On the Effectiveness of Ozone Treatments: A Silver Bullet for Plant Health?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and O3 Treatments
2.2. Analysis of Plant Physiological Parameters
2.3. RNA Extraction and Plant Gene Expression Analysis
2.4. Statistical Analysis
3. Results
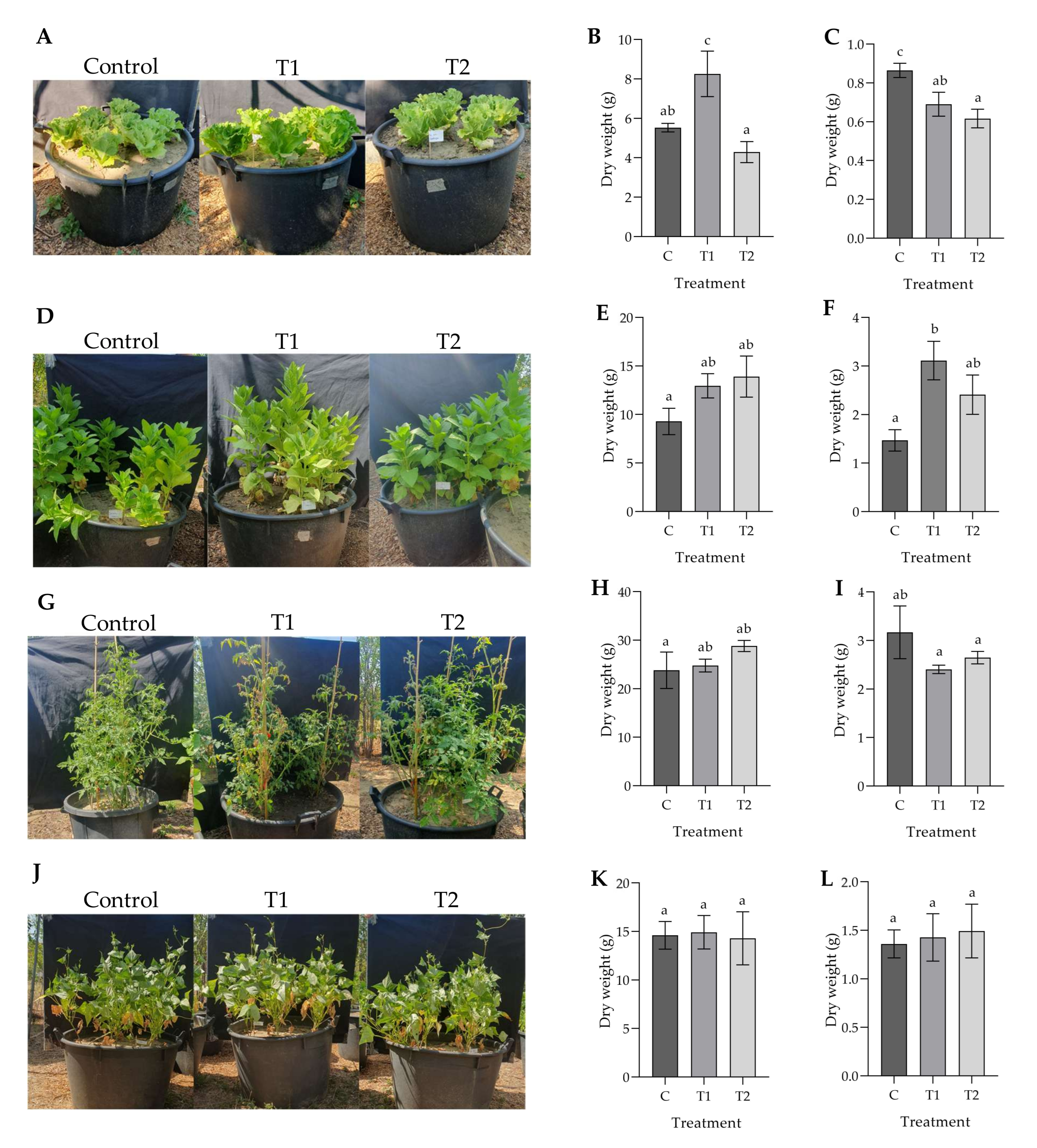
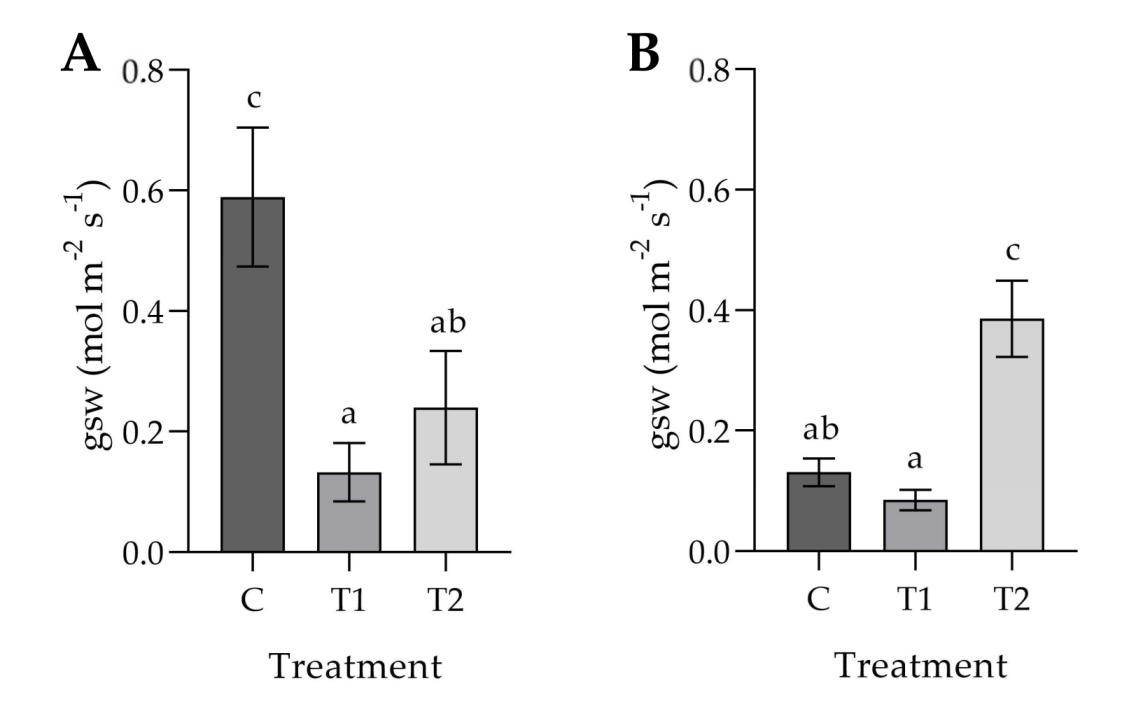
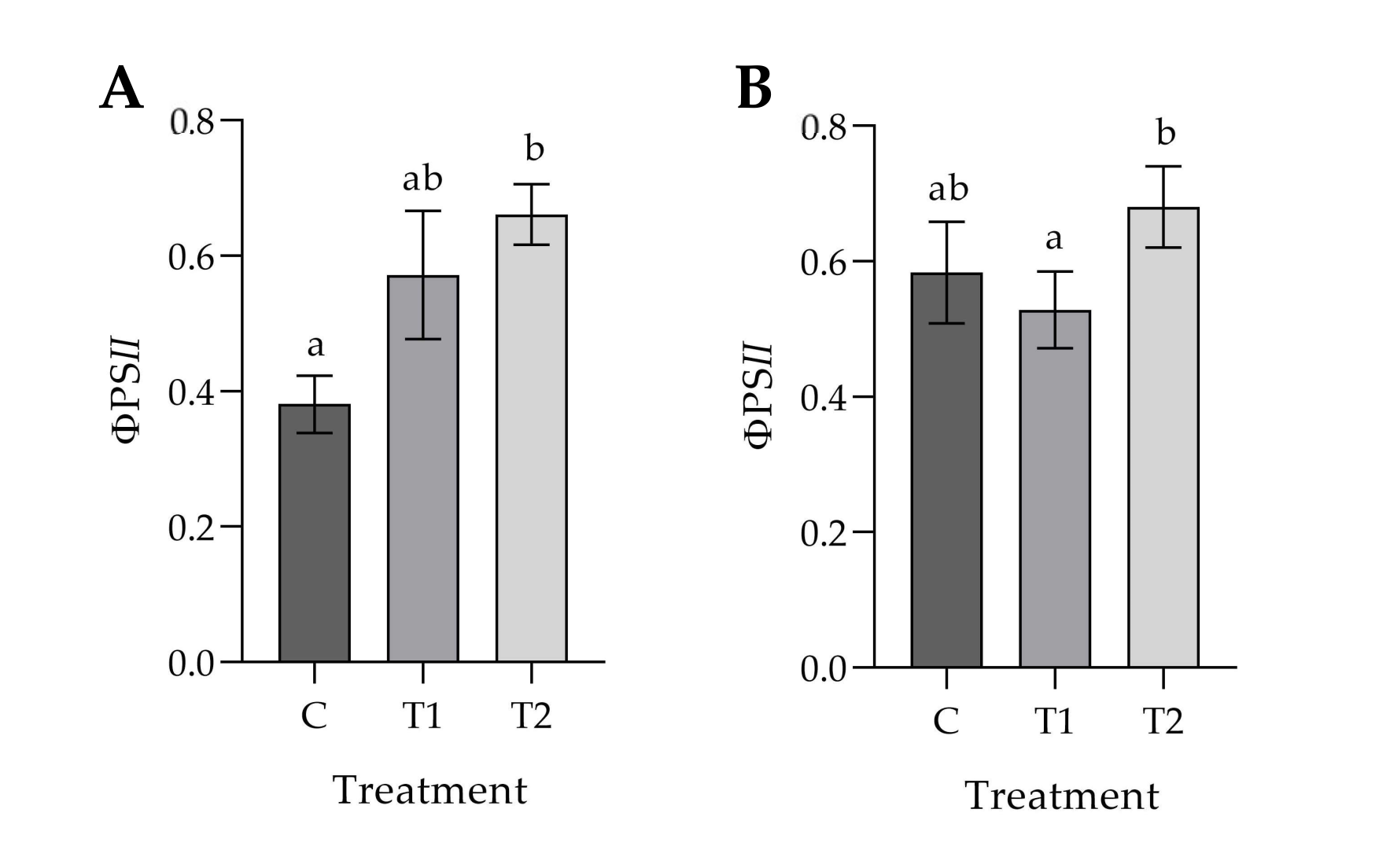
3.1. Effects of Ozonated Water on the Development and Physiology of Potted Plants
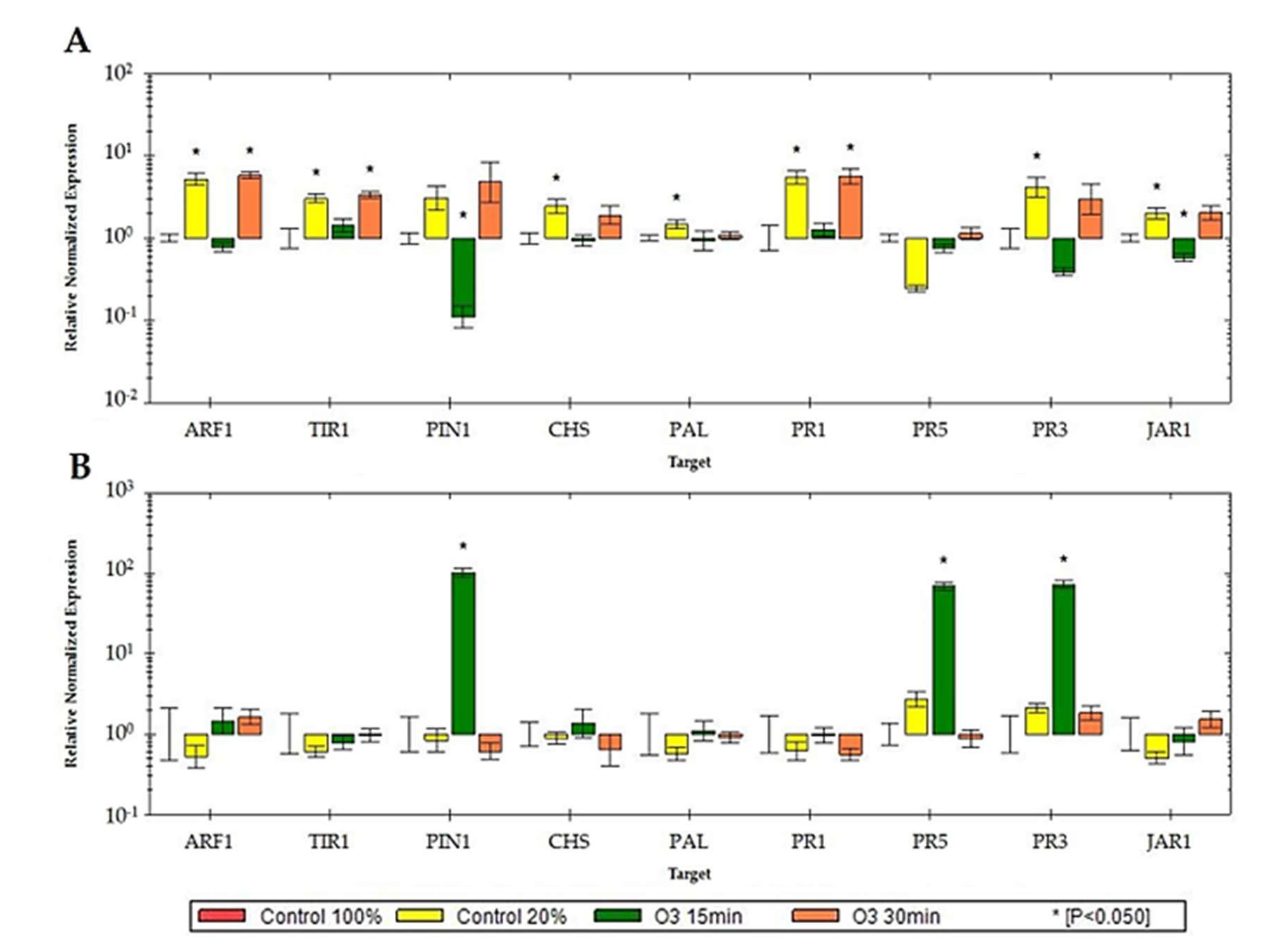
3.2. Impact of Ozonated Water on the Expression of Defense-Related Genes in Potted Plants
3.3. Effects of Ozonated Water on the Development and Physiology of Hydroponically Grown Lettuce Under Nutritional Stress
3.4. Impact of Ozonated Water on the Expression of Defense Related Genes in Hydroponically Grown Lettuce
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Population Prospects—Population Division—United Nations. Available online: https://population.un.org/wpp/graphs?loc=900&type=Probabilistic%20Projections&category=Population&subcategory=1_Total%20Population (accessed on 21 November 2024).
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate Change and the Need for Agricultural Adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef]
- Arora, N.K. Impact of Climate Change on Agriculture Production and Its Sustainable Solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Vlaiculescu, A.; Varrone, C. Chapter 14—Sustainable and Eco-Friendly Alternatives to Reduce the Use of Pesticides. In Pesticides in the Natural Environment; Singh, P., Singh, S., Sillanpää, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–364. ISBN 978-0-323-90489-6. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/RP/visualize (accessed on 21 November 2024).
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment. In Plant, Soil and Microbes; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 253–269. ISBN 978-3-319-27453-9. [Google Scholar]
- Ozone Properties. Available online: https://ozonesolutions.com/blog/ozone-properties/ (accessed on 21 November 2024).
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2012; ISBN 978-1-78040-083-9. [Google Scholar]
- Remondino, M.; Valdenassi, L. Different Uses of Ozone: Environmental and Corporate Sustainability. Literature review and case study. Sustainability 2018, 10, 4783. [Google Scholar] [CrossRef]
- Martínez, S.B.; Pérez-Parra, J.; Suay, R. Use of Ozone in Wastewater Treatment to Produce Water Suitable for Irrigation. Water Resour. Manag. 2011, 25, 2109–2124. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Risoli, S.; Lauria, G. Ozonated Water Application as an Innovative Tool for Elicitation of Plant Defense Response: A Minireview. Curr. Opin. Environ. Sci. Health 2022, 28, 100375. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Yoshii, M.; Isobe, T.; Park, J.-S.; Kurata, K.; Fujiwara, K. Nutrient Solution Prepared with Ozonated Water Does Not Damage Early Growth of Hydroponically Grown Tomatoes. Ozone Sci. Eng. 2009, 31, 21–27. [Google Scholar] [CrossRef]
- Díaz-López, M.; Siles, J.A.; Ros, C.; Bastida, F.; Nicolás, E. The Effects of Ozone Treatments on the Agro-Physiological Parameters of Tomato Plants and the Soil Microbial Community. Sci. Total Environ. 2022, 812, 151429. [Google Scholar] [CrossRef] [PubMed]
- Díaz-López, M.; Galera, L.; Bastida, F.; Nicolás, E. Tomato Growth and Physiology as Well as Soil Physicochemical and Biological Properties Affected by Ozonated Water in a Saline Agroecosystem. Sci. Total Environ. 2024, 906, 167472. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A. Understanding and Improving Global Crop Response to Ozone Pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, P.; Kollist, H.; Langebartels, C.; Kangasjärvi, J. Plant Responses to Ozone. In Antioxidants and Reactive Oxygen Species in Plants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 268–292. ISBN 978-0-470-98856-5. [Google Scholar]
- Tahamolkonan, M.; Ghahsareh, A.M.; Ashtari, M.K.; Honarjoo, N. Tomato (Solanum lycopersicum) Growth and Fruit Quality Affected by Organic Fertilization and Ozonated Water. Protoplasma 2022, 259, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Labair, H.; Nemmich, S.; Chiali Charif, K.; Ramdani, N.; Nassour, K.; Reguig, M.; Tilmatine, A. Investigating the Synergistic Impact of Ozonated Water Irrigation and Organic Fertilization on Tomato Growth. Emir. J. Food Agric. 2024, 36, 1–9. [Google Scholar] [CrossRef]
- Najarian, M.; Mohammadi-Ghehsareh, A.; Fallahzade, J.; Peykanpour, E. Responses of Cucumber (Cucumis sativus L.) to Ozonated Water under Varying Drought Stress Intensities. J. Plant Nutr. 2018, 41, 1–9. [Google Scholar] [CrossRef]
- Xu, J.-P.; Yu, Y.-C.; Zhang, T.; Ma, Q.; Yang, H.-B. Effects of Ozone Water Irrigation and Spraying on Physiological Characteristics and Gene Expression of Tomato Seedlings. Hortic Res 2021, 8, 180. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Li, Y.; Wang, Q. Effect of Different Concentrations of Ozone on in Vitro Plant Pathogens Development, Tomato Yield and Quality, Photosynthetic Activity and Enzymatic Activities. Ozone Sci. Eng. 2019, 41, 531–540. [Google Scholar] [CrossRef]
- Veronico, P.; Paciolla, C.; Sasanelli, N.; De Leonardis, S.; Melillo, M.T. Ozonated Water Reduces Susceptibility in Tomato Plants to Meloidogyne incognita by the Modulation of the Antioxidant System. Mol. Plant Pathol. 2017, 18, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Prigigallo, M.I.; Melillo, M.T.; Bubici, G.; Dobrev, P.I.; Vankova, R.; Cillo, F.; Veronico, P. Ozone Treatments Activate Defence Responses against Meloidogyne incognita and Tomato Spotted Wilt Virus in Tomato. Pest Manag. Sci. 2019, 75, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, Y.; Yan, H.; Li, C.; Ge, F. O3-Induced Priming Defense Associated with the Abscisic Acid Signaling Pathway Enhances Plant Resistance to Bemisia tabaci. Front. Plant Sci. 2020, 11, 93. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Tegli, S.; Bini, L.; Calamai, S.; Cerboneschi, M.; Biancalani, C. A MATE Transporter Is Involved in Pathogenicity and IAA Homeostasis in the Hyperplastic Plant Pathogen Pseudomonas savastanoi pv. nerii. Microorganisms 2020, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gardoni, D.; Vailati, A.; Canziani, R. Decay of Ozone in Water: A Review. Ozone Sci. Eng. 2012, 34, 233–242. [Google Scholar] [CrossRef]
- Colunje, J.; Garcia-Caparros, P.; Moreira, J.F.; Lao, M.T. Effect of Ozonated Fertigation in Pepper Cultivation under Greenhouse Conditions. Agronomy 2021, 11, 544. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, C.; Song, W. Effect of Ozonated Nutrient Solution on the Growth and Root Antioxidant Capacity of Substrate and Hydroponically Cultivated Lettuce (Lactuca sativa). Ozone Sci. Eng. 2020, 42, 286–292. [Google Scholar] [CrossRef]
- Berry, J.A.; Beerling, D.J.; Franks, P.J. Stomata: Key Players in the Earth System, Past and Present. Curr. Opin. Plant Biol. 2010, 13, 232–239. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Kangasjärvi, J. Plant Signalling in Acute Ozone Exposure. Plant Cell Environ. 2015, 38, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Aguayo, E. Effects of Ozonated Water Irrigation on the Quality of Grafted Watermelon Seedlings. Sci. Hortic. 2020, 261, 109047. [Google Scholar] [CrossRef]
- Sudhakar, N.; Nagendra-Prasad, D.; Mohan, N.; Murugesan, K. Induction of Systemic Resistance in Lycopersicon esculentum Cv. PKM1 (Tomato) against Cucumber Mosaic Virus by Using Ozone. J. Virol. Methods 2007, 139, 71–77. [Google Scholar] [CrossRef]
- Sharma, Y.K.; Léon, J.; Raskin, I.; Davis, K.R. Ozone-Induced Responses in Arabidopsis thaliana: The Role of Salicylic Acid in the Accumulation of Defense-Related Transcripts and Induced Resistance. Proc. Natl. Acad. Sci. USA 1996, 93, 5099–5104. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-Related Proteins and Peptides as Promising Tools for Engineering Plants with Multiple Stress Tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Meddya, S.; Meshram, S.; Sarkar, D.; S, R.; Datta, R.; Singh, S.; Avinash, G.; Kumar Kondeti, A.; Savani, A.K.; Thulasinathan, T. Plant Stomata: An Unrealized Possibility in Plant Defense against Invading Pathogens and Stress Tolerance. Plants 2023, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Tamaoki, M. The Role of Phytohormone Signaling in Ozone-Induced Cell Death in Plants. Plant Signal. Behav. 2008, 3, 166. [Google Scholar] [CrossRef]
- Paolacci, A.R.; D’Ovidio, R.; Marabottini, R.; Nali, C.; Lorenzini, G.; Abenavoli, M.R.; Badiani, M. Research Note: Ozone Induces a Differential Accumulation of Phenyalanine Ammonia-Lyase, Chalcone Synthase and Chalcone Isomerase RNA Transcripts in Sensitive and Resistant Bean Cultivars. Funct. Plant Biol. 2001, 28, 425. [Google Scholar] [CrossRef]
- Pazarlar, S.; Cetinkaya, N.; Bor, M.; Ozdemir, F. Ozone Triggers Different Defence Mechanisms against Powdery Mildew (Blumeria graminis DC. Speer f. sp. tritici) in Susceptible and Resistant Wheat Genotypes. Funct. Plant Biol. 2017, 44, 1016. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Goto, E. Ozone Control as a Novel Method to Improve Health-Promoting Bioactive Compounds in Red Leaf Lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1045239. [Google Scholar] [CrossRef] [PubMed]
- Marchica, A.; Ascrizzi, R.; Flamini, G.; Cotrozzi, L.; Tonelli, M.; Lorenzini, G.; Nali, C.; Pellegrini, E. Ozone as Eustress for Enhancing Secondary Metabolites and Bioactive Properties in Salvia officinalis. Ind. Crops Prod. 2021, 170, 113730. [Google Scholar] [CrossRef]
- Del Bianco, M.; Kepinski, S. Context, Specificity, and Self-Organization in Auxin Response. Cold Spring Harb. Perspect. Biol. 2011, 3, a001578. [Google Scholar] [CrossRef]
- Waller, F.; Furuya, M.; Nick, P. OsARF1, an Auxin Response Factor from Rice, Is Auxin-Regulated and Classifies as a Primary Auxin Responsive Gene. Plant Mol. Biol. 2002, 50, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2regulate Senescence and Floral Organ Abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic Acid Inhibits Pathogen Growth in Plants through Repression of the Auxin Signaling Pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. Linking Development to Defense: Auxin in Plant–Pathogen Interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef]
- Campos, M.L.; Kang, J.-H.; Howe, G.A. Jasmonate-Triggered Plant Immunity. J. Chem. Ecol. 2014, 40, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Franco, O.L. Pathogenesis-Related Proteins (PRs) with Enzyme Activity Activating Plant Defense Responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef] [PubMed]



| Components | Volume (mL) |
|---|---|
| 1 M KH2PO4 | 1.0 |
| 1 M KNO3 | 6.0 |
| 1 M Ca(NO3)2·4H2O | 4.0 |
| 1 M MgSO4·7H2O | 2.0 |
| Micronutrient solution * | 1.0 |
| Fe-EDDHA 60 g/L | 1.0 |
| Distilled H2O | Up to 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastacaldi, C.; Gaudioso, D.; Beltrami, C.; Gunnella, B.; Tegli, S. On the Effectiveness of Ozone Treatments: A Silver Bullet for Plant Health? Agronomy 2025, 15, 567. https://doi.org/10.3390/agronomy15030567
Pastacaldi C, Gaudioso D, Beltrami C, Gunnella B, Tegli S. On the Effectiveness of Ozone Treatments: A Silver Bullet for Plant Health? Agronomy. 2025; 15(3):567. https://doi.org/10.3390/agronomy15030567
Chicago/Turabian StylePastacaldi, Chiara, Dario Gaudioso, Cosimo Beltrami, Benedetta Gunnella, and Stefania Tegli. 2025. "On the Effectiveness of Ozone Treatments: A Silver Bullet for Plant Health?" Agronomy 15, no. 3: 567. https://doi.org/10.3390/agronomy15030567
APA StylePastacaldi, C., Gaudioso, D., Beltrami, C., Gunnella, B., & Tegli, S. (2025). On the Effectiveness of Ozone Treatments: A Silver Bullet for Plant Health? Agronomy, 15(3), 567. https://doi.org/10.3390/agronomy15030567








