2019, 9, 4223. [Google Scholar] [CrossRef]
- Li, B.; Peng, L.; Cao, Y.; Liu, S.; Zhu, Y.; Dou, J.; Yang, Z.; Zhou, C. Insights into Cold Plasma Treatment on the Cereal and Legume Proteins Modification: Principle, Mechanism, and Application. Foods 2024, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant Growth Promotion Effect of Plasma Activated Water on Lactuca sativa L. Cultivated in Two Different Volumes of Substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Z.; Ma, T. The Effects of Plasma-Activated Water Treatment on the Growth of Tartary Buckwheat Sprouts. Front. Nutr. 2022, 9, 849615. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, S.; Khacef, A. Enhanced Seed Germination and Plant Growth by Atmospheric Pressure Cold Air Plasma: Combined Effect of Seed and Water Treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma Agriculture: Review from the Perspective of the Plant and Its Ecosystem. Plasma Process. Polym. 2021, 18, 2000162. [Google Scholar] [CrossRef]
- Kalachova, T.; Jindřichová, B.; Pospíchalová, R.; Fujera, J.; Artemenko, A.; Jančík, J.; Antonova, A.; Kylián, O.; Prukner, V.; Burketová, L.; et al. Plasma Treatment Modifies Element Distribution in Seed Coating and Affects Further Germination and Plant Growth Through Interaction with Soil Microbiome. J. Agric. Food Chem. 2024, 72, 5609–5624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a New Atmospheric Pressure Plasma Device and Application on Tomato Seeds. Agric. Sci. 2011, 2, 23. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Kong, T.-K.; Lin, C.-M.; Chen, H.-L. The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach. Appl. Sci. 2021, 11, 5304. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas Sarkar, M.; Masi, A.; Rakwal, R.; Agrawal, G.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants–Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Javed, R.; Mumtaz, S.; Choi, E.H.; Han, I. Effect of Plasma-Treated Water with Magnesium and Zinc on Growth of Chinese Cabbage. Int. J. Mol. Sci. 2023, 24, 8426. [Google Scholar] [CrossRef] [PubMed]
- Ka, D.H.; Priatama, R.A.; Park, J.Y.; Park, S.J.; Kim, S.B.; Lee, I.A.; Lee, Y.K. Plasma-Activated Water Modulates Root Hair Cell Density via Root Developmental Genes in Arabidopsis thaliana L. Appl. Sci. 2021, 11, 2240. [Google Scholar] [CrossRef]
- Than, H.; Pham, T.; Nguyen, D.; Pham, T.; Khacef, A. Non-Thermal Plasma Activated Water for Increasing Germination and Plant Growth of Lactuca sativa L. Plasma Chem. Plasma Process. 2022, 42, 73–89. [Google Scholar] [CrossRef]
- Lukacova, Z.; Svubova, R.; Selvekova, P.; Hensel, K. The Effect of Plasma Activated Water on Maize (Zea mays L.) under Arsenic Stress. Plants 2021, 10, 1899. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, F.; Krüger, A.; Scholz, C.; Brust, H.; Stöhr, C. Long-Term Effects of Cold Atmospheric Plasma-Treated Water on the Antioxidative System of Hordeum vulgare. J. Plant Growth Regul. 2023, 42, 3274–3290. [Google Scholar] [CrossRef]
- Cortese, E.; Settimi, A.G.; Pettenuzzo, S.; Cappellin, L.; Galenda, A.; Famengo, A.; Dabalà, M.; Antoni, V.; Navazio, L. Plasma-Activated Water Triggers Rapid and Sustained Cytosolic Ca2+ Elevations in Arabidopsis thaliana. Plants 2021, 10, 2516. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.-H.; Sun, D.-W. Enhancement of Wheat Seed Germination, Seedling Growth and Nutritional Properties of Wheat Plantlet Juice by Plasma Activated Water. J. Plant Growth Regul. 2023, 42, 2006–2022. [Google Scholar] [CrossRef] [PubMed]
- Matra, K.; Tanakaran, Y.; Luang-In, V.; Theepharaksapan, S. Enhancement of Lettuce Growth by PAW Spray Gliding Arc Plasma Generator. IEEE Trans. Plasma Sci. 2022, 50, 1430–1439. [Google Scholar] [CrossRef]
- Laurita, R.; Gozzi, G.; Tappi, S.; Capelli, F.; Bisag, A.; Laghi, G.; Gherardi, M.; Cellini, B.; Abouelenein, D.; Vittori, S.; et al. Effect of Plasma Activated Water (PAW) on Rocket Leaves Decontamination and Nutritional Value. Innov. Food Sci. Emerg. Technol. 2021, 73, 102805. [Google Scholar] [CrossRef]
- Takahashi, K.; Saito, Y.; Oikawa, R.; Okumura, T.; Takaki, K.; Fujio, T. Development of Automatically Controlled Corona Plasma System for Inactivation of Pathogen in Hydroponic Cultivation Medium of Tomato. J. Electrost. 2018, 91, 61–69. [Google Scholar] [CrossRef]
- Turkan, I. ROS and RNS: Key Signalling Molecules in Plants. J. Exp. Bot. 2018, 69, 3313–3315. [Google Scholar] [CrossRef] [PubMed]
- Moghanloo, M.; Iranbakhsh, A.; Ebadi, M.; Nejad Satari, T.; Oraghi Ardebili, Z. Seed Priming with Cold Plasma and Supplementation of Culture Medium with Silicon Nanoparticle Modified Growth, Physiology, and Anatomy in Astragalus Fridae as an Endangered Species. Acta Physiol. Plant. 2019, 41, 54. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Ebadi, M. Seed Priming with Cold Plasma Improved Early Growth, Flowering, and Protection of Cichorium intybus Against Selenium Nanoparticle. J. Theor. Appl. Phys. 2020, 14, 113–119. [Google Scholar] [CrossRef]
- Date, M.; Rivero, W.; Tan, J.; Specca, D.; Simon, J.; Salvi, D.; Karwe, M. Growth of Hydroponic Sweet Basil (O. basilicum L.) Using Plasma-Activated Nutrient Solution (PANS). Agriculture 2023, 13, 443. [Google Scholar] [CrossRef]
- Monden, K.; Kamiya, T.; Sugiura, D.; Suzuki, T.; Nakagawa, T.; Hachiya, T. Root-Specific Activation of Plasma Membrane H+-ATPase 1 Enhances Plant Growth and Shoot Accumulation of Nutrient Elements under Nutrient-Poor Conditions in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2022, 621, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, D.; Fang, X.; Tang, X.; Zhao, L.; Zhang, L.; Liu, L.; Wang, G. Effect of Low-Temperature Plasma on Forage Maize (Zea mays Linn.) Seeds Germination and Characters of the Seedlings. In Proceedings of the Computer and Computing Technologies in Agriculture VIII: 8th IFIP WG 5.14 International Conference, CCTA 2014, Beijing, China, 16–19 September 2014; Springer: Berlin/Heidelberg, Germany, 2015; pp. 437–443. [Google Scholar]
- Chuea-Uan, S.; Boonyawan, D.; Sawangrat, C.; Thanapornpoonpong, S.-N. Using Plasma-Activated Water Generated by an Air Gliding Arc as a Nitrogen Source for Rice Seed Germination. Agronomy 2023, 14, 15. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma Activated Water: The next Generation Eco-Friendly Stimulant for Enhancing Plant Seed Germination, Vigor and Increased Enzyme Activity, a Study on Black Gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. A Nitrogen Alternative: Use of Plasma Activated Water as Nitrogen Source in Hydroponic Solution for Radish Growth. arXiv 2024, arXiv:2404.16910. [Google Scholar]
- Veerana, M.; Ketya, W.; Choi, E.-H.; Park, G. Non-Thermal Plasma Enhances Growth and Salinity Tolerance of Bok Choy (Brassica rapa Subsp. chinensis) in Hydroponic Culture. Front. Plant Sci. 2024, 15, 1445791. [Google Scholar] [CrossRef]
- Takahashi, K.; Kawamura, S.; Takada, R.; Takaki, K.; Satta, N.; Fujio, T. Influence of a Plasma-Treated Nutrient Solution Containing 2, 4-Dichlorobenzoic Acid on the Growth of Cucumber in a Hydroponic System. J. Appl. Phys. 2021, 129, 143301. [Google Scholar] [CrossRef]
- Song, J.-S.; Jung, S.; Jee, S.; Yoon, J.W.; Byeon, Y.S.; Park, S.; Kim, S.B. Growth and Bioactive Phytochemicals of Panax Ginseng Sprouts Grown in an Aeroponic System Using Plasma-Treated Water as the Nitrogen Source. Sci. Rep. 2021, 11, 2924. [Google Scholar] [CrossRef]
- Capitelli, M.; Ferreira, C.M.; Gordiets, B.F.; Osipov, A.I. Plasma Kinetics in Atmospheric Gases; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 31, ISBN 3-662-04158-8. [Google Scholar]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008; ISBN 1-139-47173-2. [Google Scholar]
- Fridman, A.; Nester, S.; Kennedy, L.A.; Saveliev, A.; Mutaf-Yardimci, O. Gliding Arc Gas Discharge. Prog. Energy Combust. Sci. 1999, 25, 211–231. [Google Scholar] [CrossRef]
- Li, J.; Lan, C.; Nie, L.; Liu, D.; Lu, X. Distributed Plasma-Water-Based Nitrogen Fixation System Based on Cascade Discharge: Generation, Regulation, and Application. Chem. Eng. J. 2023, 478, 147483. [Google Scholar] [CrossRef]
- Chiappim, W.; Sampaio, A.; Miranda, F.; Petraconi, G.; da Silva Sobrinho, A.; Cardoso, P.; Kostov, K.; Koga-Ito, C.; Pessoa, R. Nebulized Plasma-Activated Water Has an Effective Antimicrobial Effect on Medically Relevant Microbial Species and Maintains Its Physicochemical Properties in Tube Lengths from 0.1 up to 1.0 m. Plasma Process. Polym. 2021, 18, 2100010. [Google Scholar] [CrossRef]
- Sakiyama, Y.; Graves, D.B. Corona-Glow Transition in the Atmospheric Pressure RF-Excited Plasma Needle. J. Phys. D Appl. Phys. 2006, 39, 3644. [Google Scholar] [CrossRef]
- Cornell, K.A.; White, A.; Croteau, A.; Carlson, J.; Kennedy, Z.; Miller, D.; Provost, M.; Goering, S.; Plumlee, D.; Browning, J. Fabrication and Performance of a Multidischarge Cold-Atmospheric Pressure Plasma Array. IEEE Trans. Plasma Sci. 2021, 49, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, D. Non-Thermal Plasma Nitrogen Fixation Based on Micron Droplets and Its Application in Aeroponics. Acta Pet. Sin. Pet. Process. Sect. 2023, 39, 1184–1193. [Google Scholar]
- Tarabova, B. Investigation of Cold Air Plasma Generation of Aqueous Reactive Oxygen and Nitrogen Species with Focus on Their Detection and Related Antibacterial Effects; Comenius University: Bratislava, Slovakia, 2019. [Google Scholar]
- Zhou, D.; Zhou, R.; Zhou, R.; Liu, B.; Zhang, T.; Xian, Y.; Cullen, P.J.; Lu, X.; Ostrikov, K. Sustainable Ammonia Production by Non-Thermal Plasmas: Status, Mechanisms, and Opportunities. Chem. Eng. J. 2021, 421, 129544. [Google Scholar] [CrossRef]
- Kruszelnicki, J.; Lietz, A.M.; Kushner, M.J. Atmospheric Pressure Plasma Activation of Water Droplets. J. Phys. D Appl. Phys. 2019, 52, 355207. [Google Scholar]
- Toth, J.R.; Abuyazid, N.H.; Lacks, D.J.; Renner, J.N.; Sankaran, R.M. A Plasma-Water Droplet Reactor for Process-Intensified, Continuous Nitrogen Fixation at Atmospheric Pressure. ACS Sustain. Chem. Eng. 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 5360. [Google Scholar] [CrossRef]
- Tomeková, J.; Kyzek, S.; Medvecká, V.; Gálová, E.; Zahoranová, A. Influence of Cold Atmospheric Pressure Plasma on Pea Seeds: DNA Damage of Seedlings and Optical Diagnostics of Plasma. Plasma Chem. Plasma Process. 2020, 40, 1571–1584. [Google Scholar] [CrossRef]
- Manickam, P.; Mariappan, S.A.; Murugesan, S.M.; Hansda, S.; Kaushik, A.; Shinde, R.; Thipperudraswamy, S. Artificial Intelligence (AI) and Internet of Medical Things (IoMT) Assisted Biomedical Systems for Intelligent Healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Salah, M.A.; Ali, M.; Ali, B.; Saleem, M.H.; Samarasinghe, K.; De Silva, S.; Ercisli, S.; Iqbal, N.; Anas, M. Advancing Agriculture: Harnessing Smart Nanoparticles for Precision Fertilization. BioNanoScience 2024, 14, 3846–3863. [Google Scholar] [CrossRef]
- Shejan, M.E.; Bhuiyan, S.M.Y.; Schoen, M.P.; Mahamud, R. Assessment of Machine Learning Techniques for Simulating Reacting Flow: From Plasma-Assisted Ignition to Turbulent Flame Propagation. Energies 2024, 17, 4887. [Google Scholar] [CrossRef]
- Lan, C.; Yin, Y.; Liu, D.; Lu, X. Nanosecond Pulse Plasma Activation of Micron-Sized Mist Droplets. Plasma Process. Polym. 2024, 21, e2400113. [Google Scholar] [CrossRef]
- Sysolyatina, E.V.; Lavrikova, A.Y.; Loleyt, R.A.; Vasilieva, E.V.; Abdulkadieva, M.A.; Ermolaeva, S.A.; Sofronov, A.V. Bidirectional Mass Transfer-Based Generation of Plasma-Activated Water Mist with Antibacterial Properties. Plasma Process. Polym. 2020, 17, 2000058. [Google Scholar] [CrossRef]
- Kaneko, T.; Takashima, K.; Sasaki, S. Integrated Transport Model for Controlled Delivery of Short-Lived Reactive Species via Plasma-Activated Liquid with Practical Applications in Plant Disease Control. Plasma Chem. Plasma Process. 2024, 44, 1165–1201. [Google Scholar] [CrossRef]
- He, J.; Ortiz, S.; Attri, S.; Bailey, C.; Rabinovich, A.; Fridman, A.; Fridman, G.; Sales, C.M. Comparing Inactivation of Escherichia coli O157:H7 on Fresh Produce Using Plasma-Activated Mist. Innov. Food Sci. Emerg. Technol. 2024, 93, 103634. [Google Scholar] [CrossRef]
- Gierczik, K.; Vukušić, T.; Kovács, L.; Székely, A.; Szalai, G.; Milošević, S.; Kocsy, G.; Kutasi, K.; Galiba, G. Plasma-Activated Water to Improve the Stress Tolerance of Barley. Plasma Process. Polym. 2020, 17, 1900123. [Google Scholar] [CrossRef]
- Ziuzina, D.; Patil, S.; Cullen, P.J.; Keener, K.; Bourke, P. Atmospheric Cold Plasma Inactivation of Escherichia coli, Salmonella enterica Serovar Typhimurium and Listeria Monocytogenes Inoculated on Fresh Produce. Food Microbiol. 2014, 42, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Mitsugi, F.; Abiru, T.; Ikegami, T.; Ebihara, K.; Aoqui, S.-I.; Nagahama, K. Influence of Ozone Generated by Surface Barrier Discharge on Nematode and Plant Growth. IEEE Trans. Plasma Sci. 2016, 44, 3071–3076. [Google Scholar] [CrossRef]
- Mitsugi, F.; Abiru, T.; Ikegami, T.; Ebihara, K.; Nagahama, K. Treatment of Nematode in Soil Using Surface Barrier Discharge Ozone Generator. IEEE Trans. Plasma Sci. 2017, 45, 3076–3081. [Google Scholar] [CrossRef]
- Ueda, Y.; Konishi, M.; Yanagisawa, S. Molecular Basis of the Nitrogen Response in Plants. Soil Sci. Plant Nutr. 2017, 63, 329. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A Review of Advanced Techniques for Detecting Plant Diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Kamala, K.L.; Alex, S.A. Apple Fruit Disease Detection for Hydroponic Plants Using Leading Edge Technology Machine Learning and Image Processing. In Proceedings of the 2021 2nd International Conference on Smart Electronics and Communication (ICOSEC), Trichy, India, 7–9 October 2021; pp. 820–825. [Google Scholar]
- Musa, A.; Hassan, M.; Hamada, M.; Aliyu, F. Low-Power Deep Learning Model for Plant Disease Detection for Smart-Hydroponics Using Knowledge Distillation Techniques. J. Low Power Electron. Appl. 2022, 12, 24. [Google Scholar] [CrossRef]
- Raju, S.R.; Dappuri, B.; Varma, P.R.K.; Yachamaneni, M.; Verghese, D.M.G.; Mishra, M.K. Research Article Design and Implementation of Smart Hydroponics Farming Using IoT-Based AI Controller with Mobile Application System. J. Nanomater. 2022, 2022, 4435591. [Google Scholar] [CrossRef]
- Elsherbiny, O.; Elaraby, A.; Alahmadi, M.; Hamdan, M.; Gao, J. Rapid Grapevine Health Diagnosis Based on Digital Imaging and Deep Learning. Plants 2024, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Gul, Z.; Bora, S. Exploiting Pre-Trained Convolutional Neural Networks for the Detection of Nutrient Deficiencies in Hydroponic Basil. Sensors 2023, 23, 5407. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, Y.V.; Chittoor, P.K.; Bharatiraja, C.; Verma, R.; Sathiyasekar, K. Sensor Fusion Based Intelligent Hydroponic Farming and Nursing System. IEEE Sens. J. 2022, 22, 14584–14591. [Google Scholar] [CrossRef]
- Mehra, M.; Saxena, S.; Sankaranarayanan, S.; Tom, R.J.; Veeramanikandan, M. IoT Based Hydroponics System Using Deep Neural Networks. Comput. Electron. Agric. 2018, 155, 473–486. [Google Scholar] [CrossRef]
- Adidrana, D.; Surantha, N. Hydroponic Nutrient Control System Based on Internet of Things and K-Nearest Neighbors. In Proceedings of the 2019 International Conference on Computer, Control, Informatics and its Applications (IC3INA), Tangerang, Indonesia, 23–24 October 2019; pp. 166–171. [Google Scholar]
- Elsherbiny, O.; Gao, J.; Ma, M.; Qureshi, W.A.; Mosha, A.H. Developing an IoT-Driven Delta Robot to Stimulate the Growth of Mulberry Branch Cuttings Cultivated Aeroponically Using Machine Vision Technology. Comput. Electron. Agric. 2025, 232, 110111. [Google Scholar] [CrossRef]
- Cedric, L.S.; Adoni, W.Y.H.; Aworka, R.; Zoueu, J.T.; Mutombo, F.K.; Krichen, M.; Kimpolo, C.L.M. Crops Yield Prediction Based on Machine Learning Models: Case of West African Countries. Smart Agric. Technol. 2022, 2, 100049. [Google Scholar] [CrossRef]
- Mokhtar, A.; El-Ssawy, W.; He, H.; Al-Anasari, N.; Sammen, S.S.; Gyasi-Agyei, Y.; Abuarab, M. Using Machine Learning Models to Predict Hydroponically Grown Lettuce Yield. Front. Plant Sci. 2022, 13, 706042. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Yanes, A.; Martinez, P.; Ahmad, R. Real-Time Growth Rate and Fresh Weight Estimation for Little Gem Romaine Lettuce in Aquaponic Grow Beds. Comput. Electron. Agric. 2020, 179, 105827. [Google Scholar] [CrossRef]
- Debroy, P.; Seban, L. A Tomato Fruit Biomass Prediction Model for Aquaponics System Using Machine Learning Algorithms. IFAC-PapersOnLine 2022, 55, 709–714. [Google Scholar] [CrossRef]
- Rathor, A.S.; Choudhury, S.; Sharma, A.; Nautiyal, P.; Shah, G. Empowering Vertical Farming through IoT and AI-Driven Technologies: A Comprehensive Review. Heliyon 2024, 10, e34998. [Google Scholar] [CrossRef]
- Bijjahalli, S.; Sabatini, R.; Gardi, A. Advances in Intelligent and Autonomous Navigation Systems for Small UAS. Prog. Aerosp. Sci. 2020, 115, 100617. [Google Scholar] [CrossRef]
- Roffi, T.M.; Jamhari, C. Internet of Things Based Automated Monitoring for Indoor Aeroponic System. Int. J. Electr. Comput. Eng. IJECE 2023, 13, 270–277. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Khan, I.H.; Suman, R. Understanding the Potential Applications of Artificial Intelligence in Agriculture Sector. Adv. Agrochem. 2023, 2, 15–30. [Google Scholar] [CrossRef]
- Bhakta, I.; Phadikar, S.; Majumder, K. State-of-the-Art Technologies in Precision Agriculture: A Systematic Review. J. Sci. Food Agric. 2019, 99, 4878–4888. [Google Scholar] [CrossRef] [PubMed]
- Dhope, V.; Chavan, A.; Hadmode, N.; Godase, V. Smart plant monitoring system. Int. J. Creat. Res. Thoughts 2024, 12, 2320–2882. [Google Scholar]
- Parween, S.; Pal, A.; Snigdh, I.; Kumar, V. An IoT and Machine Learning-Based Crop Prediction System for Precision Agriculture; Springer: Berlin/Heidelberg, Germany, 2021; pp. 9–16. [Google Scholar]
- Durai, S.K.S.; Shamili, M.D. Smart Farming Using Machine Learning and Deep Learning Techniques. Decis. Anal. J. 2022, 3, 100041. [Google Scholar] [CrossRef]
- Shaikh, T.A.; Rasool, T.; Lone, F.R. Towards Leveraging the Role of Machine Learning and Artificial Intelligence in Precision Agriculture and Smart Farming. Comput. Electron. Agric. 2022, 198, 107119. [Google Scholar] [CrossRef]
- Jeyabharath, R.; Tamilvani, P.; Karthikeyan, G.; Vijayakumar, P.; Rohini, J.; Hussaini, M. Smart Aeroponic Farms with IoT-Enabled Efficient Automation and Monitoring. In Proceedings of the 2024 2nd International Conference on Artificial Intelligence and Machine Learning Applications Theme: Healthcare and Internet of Things (AIMLA), Namakkal, India, 15–16 March 2024; pp. 1–7. [Google Scholar]
- Jagadesh, M.; Karthik, M.; Manikandan, A.; Nivetha, S.; Kumar, R.P. IoT Based Aeroponics Agriculture Monitoring System Using Raspberry Pi. Int. J. Creat. Res. Thoughts 2018, 6, 601–608. [Google Scholar]
- Niswar, M.; Tahir, Z.; Wey, C.Y. Design and Implementation of IoT-Based Aeroponic Farming System. In Proceedings of the 2022 IEEE International Conference on Cybernetics and Computational Intelligence (CyberneticsCom), Malang, Indonesia, 16–18 June 2022; pp. 308–311. [Google Scholar]
- Lucero, L.; Lucero, D.; Ormeno-Mejia, E.; Collaguazo, G. Automated Aeroponics Vegetable Growing System. Case Study Lettuce. In Proceedings of the 2020 IEEE Andescon, Quito, Ecuador, 13–16 October 2020; pp. 1–6. [Google Scholar]
- Jamhari, C.; Wibowo, W.; Rahma, A.; Roffi, T.M. Design and Implementation of IoT System for Aeroponic Chamber Temperature Monitoring. In Proceedings of the 2020 Third International Conference on Vocational Education and Electrical Engineering (ICVEE), Surabaya, Indonesia, 3–4 October 2020; pp. 1–4. [Google Scholar]
- Vadivel, M. Aeroponics System Using IoT for Smart Farming. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 1607–1611. [Google Scholar] [CrossRef]
- Eka Putri, R.; Fauzia, W.; Cherie, D. Monitoring and Control System Development on IoT-Based Aeroponic Growth of Pakcoy (Brassica rapa L.). J. Keteknikan Pertan. 2023, 11, 222–239. [Google Scholar] [CrossRef]
- Francis, F.; Vishnu, P.L.; Jha, M.; Rajaram, B. IOT-Based Automated Aeroponics System. In Intelligent Embedded Systems: Select Proceedings of ICNETS2; Thalmann, D., Subhashini, N., Mohanaprasad, K., Murugan, M., Eds.; Springer: Singapore, 2018; Volume 492, pp. 337–345. [Google Scholar]
- Kim, J.; Park, H.; Seo, C.; Kim, H.; Choi, G.; Kim, M.; Kim, B.; Lee, W. Sustainable and Inflatable Aeroponics Smart Farm System for Water Efficiency and High-Value Crop Production. Appl. Sci. 2024, 14, 4931. [Google Scholar] [CrossRef]
- Rajendiran, G.; Rethnaraj, J. A Machine Learning Approach for Aeroponic Lettuce Crop Growth Monitoring System. In Proceedings of the International Conference on Intelligent Sustainable Systems, Trichy, India, 23–25 August 2023; pp. 99–116, ISBN 978-981-99-1725-9. [Google Scholar]
- Åström, O.; Hedlund, H.; Sopasakis, A. Machine-Learning Approach to Non-Destructive Biomass and Relative Growth Rate Estimation in Aeroponic Cultivation. Agriculture 2023, 13, 801. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Vervloessem, E.; Nikiforov, A.; Bogaerts, A. Nitrogen Fixation with Water Vapor by Nonequilibrium Plasma: Toward Sustainable Ammonia Production. ACS Sustain. Chem. Eng. 2020, 8, 2996–3004. [Google Scholar] [CrossRef]
- Lai, W.-I.; Chen, Y.-Y.; Sun, J.-H. Ensemble Machine Learning Model for Accurate Air Pollution Detection Using Commercial Gas Sensors. Sensors 2022, 22, 4393. [Google Scholar] [CrossRef] [PubMed]
- Tozlu, B.H. A Fast and Cost-Effective Electronic Nose Model for Methanol Detection Using Ensemble Learning. Chemosensors 2024, 12, 225. [Google Scholar] [CrossRef]
- Rahardja, U.; Aini, Q.; Manongga, D.; Sembiring, I.; Girinzio, I.D. Implementation of Tensor Flow in Air Quality Monitoring Based on Artificial Intelligence. Int. J. Artif. Intell. Res. 2023, 6, 1. [Google Scholar]
- Li, Y.; Guo, S.; Wang, B.; Sun, J.; Zhao, L.; Wang, T.; Yan, X.; Liu, F.; Sun, P.; Wang, J. Machine Learning-assisted Wearable Sensor Array for Comprehensive Ammonia and Nitrogen Dioxide Detection in Wide Relative Humidity Range. InfoMat 2024, 6, e12544. [Google Scholar]
- Bruno, C.; Licciardello, A.; Nastasi, G.A.M.; Passaniti, F.; Brigante, C.; Sudano, F.; Faulisi, A.; Alessi, E. Embedded Artificial Intelligence Approach for Gas Recognition in Smart Agriculture Applications Using Low Cost Mox Gas Sensors. In Proceedings of the 2021 Smart Systems Integration (SSI), Grenoble, France, 27–29 April 2021; pp. 1–5. [Google Scholar]
- Mahapatra, C. Recent Advances in Medical Gas Sensing with Artificial Intelligence–Enabled Technology. Med. Gas Res. 2025, 15, 318–326. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lee, Y.-C.; Tee, J.-H.; Lee, M.-T.; Ting, C.-K.; Juang, J.-Y. AI-Powered Precursor Quantification in Atmospheric Pressure Plasma Jet Thin Film Deposition via Optical Emission Spectroscopy. Plasma Sources Sci. Technol. 2024, 33, 105015. [Google Scholar] [CrossRef]
- Chan, K.J.; Stancampiano, A.; Skinner, K.N.; Robert, E.; Mesbah, A. A Cold Atmospheric Plasma Sensor for Identification and Differentiation of Biological Tissues. IEEE Trans. Radiat. Plasma Med. Sci. 2024. [Google Scholar] [CrossRef]
- Özdemir, G.D.; Özdemir, M.A.; Şen, M.; Ercan, U.K. Machine Learning to Predict Oxidative Strength of Cold Atmospheric Plasma Activated Water via Paper-Based Sensor. In Proceedings of the 2022 Medical Technologies Congress (TIPTEKNO), Antalya, Turkey, 31 October–2 November 2022; pp. 1–4. [Google Scholar]
- Islam, T.; Rabbi, F.; Ahmed, R.; Rahman, M.M.; Ahmed, M. IoT Based Air Components Collection for Machine Learning Reinforcement. Doctoral Dissertation, Brac University, Dhaka, Bangladesh, 2022. [Google Scholar]
- Listyarini, S.; Warlina, L.; Sambas, A. The Air Quality Monitoring Tool Based on Internet of Things to Monitor Pollution Emissions Continuously. Environ. Ecol. Res. 2022, 10, 824–829. [Google Scholar] [CrossRef]
- Pizarro Mujica, A.F. Design an Implementation of a Gas Sensing Device Capable of Sending Real Time Data via Narrow Band-IoT. Thesis, Universidad de Chile, Santiago, Chile, 2024. Available online: https://repositorio.uchile.cl/xmlui/handle/2250/200772 (accessed on 19 February 2025).
- Maulini, R.; Sahlinal, D.; Arifin, O. Monitoring of pH, Amonia (NH3) and Temperature Parameters Aquaponic Water in the 4.0 Revolution Era. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1012, p. 012087. [Google Scholar] [CrossRef]
- Mohammed, A.; Shafiq, O.; Muhammad, S.; Abdulla, A. Air Polluted: Ammonia and CO2 Measurement by Using Arduino. In Proceedings of the 4th International Conference on Architectural & Civil Engineering Sciences, Suzhou, China, 24–26 March 2023; Cihan University-Erbil: Erbil Governorate, Iraq, 2023; pp. 73–77. [Google Scholar]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.-K. Chlorophyll Fluorescence Imaging for Early Detection of Drought and Heat Stress in Strawberry Plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef]
- Nayak, A.; Chakraborty, S.; Swain, D.K. Application of Smartphone-Image Processing and Transfer Learning for Rice Disease and Nutrient Deficiency Detection. Smart Agric. Technol. 2023, 4, 100195. [Google Scholar] [CrossRef]
- Castro-Valdecantos, P.; Egea, G.; Borrero, C.; Pérez-Ruiz, M.; Avilés, M. Detection of Fusarium Wilt-Induced Physiological Impairment in Strawberry Plants Using Hyperspectral Imaging and Machine Learning. Precis. Agric. 2024, 25, 2958–2976. [Google Scholar] [CrossRef]
- Sang, W.; Cui, J.; Mei, L.; Zhang, Q.; Li, Y.; Li, D.; Zhang, W.; Li, Z. Degradation of Liquid Phase N, N-Dimethylformamide by Dielectric Barrier Discharge Plasma: Mechanism and Degradation Pathways. Chemosphere 2019, 236, 124401. [Google Scholar] [CrossRef] [PubMed]
- Hawtof, R.; Ghosh, S.; Guarr, E.; Xu, C.; Mohan Sankaran, R.; Renner, J.N. Catalyst-Free, Highly Selective Synthesis of Ammonia from Nitrogen and Water by a Plasma Electrolytic System. Sci. Adv. 2019, 5, eaat5778. [Google Scholar] [CrossRef]
- He, J. Indirect Non-Thermal Plasma Treatment in Public Health, Agricultural and Food Safety Applications; Drexel University: Philadelphia, PA, USA, 2023; ISBN 979-8-3805-8405-0. [Google Scholar]
- Rout, S.; Tripathy, S.; Srivastav, P.P. Effect of Cold Plasma for Modulating Macromolecules and Bioactive Composition of Food: Unveiling Mechanisms and Synergies with Other Emerging Techniques. Food Biosci. 2024, 61, 104545. [Google Scholar] [CrossRef]




| Type | Reactive Species | Half-Life Time | Analytical Approaches | Reference |
|---|---|---|---|---|
| ROS | Hydroxyl (·OH) | 10−9~10−10 s | ESR, BA method | [76] |
| Singlet oxygen (1O2) | 4.4 μs | ESR | [77] | |
| Superoxide (O2−·) | 10−9 s | ESR | [78] | |
| Hydroperoxyl (HO2−) | N/A | ESR | [79] | |
| Hydrogen Peroxide (H2O2), | Stable | Test strips, UV–vis, FTIR | [80] | |
| Ozone (O3) | s | Indigo degradation, certified kit | [81] [82] | |
| RNS | Nitric oxide (NO) | s | ESR | [83] |
| Peroxynitrite (ONOO−) | 10−3 s | Ion chromatography | [84] | |
| Nitrite acid (HNO2), Nitrate acid (HNO3) | Stable | Nitrite assay kit, UV–vis, ion chromatography | [85] | |
| Ammonium ions (NH4+) | Stable | UV–vis, ion chromatography | [10] | |
| Nitrous acid (HNO2) | s | N/A | [86] |
| Crop | Application | Importance | Significance | Reference |
|---|---|---|---|---|
| Radish | Plasma-activated water (PAW) | Enhanced growth and nutrient uptake | 30% longer roots, 50% higher biomass | [133] |
| Sweet basil | Plasma-activated nutrient solution (PANS) | Growth enhancement and algae reduction | Increased growth; algae reduced by 24% | [128] |
| Bok choy | Plasma-treated nutrient solutions | Salinity stress tolerance and improved growth | 80.5% higher dry weight | [134] |
| Green oak lettuce | PAW for nitrate generation | Alternative to chemical fertilizers | Plasma nitrate yields comparable to commercial nitrate | [12] |
| Cucumber | Plasma for decomposing allelochemicals | Growth despite chemical inhibitors | DCBA levels significantly reduced | [135] |
| Plant | Treatments Used/Technology | Technology | Key Findings and Performances | References |
|---|---|---|---|---|
| Maize | IoT, temp and humidity control, real-time monitoring | IoT-based sensors | Automates nutrient delivery, reduces labor, optimizes resources, real-time alerts | [186] |
| Crop not specified | Irrigation, nutrient, and climate control | IoT, Raspberry Pi | IoT-based auto-monitoring and control | [187] |
| Tomato (Solanumly copersicum) | Climate, water, evapotranspiration control | IoT for Evapotranspiration Monitoring | Uses microcontroller for evapotranspiration | [188] |
| Basil (Ocimum basilicum) | Nutrient misting, humidity control, real-time monitoring | IoT-based monitoring system | Real-time data collection and control | [52] |
| Green leaf lettuce | Nutrient misting, temp, humidity, irrigation control | Arduino-based IoT system | 40% increase in leaves, 400% in root growth | [189] |
| Mustard greens (Brassica juncea) | pH control, automated irrigation, climate control | IoT-based system | Optimizes temperature and humidity for growth | [190] |
| Pakcoy (Brassica rapa. L.) | Climate control, nutrient automation, real-time analysis | IoT for smart farming | Regulates growth and mist environment | [191] |
| Pakcoy (Brassica rapa. L.) | NodeMCU, DHT22, TDS sensors, Blynk app | NodeMCU, Blynk | Superior sensor precision, similar growth to control group | [192] |
| Crop not specified | Temp and humidity control, LED lights, IoT-based monitoring | IoT-based system | Enhances growth and stabilizes conditions | [193] |
| Lettuce | Misting, sealed environment for water and nutrient delivery | Smart farm system | Reduces water usage, optimal conditions for urban environments | [194] |
| Lettuce | IoT sensors, machine learning for automation | IoT sensors, machine learning | Improves yield prediction and automates growth | [195] |
| Crop not specified | Multi-variate regression, ResNet-50 for growth estimation | Machine learning, neural networks | Multi-variate regression best for biomass, ResNet-50 for growth rate estimation | [196] |
| Ipomoea reptans | LED lighting, IoT control for temp, humidity, light | Wemos D1 mini, Thing Speak | Controlled temperature and improved growth quality | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, W.A.; Gao, J.; Elsherbiny, O.; Mosha, A.H.; Tunio, M.H.; Qureshi, J.A. Boosting Aeroponic System Development with Plasma and High-Efficiency Tools: AI and IoT—A Review. Agronomy 2025, 15, 546. https://doi.org/10.3390/agronomy15030546
Qureshi WA, Gao J, Elsherbiny O, Mosha AH, Tunio MH, Qureshi JA. Boosting Aeroponic System Development with Plasma and High-Efficiency Tools: AI and IoT—A Review. Agronomy. 2025; 15(3):546. https://doi.org/10.3390/agronomy15030546
Chicago/Turabian StyleQureshi, Waqar Ahmed, Jianmin Gao, Osama Elsherbiny, Abdallah Harold Mosha, Mazhar Hussain Tunio, and Junaid Ahmed Qureshi. 2025. "Boosting Aeroponic System Development with Plasma and High-Efficiency Tools: AI and IoT—A Review" Agronomy 15, no. 3: 546. https://doi.org/10.3390/agronomy15030546
APA StyleQureshi, W. A., Gao, J., Elsherbiny, O., Mosha, A. H., Tunio, M. H., & Qureshi, J. A. (2025). Boosting Aeroponic System Development with Plasma and High-Efficiency Tools: AI and IoT—A Review. Agronomy, 15(3), 546. https://doi.org/10.3390/agronomy15030546









