Influence of Rhizosphere Temperature and Humidity Regulation on Rooting, Mortality, and Transplant Survival of Aeroponically Rapid Growth Mulberry Cutting
Abstract
:1. Introduction
2. Materials and Methods
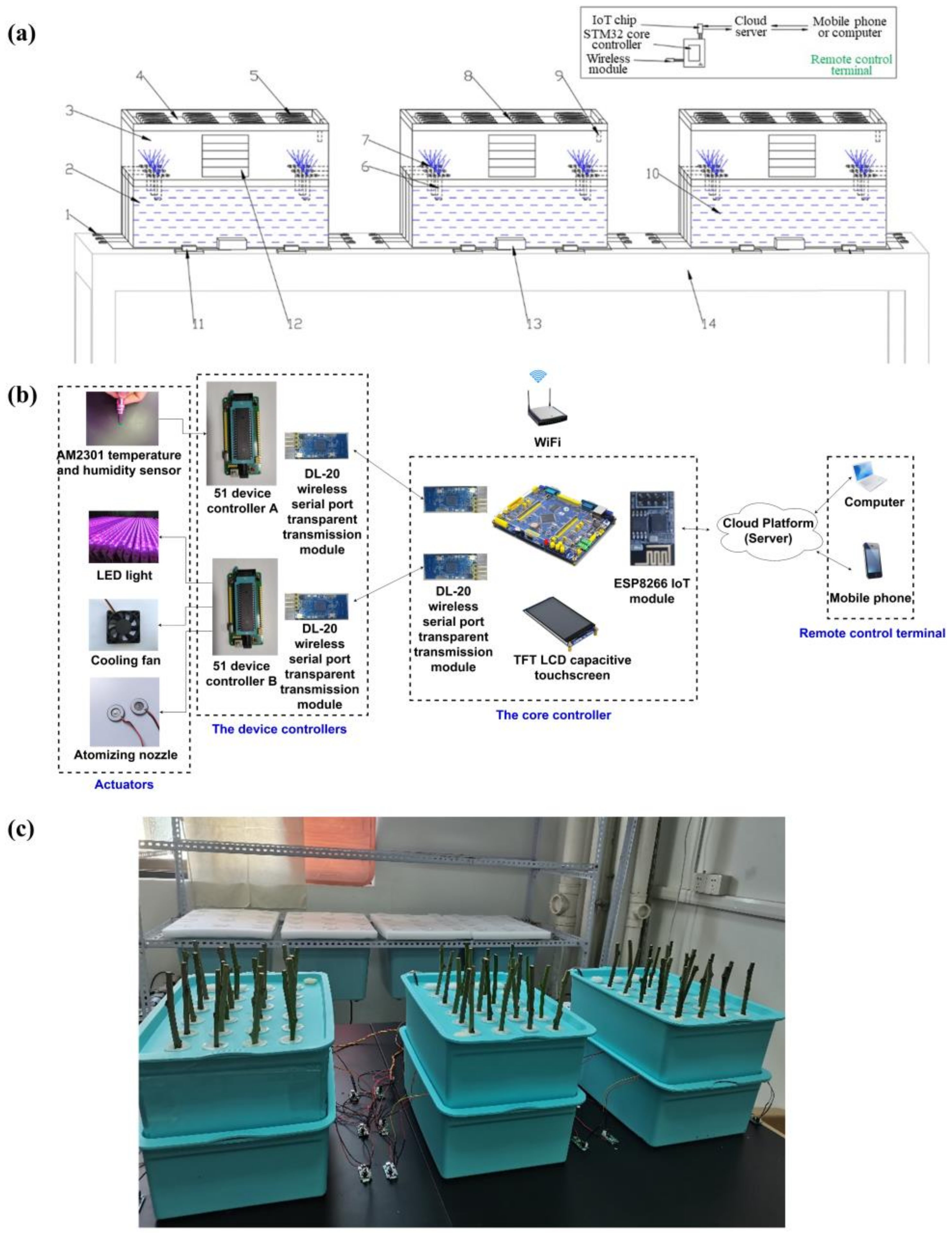
2.1. Design of Aeroponically Rapid Propagation Experimental Test Bench
2.2. Temperature and Humidity Monitoring and Control System
2.3. Experimental Site and Plant Material
2.4. Experimental Arrangement
2.5. Experimental Indices
2.5.1. Indices of Cultivation
- The computation methodologies for callus rooting and mortality rates remained consistent across treatment groups. In each treatment group, callus formation at the incision site, root system development at the base, and the count of deceased cuttings were compared to calculate the callus, rooting, and mortality rates.
- Root length was non-invasively measured using a vernier caliper, and root count was recorded directly. The subsequent determination of average root length and average root number followed.
- Upon experiment completion, five randomly selected rooted plants underwent root clipping. The fresh weight of the roots was assessed using an electronic balance model JM-B6002 (Max = 600 g, d = 0.01 g, e = 10 d). After measuring the fresh weight, the roots were placed in an envelope and dried for three hours in an 85 °C dryer. The dry weight was measured using a high-precision electronic balance, Sartorius model BS223S (Max 220 g, d = 0.001 g).
2.5.2. Transplanting Survival Rate
2.5.3. Droplet Adhesion Amount
2.6. Statistical Analyses
3. Results and Discussion
3.1. Rapid Propagation and Transplanting Results
- a.
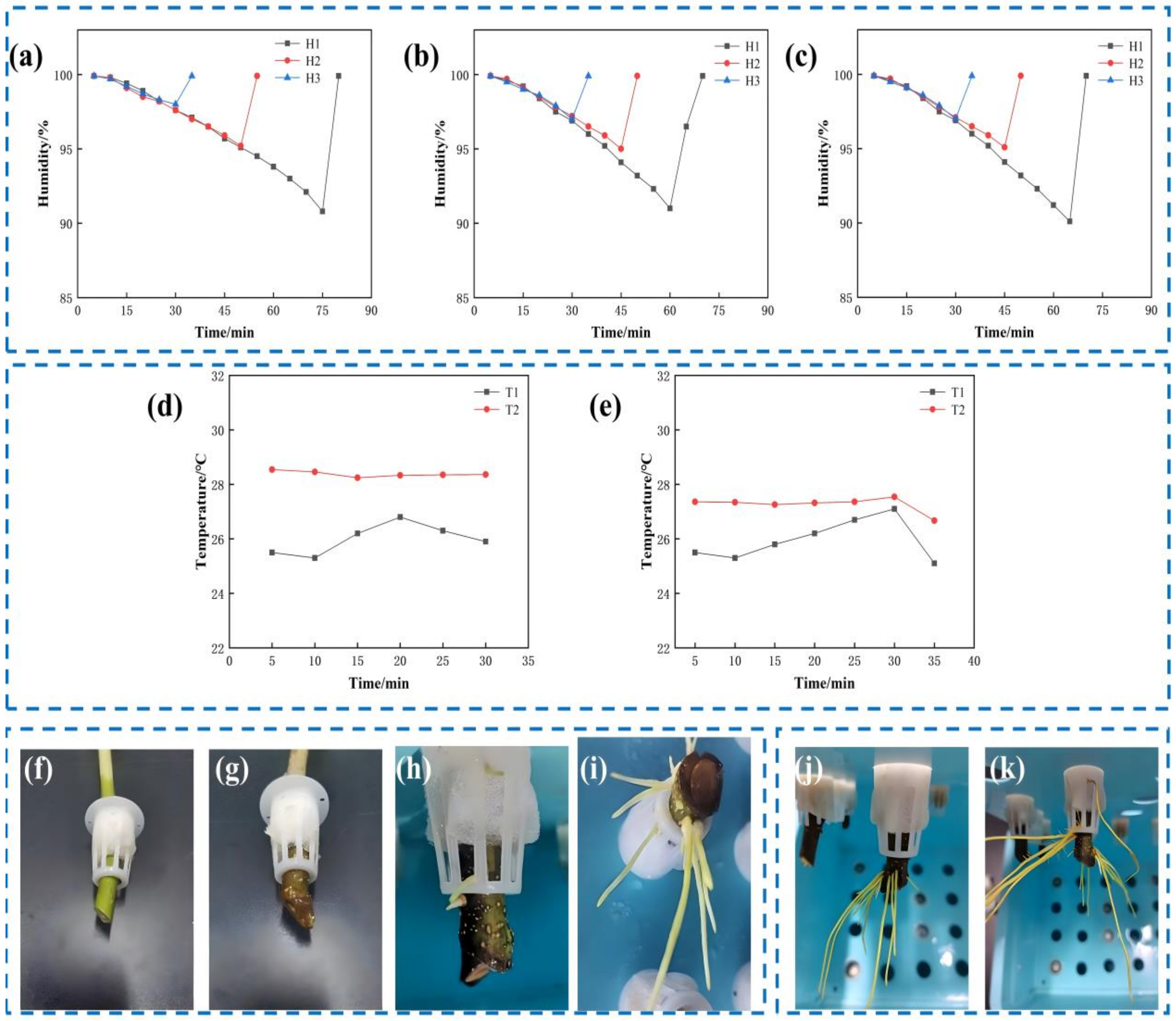
- Effect of humidity regulation on rooting of cuttings
- b.
- Effect of temperature regulation on rooting of cuttings
- c.
- Effect of temperature and humidity regulation on rooting of cuttings
3.2. The Impact of Aeroponically Rapid Propagation Transplanting Survival Rate Statistics
3.3. Study the Relationship Between the Droplet Adhesion Amount, Mildew, and Rot of Cuttings
3.3.1. Distribution of Moldy and Rotten Cuttings in Aeroponically Rapid Propagation System
3.3.2. Result Analysis of Droplet Adhesion
4. Conclusions
- Develop intelligent systems to regulate the plant’s aeroponically rapid propagation process precisely.
- Investigate temperature and humidity conditions with greater detail and specificity, narrowing down the regulation ranges. Explore other environmental factors, such as light intensity, nutrient solution composition, and concentrations.
- Address the issue of uneven droplet adhesion by redesigning aeroponically rapid propagation devices to improve cutting survival rates.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H1 | Humidity-regulated aerosol rapid cultivation experiment with 90–100% humidity |
| H2 | Humidity-regulated aerosol rapid cultivation experiment with 95–100% humidity |
| H3 | Humidity-regulated aerosol rapid cultivation experiment, and spraying for 2 min every 30 min |
| HCK | Humidity-regulated soil cultivation experiment and regular watering |
| T1 | Temperature-regulated aerosol rapid cultivation experiment with 25–27 °C temperature and spray for 2 min every 30 min |
| T2 | No temperature regulation, with room temperature and spraying for 2 min every 30 min |
| TCK | Temperature-regulated soil cultivation with room temperature and regular watering |
| TH | Temperature and humidity co-regulated aerosol rapid cultivation experiment with 90–100% humidity and 25–27 °C temperature |
| THCK | No temperature and humidity co-regulated aerosol rapid cultivation experiment, with room temperature and spraying for 2 min every 30 min |
| ANOVA | Analysis of variance |
References
- Nazari, B.; Liaghat, A.; Akbari, M.R.; Keshavarz, M. Irrigation water management in Iran: Implications for water use efficiency improvement. Agric. Water Manag. 2018, 208, 7–18. [Google Scholar] [CrossRef]
- Gupta, G.S. Land degradation and challenges of food security. Rev. Eur. Stud. 2019, 11, 63. [Google Scholar] [CrossRef]
- Seppelt, R.; Arndt, C.; Beckmann, M.; Martin, E.A.; Hertel, T.W. Deciphering the biodiversity–production mutualism in the global food security debate. Trends Ecol. Evol. 2020, 35, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Shan, V.; Singh, S.K.; Haritash, A.K. Water Crisis in the Asian countries: Status and future trends. In Resilience, Response, and Risk in Water Systems: Shifting Management and Natural Forcings Paradigms; Springer: Singapore, 2020; pp. 173–194. [Google Scholar]
- Vargas, D.C.M.; Hoyos, C.P.Q.; Manrique, O.L.H. The water-energy-food nexus in biodiversity conservation: A systematic review around sustainability transitions of agricultural systems. Heliyon 2023, 9, e17016. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J.M.; Regolo, L.; Sánchez-González, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, M. Organic vs. conventional plant-based foods: A review. Food Chem. 2022, 383, 132352. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Zhu, Q.; Niu, Y. Further investigating the performance of crop water stress index for maize from baseline fluctuation, effects of environmental factors, and variation of critical value. Agric. Water Manag. 2023, 285, 108349. [Google Scholar] [CrossRef]
- Nishchitha, G.A.; Bhaskar, R.N.; Bharathi, V.P.; Anusha, H.G. Aeroponic system is boon for rainfed mulberry cultivation and enhance productivity. J. Pharm. Innov. J. 2023, 12, 4480–4484. [Google Scholar]
- Tokunaga, H.; Anh, N.H.; Dong, N.V.; Ham, L.H.; Hanh, N.T.; Hung, N.; Ishitani, M.; Tuan, L.N.; Utsumi, Y.; Vu, N.A.; et al. An efficient method of propagating cassava plants using aeroponic culture. J. Crop Improv. 2020, 34, 64–83. [Google Scholar] [CrossRef]
- Spinoff, N. Progressive plant growing has business blooming. In Environmental and Agricultural Resources; NASA Spinoff: New York, NY, USA, 2006; pp. 64–77. [Google Scholar]
- Buckseth, T.; Sharma, A.K.; Pandey, K.K.; Singh, B.P.; Muthuraj, R. Methods of pre-basic seed potato production with special reference to aeroponics—A review. Sci. Hortic. 2016, 204, 79–87. [Google Scholar] [CrossRef]
- Mohamed, T.M.K.; Gao, J.; Abuarab, M.E.; Kassem, M.; Wasef, E.; El-Ssawy, W. Applying different magnetic water densities as irrigation for aeroponically and hydroponically grown strawberries. Agriculture 2022, 12, 819. [Google Scholar] [CrossRef]
- Jing, L.; Wei, X. Spray deposition and distribution on rice as affected by a boom sprayer with a canopy-opening Device. Agriculture 2022, 13, 94. [Google Scholar] [CrossRef]
- Reddy, M.C.; Indu, K.; Bhargavi, C.; Rajendra, M.P.; Babu, B.H. A review on vegetative propagation and applications in forestry. J. Plant Dev. Sci. 2022, 14, 265–272. [Google Scholar]
- Regas, T.; Han, J.H.; Pauli, C.S.; Park, S.H. Employing aeroponic systems for the clonal propagation of Cannabis. J. Vis. Exp. 2021, 178, e63117. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, S.; Chang, H.; Li, G.; Son, Y. Increased soil temperature stimulates changes in carbon, nitrogen, and mass loss in the fine roots of Pinus koraiensis under experimental warming and drought. Turk. J. Agric. For. 2019, 43, 80–87. [Google Scholar] [CrossRef]
- Bergonci, T.; Fomsgaard, I.S.; Kjaer, K.H.; Paponov, I.A. Hormone–Flavonoid Patterns in Two Genotypes of Campanula portenschlagiana with Distinct Adventitious Rooting Competence. Horticulturae 2023, 9, 121. [Google Scholar] [CrossRef]
- Shabbir, A.; Mao, H.; Ullah, I.; Buttar, N.A.; Ajmal, M.; Solangi, K.A. Improving water use efficiency by optimizing the root distribution patterns under varying drip emitter density and drought stress for cherry tomato. Agronomy 2020, 11, 3. [Google Scholar] [CrossRef]
- Shibuya, T.; Tsukuda, S.; Tokuda, A.; Shiozaki, S.; Endo, R.; Kitaya, Y. Effects of warming basal ends of Carolina poplar (Populus × canadensis Moench.) softwood cuttings at controlled low-air-temperature on their root growth and leaf damage after planting. J. For. Res. 2013, 18, 279–284. [Google Scholar] [CrossRef]
- Rusnak, T.; Braun, L. The Effects of Relative Humidity and Substrate Moisture on Rooting of Hybrid Hazelnuts from Hardwood Stem Cuttings. J. Environ. Hortic. 2017, 35, 156–160. [Google Scholar] [CrossRef]
- Tik, L.B.; Khuan, C.T.; Palaniappan, S. Monitoring of an aeroponic greenhouse with a sensor network. Int. J. Comput. Sci. Netw. Secur. 2009, 40, 240–246. [Google Scholar]
- Domingues, D.S.; Takahashi, H.W.; Camara, C.; Nixdorf, S.L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012, 84, 53–61. [Google Scholar] [CrossRef]
- Herman, R.A.; Ayepa, E.; Fometu, S.S.; Shittu, S.; Davids, J.S.; Wang, J. Mulberry fruit post-harvest management: Techniques, composition and influence on quality traits-A review. Food Control 2022, 140, 109126. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Bae, S.H.; Suh, H.J. Antioxidant activities of five different mulberry cultivars in Korea. LWT-Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Kumar, A.; Palni, L.M.S. The effect of light source and gelling agent on micropropagation of Rosa damascena Mill. and Rhynchostylis retusa (L.) Bl. J. Pomol. Hortic. Sci. 2003, 78, 786–792. [Google Scholar]
- Lebude, A.V.; Goldfarb, B.; Blazich, F.A.; Wise, F.C.; Frampton, J. Mist, substrate water potential and cutting water potential influence rooting of stem cuttings of loblolly pine. Tree Physiol. 2004, 24, 823–831. [Google Scholar] [CrossRef]
- Devi, S.; Singh, S.; Kaur, R.P.; Shah, M.A.; Singh, R.K. Sprouting Behavior of Aeroponic Minitubers of Different Varieties. Potato J. 2019, 46, 67–72. [Google Scholar]
- Kuncoro CB, D.; Sutandi, T.; Adristi, C.; Kuan, Y.D. Aeroponics root chamber temperature conditioning design for smart mini-tuber potato seed cultivation. Sustainability 2021, 13, 5140. [Google Scholar] [CrossRef]
- Aluh Nikmatullah, A.N.; Agus Purbathin Hadi, A.P.H. Utilisation of Apical Stem Cutting for Fast Propagation of White Potato Seed Tubers. Akad. Sains Malays. Sci. J. 2021, 14, 75–86. [Google Scholar]
- Sharma, U.; Kataria, V.; Shekhawat, N.S. Aeroponics for adventitious rhizogenesis in evergreen haloxeric tree Tamarix aphylla (L.) Karst.: Influence of exogenous auxins and cutting type. Physiol. Mol. Biol. Plants 2018, 24, 167–174. [Google Scholar] [CrossRef]
- Mohamed, T.M.K.; Gao, J.; Tunio, M. Development and experiment of the intelligent control system for rhizosphere temperature of aeroponic lettuce via the Internet of Things. Int. J. Agric. Biol. Eng. 2022, 15, 225–233. [Google Scholar]
- Dabbour, M.; He, R.; Mintah, B.; Ma, H. Antioxidant activities of sunflower protein hdrolysates treated with dual-frequency ultrasonic: Optimization study. J. Food Process Eng. 2019, 42, e13084. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kaptso, G.K.; Kwaw, E.; Cheno, R.W.; Xiao, L.; Osae, R.; Wu, M.; Farooq, M. Process analysis of mulberry (Morus alba) leaf extract encapsulation: Effects of spray drying conditions on bioactive encapsulated powder quality. Food Bioprocess Technol. 2019, 12, 122–146. [Google Scholar] [CrossRef]
- Liao, J.; Luo, X.; Wang, P.; Zhou, Z.; O’Donnell, C.C.; Zang, Y.; Hewitt, A.J. Analysis of the influence of different parameters on droplet characteristics and droplet size classification categories for air induction nozzle. Agronomy 2020, 10, 256. [Google Scholar] [CrossRef]
- Giertych, M.J.; Suszka, J. Influence of cutting off distal ends of Quercus robur acorns on seedling growth and their infection by the fungus Erysiphe alphitoides in different light conditions. Dendrobiology 2010, 64, 73–77. [Google Scholar]
- Li, Y.; Ma, Y.; Xu, M.; Yaqoob, S.; Aregbe, A.Y.; Xiong, Y. Impact of fermentation through Wickerhamomyces anomalus and Saccharomyces cerevisiae on aroma and quality of mulberry wine. Int. J. Food Sci. Technol. 2024, 59, 971–984. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, J.; Tunio, M.H.; Wang, L. Study on the Identification of Mildew Disease of Cuttings at the Base of Mulberry Cuttings by Aeroponics Rapid Propagation Based on a BP Neural Network. Agronomy 2022, 13, 106. [Google Scholar] [CrossRef]


| Groups | Type | Condition |
|---|---|---|
| Humidity regulation experiment | ||
| H1 | 90% ≤ Humidity ≤ 100% | |
| H2 | Aeroponic rapid cultivation | 95% ≤ Humidity ≤ 100% |
| H3 | Spray for 2 min every 30 min | |
| HCK | Soil cultivation | Regular watering |
| Temperature regulation experiment | ||
| T1 | Aeroponic rapid cultivation | Spray for 2 min every 30 min, 25 °C < Temperature < 27 °C |
| T2 | Spray for 2 min every 30 min, There is no temperature Regulation, Room temperature | |
| TCK | Soil cultivation | Regular watering, Room temperature |
| Temperature and humidity regulation experiment | ||
| TH | Aeroponic rapid cultivation | 90% ≤ Humidity ≤ 100% 25 °C < Temperature < 27 °C |
| THCK | Spray for 2 min every 30 min, There is no temperature Regulation, Room temperature | |
| Groups | The Rooting Rate/% | The Callus Rate/% | The Average Root Number | The Average Root Length/mm | The Mortality Rate/% | The Fresh Weight/g | The Dry Weight/g |
|---|---|---|---|---|---|---|---|
| Humidity regulation experiment | |||||||
| H1 | 54 ± 2 a | 77 ± 3 a | 5.27 ± 0.08 b | 72.10 ± 0.34 b | 40 ± 1 b | 0.722 ± 0.007 b | 0.062 ± 0.002 b |
| H2 | 52 ± 2 b | 71 ± 2 b | 4.35 ± 0.05 c | 67.30 ± 0.41 c | 34 ± 2 c | 0.613 ± 0.008 c | 0.053 ± 0.001 c |
| H3 | 51 ± 2 c | 70 ± 0.01 c | 5.87 ± 0.05 a | 75.70 ± 0.35 a | 43 ± 2 a | 0.772 ± 0.011 a | 0.064 ± 0.001 a |
| Temperature regulation experiment | |||||||
| T1 | 43.8 ± 2.3 a | 64.2 ± 1.9 a | 3.183 ± 0.054 a | 18.690 ± 0.265 a | 44.6 ± 2.6 a | 0.473 ± 0.005 a | 0.033 ± 0.001 a |
| T2 | 31.7 ± 2.5 b | 45.4 ± 2.6 b | 3.107 ± 0.068 b | 17.983 ± 0.620 b | 58.3 ± 2.5 b | 0.463 ± 0.006 b | 0.032 ± 0.001 b |
| Temperature and humidity regulation experiment | |||||||
| TH | 48.8 ± 0.023 a | 68.8 ± 2.3 a | 3.767 ± 0.049 a | 34.877 ± 0.214 a | 34.2 ± 1.9 a | 0.473 ± 0.005 a | 0.033 ± 0.003 a |
| THCK | 34.6 ± 0.026 b | 43.3 ± 2.5 b | 2.810 ± 0.074 b | 30.167 ± 0.047 b | 53.3 ± 2.5 b | 0.37 ± 0.009 b | 0.024 ± 0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, P.; Wang, L.; Qureshi, W.A.; Gao, J. Influence of Rhizosphere Temperature and Humidity Regulation on Rooting, Mortality, and Transplant Survival of Aeroponically Rapid Growth Mulberry Cutting. Agronomy 2025, 15, 583. https://doi.org/10.3390/agronomy15030583
Shen P, Wang L, Qureshi WA, Gao J. Influence of Rhizosphere Temperature and Humidity Regulation on Rooting, Mortality, and Transplant Survival of Aeroponically Rapid Growth Mulberry Cutting. Agronomy. 2025; 15(3):583. https://doi.org/10.3390/agronomy15030583
Chicago/Turabian StyleShen, Pengfei, Liang Wang, Waqar Ahmed Qureshi, and Jianmin Gao. 2025. "Influence of Rhizosphere Temperature and Humidity Regulation on Rooting, Mortality, and Transplant Survival of Aeroponically Rapid Growth Mulberry Cutting" Agronomy 15, no. 3: 583. https://doi.org/10.3390/agronomy15030583
APA StyleShen, P., Wang, L., Qureshi, W. A., & Gao, J. (2025). Influence of Rhizosphere Temperature and Humidity Regulation on Rooting, Mortality, and Transplant Survival of Aeroponically Rapid Growth Mulberry Cutting. Agronomy, 15(3), 583. https://doi.org/10.3390/agronomy15030583







