Breeding Black Soybeans for High Yield and First Pod Height Is a Promising Approach to Improving Thai Commercial Soybean Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
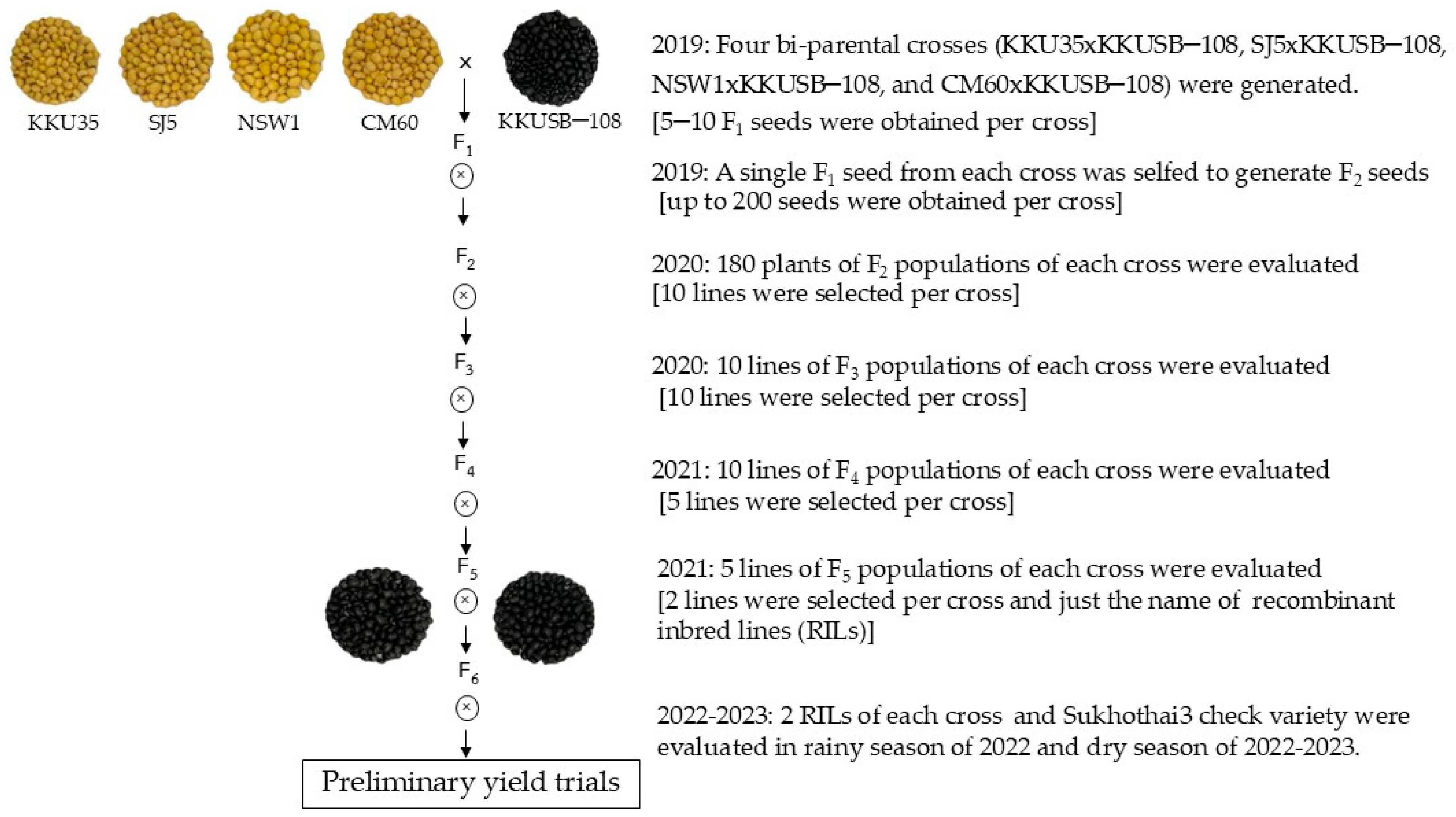
2.2. Population Development
2.3. An F2 to F5 Experimental Block and Fields
2.4. Preliminary Yield Trials
2.5. Data Collection
2.6. Data Analysis
3. Results
3.1. Population Development
3.2. Combined ANOVA
3.3. Growth Data on Black Soybean Genotypes
3.4. Yield, Yield Components, and Protein Content of Black Soybean Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.Y.; Van, K.; Kang, Y.J.; Kim, K.H.; Lee, S.H. Tracing soybean domestication history: From nucleotide to genome. Breed. Sci. 2012, 61, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops that feed the World 2. Soybean—Worldwide production, use, and constraints caused by pathogens and pests. Food Sec. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Hao, X.; Ji, X.; Zhu, Y.; Chen, X.; Yao, Y. A systematic review of black soybean (Glycine max (L.) Merr.): Nutritional composition, bioactive compounds, health benefits, and processing to application. Food Front. 2024, 5, 1188–1211. [Google Scholar] [CrossRef]
- Voora, V.; Bermudez, S.; Le, H.; Larrea, C.; Luna, E. Soybean prices and sustainability. In Global Market Report; IISD: Winnipeg, MB, Canada, 2024; pp. 1–38. Available online: https://www.iisd.org/system/files/2024-02/2024-global-market-report-soybean.pdf (accessed on 30 December 2020).
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Rana, S.; Dasila, K.; Agnihotri, V.; Pandey, A.; Pande, V. Comparative nutritional and antimicrobial analysis of Himalayan black and yellow soybean and their okara. J. Sci. Food Agric. 2022, 102, 5358–5367. [Google Scholar] [CrossRef]
- Takahashi, R.; Ohmori, R.; Kiyose, C.; Momiyama, Y.; Ohsuzu, F.; Kondo, K. Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J. Agric. Food Chem. 2005, 53, 4578–4582. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S. Therapeutic effects of polyphenols in fermented soybean and black soybean products. J. Funct. Foods 2021, 81, 104467. [Google Scholar] [CrossRef]
- Nirmal, N.; Khanashyam, A.; Mundanat, A.; Shah, K.; Babu, K.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of fruit waste for bioactive compounds and their applications in the food industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Choung, M.G.; Baek, I.Y.; Kang, S.T.; Han, W.Y.; Shin, D.C.; Moon, H.P.; Kang, K.W. Isolation and determination of anthocyanins in seed coats of black soybean (Glycin max (L.) Merr.). J. Agric. Food Chem. 2001, 49, 5848–5851. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, N.S.; Shin, S.O.; Shin, S.H.; Lim, S.G.; Suh, D.Y.; Baek, I.Y.; Park, K.Y.; Ha, Y.J. Characterization of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009, 112, 226–231. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.S.; Yoo, H.; Choung, M.G.; Sung, M.K. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 8427–8433. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 61, 923–933. [Google Scholar] [CrossRef]
- Bhartiya, A.; Aditya, J.P.; Pal, R.S.; Chandra, N.; Kant, L.; Pattanayak, A. Bhat (black soybean): A traditional legume with high nutritional and nutraceutical properties from NW Himalayan region of India. Indian J. Tradit. Knowl. 2020, 19, 307–319. [Google Scholar]
- Liao, H.F.; Chen, Y.J.; Yang, Y.C. A novel polysaccharide of black soybean promotes myelopoiesis and reconstitutes bone marrow after 5-flurouracil and irradiation-induced myelosuppression. Life Sci. 2005, 77, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Ro, H.M.; Kim, S.L.; Kim, H.S.; Chung, I.M. Analysis of isoflavone, phenolic, soyasapogenol, and tocopherol compounds in soybean [Glycine max (L.) Merrill] germplasms of different seed weights and origins. J. Agric. Food Chem. 2012, 60, 6045–6055. [Google Scholar] [CrossRef]
- Krisnawati, A.; Gatut, W.A.S.; Adie, M.M. Screening of elite black soybean lines for resistance to rust disease, Phakopsora pachyrhizi. Biodiversitas 2016, 17, 134–139. [Google Scholar] [CrossRef]
- Barrett, J.R. The Science of Soy: What Do We Really Know? Environ. Health Perspect. 2006, 114, 352–358. [Google Scholar] [CrossRef]
- Pranto, T.H.; Noman, A.A.; Mahmud, A.; Haque, A.B. Blockchain and smart contract for IoT enabled smart agriculture. PeerJ Comput. Sci. 2021, 7, e407. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Koh, K.; Youn, J.E.; Kim, H.S. Identification of anthocyanins in black soybean (Glycine max (L.) Merr) varieties. J. Food Sci. Technol. 2014, 51, 377–381. [Google Scholar] [CrossRef]
- Sohn, D.W.; Bae, W.J.; Kim, H.S.; Kim, S.W.; Kim, S.W. The anti-inflammatory and antifibrosis effects of anthocyanin extracted from black soybean on a Peyronie disease rat model. Urology 2014, 84, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Choi, T.H.; Kim, S.; Kim, S.-H.; Chang, H.W.; Choe, M.; Kwon, S.Y.; Hur, J.A.; Shin, S.C.; Chung, J.I.; et al. Anthocyanins from black soybean seed coat enhance wound healing. Ann. Plast. Surg. 2013, 71, 415–420. [Google Scholar] [CrossRef]
- Sritongtae, C.; Monkham, T.; Sanitchon, J.; Lodthong, S.; Srisawangwong, S.; Chankaew, S. Identification of Superior Soybean Cultivars through the Indication of Specific Adaptabilities within Duo-Environments for Year-Round Soybean Production in Northeast Thailand. Agronomy 2021, 11, 585. [Google Scholar] [CrossRef]
- Elsaied, G.H.; Elfatih, A.; Arif, E.M. Studying a new combine threshing rotor design. Aust. J. Basic Appl. Sci. 2009, 3, 4085–4093. [Google Scholar]
- Lee, K.J.; Kim, J.B.; Choi, H.I.; Ha, B.K.; Kang, S.Y.; Kim, D.S. Selection of soybean mutant lines with altered seed coat colour and their antioxidant activity. Plant Breed. 2015, 134, 573–579. [Google Scholar] [CrossRef]
- Yang, K.; Jeong, N.; Moon, J.K.; Lee, Y.H.; Lee, S.H.; Kim, H.M.; Hwang, C.H.; Back, K.; Palmer, R.G.; Jeong, S.C. Genetic analysis of genes controlling natural variation of seed coat and flower colors in soybean. J. Hered. 2010, 101, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.G.; Pfeiffer, T.W.; Buss, G.R.; Kilen, T.C. Qualitative Genetics Soybeans: Improvement, Production, and Uses, 3rd ed.; ASA, CSSA, and SSSA: Madison, WI, USA, 2004; pp. 137–214. [Google Scholar]
- Ly, S.; Park, B.E.; Shim, S.I.; Kim, M.C.; Moon, J.Y.; Chung, J.I. Breeding a black soybean line with green cotyledon free from lectin, KTI, P34, lipoxygenase, and stachyose. Euphytica 2024, 220, 131. [Google Scholar] [CrossRef]
- Cober, E.R.; Voldeng, H.D. Developing high-protein, high-yield soybean populations and lines. Crop Sci. 2000, 40, 39–42. [Google Scholar] [CrossRef]
- Diers, B.W.; Specht, J.; Rainey, K.M.; Cregan, P.; Song, Q.; Ramasubramanian, V.; Graef, G.; Nelson, R.; Schapaugh, W.; Wang, D.; et al. Genetic architecture of soybean yield and agronomic traits. G3 Genes Genom. Genet. 2018, 8, 3367–3375. [Google Scholar] [CrossRef]
- Martin, R.; Wilcox, J. Heritability of lowest pod height in soybeans. Crop Sci. 1973, 13, 201–203. [Google Scholar] [CrossRef]
- Kato, S.; Sayama, T.; Ishimoto, M.; Yumoto, S.; Kikuchi, A.; Nishio, T. The effect of stem growth habit on single seed weight and seed uniformity in soybean (Glycine max (L.) Merrill). Breed. Sci. 2018, 68, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.; Qin, H.; Li, Y.; Qi, H.; Li, C.; Wang, N.; Li, R.; Zhao, Y.; Huang, S.; et al. Identification of Major QTLs Associated With First Pod Height and Candidate Gene Mining in Soybean. Front. Plant Sci. 2018, 19, 1280. [Google Scholar] [CrossRef]
- Ramteke, R.; Singh, D.; Murlidharan, P. Selecting soybean (Glycine max) genotypes for insertion height of the lowest pod, the useful trait for combine harvester. Ind. J. Agric. Sci. 2012, 82, 511–515. [Google Scholar] [CrossRef]
- Kang, B.-K.; Kim, H.-T.; Choi, M.-S.; Koo, S.-C.; Seo, J.-H.; Kim, H.-S.; Shin, S.-O.; Yun, H.-T.; Oh, I.-S.; Kulkarni, K.P.; et al. Genetic and Environmental Variation of First Pod Height in Soybean [Glycine max (L.) Merr.]. Plant Breed. Biotechnol. 2017, 5, 36–44. [Google Scholar] [CrossRef]
- Costa, M.M.; Di Mauro, A.O.; Unêda-Trevisoli, S.H.; Arriel, N.H.C.; Bárbaro, I.M.; Silveira, G.D.; Muniz, F.R.S. Heritability estimation in early generations of two-way crosses in soybean. Bragantia 2008, 67, 101–108. [Google Scholar] [CrossRef]
- Orf, J.H. Breeding, genetics, and production of soybeans. In Soybeans: Chemistry, Production, Processing, and Utilization; Johnson, L.A., White, P.J., Galloway, R., Eds.; AOCS Press: Urbana, IL, USA, 2008; pp. 33–65. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, V. Soybean Breeding. In Fundamentals of Field Crop Breeding; Yadava, D.K., Dikshit, H.K., Mishra, G.P., Tripathi, S., Eds.; Springer: Singapore, 2022; pp. 907–944. [Google Scholar] [CrossRef]
- Thiex, N.J.; Anderson, S.; Gildemeister, B.; Adcock, W.; Boedigheimer , J.; Bogren, E.; Coffin, R.; Conway, K.; DeBaker, A.; Frankenius, E.; et al. Crude Fat, Diethyl Ether Extraction, in Feed, Cereal Grain, and Forage (Randall/Soxtec/Submersion Method): Collaborative Study. J. AOAC Int. 2003, 86, 888–898. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 30 December 2020).
- Song, J.; Liu, Z.; Hong, H.; Ma, Y.; Tian, L.; Li, X.; Li, Y.-H.; Guan, R.; Guo, Y.; Qiu, L.-J. Identification and Validation of Loci Governing Seed Coat Color by Combining Association Mapping and Bulk Segregation Analysis in Soybean. PLoS ONE 2016, 11, e0159064. [Google Scholar] [CrossRef]
- FAOSTAT. Database. 2020. Available online: http://www.fao.org/faostat/en/#data/SC (accessed on 2 January 2021).
- Rincker, K.; Nelson, R.; Specht, J.; Sleper, D.; Cary, T.; Cianzio, S.R.; Casteel, S.; Conley, S.; Chen, P.; Davis, V.; et al. Genetic Improvement of U.S. Soybean in Maturity Groups II, III, and IV. Crop Sci. 2014, 5, 1419–1432. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, X.; Huang, W.; Yang, J.; Zheng, Y.; Qiu, L. Stability analysis of seven agronomic traits for soybean (Glycine max (L.) Merr.) Tokachi nagaha and its derived cultivars using the AMMI model. Plant Prod Sci. 2017, 20, 499–506. [Google Scholar] [CrossRef]
- Tekrony, D.M.; Egli, D.B.; Phillips, A.D. Effect of field weathering on the viability and vigor of soybean seed. Agron. J. 1980, 71, 742–753. [Google Scholar] [CrossRef]
- Maranna, S.; Nataraj, V.; Kumawat, G.; Chandra, S.; Rajesh, V.; Ramteke, R.; Patel, R.M.; Ratnaparkhe, M.B.; Husain, S.M.; Gupta, S.; et al. Breeding for higher yield, early maturity, wider adaptability and waterlogging tolerance in soybean (Glycine max L.): A case study. Sci. Rep. 2021, 11, 22853. [Google Scholar] [CrossRef] [PubMed]
| No. | Genotypes | Type of Varieties | Maturity Type | Sources | Grain Color |
|---|---|---|---|---|---|
| 1 | KKU35 | Check variety | Late | Khon Kaen University | Yellow |
| 2 | SJ5 | Check variety | Intermediate | Department of Agriculture, Thailand | Yellow |
| 3 | NSW1 | Check variety | Early | Department of Agriculture, Thailand | Yellow |
| 4 | CM60 | Check variety | Intermediate | Department of Agriculture, Thailand | Yellow |
| 5 | Sukhothai 3 | Check variety | Intermediate | Department of Agriculture, Thailand | Black |
| 6 | KKUSB–108 | Check variety | Intermediate | Khon Kaen University | Black |
| 7 | KKU35xKKUSB–108–12–4–3 | Breeding line | Late | Khon Kaen University | Black |
| 8 | KKU35xKKUSB–108–24–5–7 | Breeding line | Late | Khon Kaen University | Black |
| 9 | SJ5xKKUSB–108–25–2–1 | Breeding line | Intermediate | Khon Kaen University | Black |
| 10 | SJ5xKKUSB–108–30–3–7 | Breeding line | Intermediate | Khon Kaen University | Black |
| 11 | NSW1xKKUSB–108–49–3–3 | Breeding line | Early | Khon Kaen University | Black |
| 12 | NSW1xKKUSB–108–49–3–6 | Breeding line | Early | Khon Kaen University | Black |
| 13 | CM60xKKUSB–108–41–1–7 | Breeding line | Intermediate | Khon Kaen University | Black |
| 14 | CM60xKKUSB–108–64–4–8 | Breeding line | Intermediate | Khon Kaen University | Black |
| Source of Variance | df | Growth Data onSoybean Genotypes | ||||
| Plant Height at 50% Flowering | Days to 50% Flowering | Plant Height at Full Maturity | Days to Full Maturity | First Pod Height | ||
| Season (S) | 1 | 158.03 ns | 824.46 ** | 91.68 ns | 12.51 * | 0.36 ns |
| Error S × rep (R) | 4 | 17.56 | 5.90 | 68.96 | 0.64 | 2.31 |
| Genotype (G) | 8 | 429.80 ** | 31.71 * | 1010.95 ** | 170.12 ** | 37.80 ** |
| S × G | 8 | 181.72 ** | 15.38 ns | 79.15 ns | 36.14 ** | 2.39 ns |
| Error S × R × G | 32 | 30.42 | 10.61 | 74.54 | 1.75 | 1.45 |
| Total | 53 | |||||
| Source of Variance | df | Yield and Yield Componentsof Soybean Genotypes | ||||
| Node/Plant | Seed/Plant | Weight of 100Grains | Yield | Protein Content | ||
| Season (S) | 1 | 72.98 ** | 1157.87 * | 14.36 * | 0.6556 ** | 362.58 ** |
| Error S × rep (R) | 4 | 2.27 | 112.82 | 1.71 | 0.0057 | 0.075 |
| Genotype (G) | 8 | 86.79 ** | 726.41 ** | 14.06 ** | 0.3856 ** | 24.24 ** |
| S × G | 8 | 64.49 ** | 999.40 ** | 2.70 ns | 0.0463 ** | 5.56 ** |
| Error S × R × G | 32 | 6.07 | 74.49 | 1.73 | 0.0054 | 0.46 |
| Total | 53 | |||||
| Varieties/Lines | Growth Data on Soybean Genotypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plant Height at 50% Flowering (cm) | Days to 50% Flowering (Day) | Plant Height at Full Maturity (cm) | Days to Full Maturity (Day) | First Pod Height (cm) | ||||||
| Rainy | Dry | Rainy | Dry | Rainy | Dry | Rainy | Dry | Rainy | Dry | |
| KKU35xKKUSB–108–12–4–3 | 63.97 b | 68.54 ab | 45.00 a | 49.00 | 81.54 ab | 81.21 a | 98.67 ab | 107.00 a | 10.96 bc | 12.11 a |
| KKU35xKKUSB–108–24–5–7 | 80.50 a | 66.39 ab | 41.00 abc | 48.66 | 88.47 a | 88.52 a | 100.00 a | 104.33 a | 15.02 a | 12.81 a |
| SJ5xKKUSB–108–25–2–1 | 85.89 a | 72.11 a | 41.00 abc | 47.66 | 94.18 a | 84.73 a | 99.00 ab | 91.67 bc | 11.65 b | 12.38 a |
| SJ5xKKUSB–108–30–3–7 | 60.68 b | 69.29 ab | 43.00 ab | 48.66 | 73.48 ab | 67.27 abc | 98.67 ab | 91.00 bc | 10.59 bc | 9.67 a |
| NSW1xKKUSB–108–49–3–3 | 53.53 b | 66.42 ab | 37.00 c | 45.33 | 60.96 b | 74.23 ab | 89.00 e | 87.00 c | 10.55 bc | 12.74 a |
| NSW1xKKUSB–108–49–3–6 | 54.53 b | 55.34 ab | 37.00 c | 44.33 | 54.89 b | 56.54 bc | 87.33 e | 86.67 c | 10.85 bc | 10.43 a |
| CM60xKKUSB–108–41–1–7 | 54.78 b | 67.95 ab | 39.00 abc | 51.00 | 81.96 ab | 75.36 ab | 94.33 d | 94.33 b | 9.31 c | 9.90 a |
| CM60xKKUSB–108–64–4–8 | 54.49 b | 69.99 ab | 39.00 bc | 53.00 | 79.88 ab | 73.94 ab | 97.00 bc | 95.67 b | 9.27 c | 9.59 a |
| Sukhothai 3 | 50.41 b | 53.53 b | 39.00 abc | 44.33 | 58.27 b | 48.37 c | 95.00 cd | 93.00 b | 4.82 d | 4.87 b |
| Mean | 62.09 | 65.51 | 40.18 | 48.00 | 74.85 | 72.24 | 95.44 | 94.51 | 10.33 | 10.50 |
| F-test | * | ** | * | ns | * | * | * | * | * | * |
| CV% | 7.84 | 9.30 | 5.08 | 8.61 | 12.62 | 10.70 | 0.93 | 1.96 | 5.90 | 15.18 |
| Varieties/Lines | Yield and Yield Components of Soybean Genotypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Node/Plant | Grain/Plant | Weight of 100 Grains (g) | Yield (T/ha) | Protein Content (%) | ||||||
| Rainy | Dry | Rainy | Dry | Rainy | Dry | Rainy | Dry | Rainy | Dry | |
| KKU35xKKUSB–108–12–4–3 | 18.76 b | 13.71 f | 123.44 b | 106.43 de | 11.28 ab | 11.86 ab | 1.40 de | 1.68 ab | 38.71 a | 42.30 abc |
| KKU35xKKUSB–108–24–5–7 | 20.94 b | 22.63 cd | 120.72 b | 131.6 bc | 10.12 b | 9.86 b | 1.46 cd | 1.77 ab | 34.245 b | 40.31 bcd |
| SJ5xKKUSB–108–25–2–1 | 31.39 a | 22.89 c | 156.11 a | 116.9 cde | 9.85 b | 9.83 b | 1.85 a | 1.86 a | 30.16 c | 41.05 abcd |
| SJ5xKKUSB–108–30–3–7 | 17.26 b | 25.00 b | 105.03 b | 118.9 cd | 11.85 ab | 14.84 a | 1.65 b | 1.72 ab | 37.525 a | 43.75 a |
| NSW1xKKUSB–108–49–3–3 | 16.08 b | 28.23 a | 101.04 b | 154.33 a | 11.87 ab | 13.93 ab | 1.30 e | 1.72 ab | 35.21 b | 42.75 ab |
| NSW1xKKUSB–108–49–3–6 | 13.91 b | 21.51 d | 105.35 b | 127.9 bc | 13.18 a | 14.33 ab | 1.52 bcd | 1.83 ab | 38.64 a | 44.25 a |
| CM60xKKUSB–108–41–1–7 | 23.30 ab | 25.49 b | 115.77 b | 136.53 b | 12.54 a | 15.23 a | 1.46 cd | 1.57 b | 35.065 b | 38.70 d |
| CM60xKKUSB–108–64–4–8 | 20.98 b | 25.45 b | 122.35 b | 134.57 b | 12.66 a | 13.68 ab | 1.60 bc | 1.61 ab | 34.355 b | 39.35 cd |
| Sukhothai 3 | 16.58 b | 15.20 e | 97.10 b | 103.09 e | 12.66 a | 11.74 ab | 1.44 f | 1.66 c | 30.015 c | 38.60 d |
| Mean | 19.91 | 22.23 | 116.32 | 125.58 | 11.78 | 12.81 | 1.44 | 1.66 | 34.88 | 41.22 |
| F-test | * | * | * | * | * | * | * | * | * | * |
| CV% | 17.37 | 1.92 | 9.55 | 4.04 | 6.34 | 13.32 | 3.6 | 5.44 | 1.53 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnarat, J.; Monkham, T.; Sanitchon, J.; Chankaew, S. Breeding Black Soybeans for High Yield and First Pod Height Is a Promising Approach to Improving Thai Commercial Soybean Varieties. Agronomy 2025, 15, 600. https://doi.org/10.3390/agronomy15030600
Chinnarat J, Monkham T, Sanitchon J, Chankaew S. Breeding Black Soybeans for High Yield and First Pod Height Is a Promising Approach to Improving Thai Commercial Soybean Varieties. Agronomy. 2025; 15(3):600. https://doi.org/10.3390/agronomy15030600
Chicago/Turabian StyleChinnarat, Jariya, Tidarat Monkham, Jirawat Sanitchon, and Sompong Chankaew. 2025. "Breeding Black Soybeans for High Yield and First Pod Height Is a Promising Approach to Improving Thai Commercial Soybean Varieties" Agronomy 15, no. 3: 600. https://doi.org/10.3390/agronomy15030600
APA StyleChinnarat, J., Monkham, T., Sanitchon, J., & Chankaew, S. (2025). Breeding Black Soybeans for High Yield and First Pod Height Is a Promising Approach to Improving Thai Commercial Soybean Varieties. Agronomy, 15(3), 600. https://doi.org/10.3390/agronomy15030600






