Abstract
This study presents the optimal light spectrum and medium composition for the in vitro induction of adventitious bulbs in T. tarda Stapf. Bulb scales, used as explants, were cultivated on 100% MS solid media with 3% or 6% sucrose and 0 or 0.5 µM BAP (6-benzyl-aminopurine) under a 16 h photoperiod and different fluorescent light treatments (white, red, blue). Darkness was used as a control. The cultures were maintained at 20 ± 2 °C for 12 weeks. The obtained results revealed that white light combined with 6% sucrose yielded the highest adventitious bulb formation, with an average of 12.1 ± 1.3 bulbs per explant. The bulbs formed directly on the surface of the explants. Red light combined with 3% sucrose and 0.5 µM BAP completely inhibited bulb formation, while darkness promoted leaf development, with a maximum of 1.3 ± 0.1 leaves per bulb, under darkness on medium with 6% sucrose. The addition of BAP, in most cases, was essential for root formation, with a maximum of 2.9 ± 0.6 roots per bulb under the influence of white light. This study demonstrates that it is possible to obtain an effective and efficient method for T. tarda propagation from bulb scales treated with 6% sucrose under white light condition. This method offers the potential for the commercial cultivation of this ornamental species.
1. Introduction
Tulipa tarda Stapf. is a species of botanical tulip native to Central Asia [1]. The species is becoming increasingly popular on the bulb plant market, mainly due to its ability to remain and bloom in one place, in the ground, for many years. The growing interest is also due to its low, multi-flowered stems and beautiful, star-shaped, yellow flowers, which appear in early spring (mid-April) [2]. Thanks to these features, the Tarda tulip is widely used in both green areas and home gardens as a bedding and potted plant.
Tulips are among the economically important genera that account for most of the world’s bulb production [3]. They are commercially propagated through asexual reproduction by using bulbs [4,5]. Bulbs undergo several stages of development, namely, juvenile vegetative, adult vegetative, and reproductive. Flower initiation happens once the bulb enters the adult vegetative phase, which occurs after spending a few years [4,5] in the juvenile phase. The transition to the reproductive phase is triggered by high temperatures, prompting the formation of flower buds. Afterward, dormancy sets in, requiring an extended period of cold to break the dormancy and ready the bulb for spring growth [5,6]. The average rate of tulip propagation amounts to two to three [4]. Due to the low efficiency of traditional tulip reproduction [7,8,9], more effective methods based on in vitro techniques are being sought. One of the most popular techniques is organogenesis [10], which enables the large-scale reproduction of plants (including ornamental geophytes) [3,11,12]. It has already been used in the micropropagation of various tulips, resulting in promising reproductive efficiency, during which the time needed to cultivate tulips was shortened and the large number of obtained plants were of high quality and free of pathogens [13,14,15,16].
The selection of an appropriate explant plays a key role in successful in vitro culture [3,17,18]. In the case of tulip organogenesis, the explant may come from various parts of the plant, including bulb scales [15,19,20,21,22].
Tulip organogenesis is also strictly dependent on the composition of the medium. The concentration and type of exogenous plant growth regulators (PGRs) play a special role in bulb induction [23]. The main PGR is cytokinin BAP, which is often applied in various experiments with geophytes [23,24,25,26,27,28]. BAP also promoted bulb formation in T. tarda cultures [15]. An equally important component of the medium is sucrose and its appropriate concentration, which also determine the formation of tulip bulbs [3,22,24,29,30]. Usually, tuberization is promoted by increasing the sucrose level to 6% [23,28,31].
In vitro bulb regeneration is also light-dependent [22,32,33,34]. Despite LED lights being widely used today, most experiments with adventitious organogenesis have used monochromatic fluorescent lamps [3]. According to Lian et al. [35] and Prokopiuk et al. [27], the formation of Lilium storage organs depends on blue light combined with red. Red light itself enhances the formation of adventitious bulbs in Hyacinthus sp. cultures [36,37]. Bach and Pawłowska [38] claimed that the quality of light and spectral sequence may influence the morphogenesis of ornamental geophytes, while Jasenovska et al. [39] confirmed that various species respond differently to different types of light.
Despite the development of many protocols for the effective micropropagation of tulips, they are still not propagated in vitro on a commercial scale [3].
This research focused on determining the best light conditions, depending on the applied sucrose concentration and the presence of BAP, for the formation of T. tarda adventitious bulbs in vitro.
2. Materials and Methods
Tulipa tarda Stapf. used in the experiment was obtained from the University of Agriculture’s in vitro collection, in Kraków. In vitro cultures were initiated in October 2019 and mass-propagated from seeds according to the protocol of Maślanka and Bach [15]. Single adventitious bulb scales (approximately 9 mm length, 4 mm width), cut lengthwise (V-shaped), were used as explants (Figure 1A). The explants were plated in Petri dishes (90 × 25 mm, without ventilation system), on four 25 mL MS [40] solid media (0.5% Lab-agar, Biocorp, Warszawa, Poland) containing either 0 or 0.5 μM BAP (Sigma-Aldrich, Poznań, Poland), and 3% or 6% sucrose (Avantor Performance Materials, Gliwice, Poland). The media were adjusted to a pH of 5.8. The MS basal medium included vitamins. No inositol or antioxidants were used. The cultures were maintained under a 16 h photoperiod under various light spectra (fluorescent lamps, PPFD 30 μmol·m−2·s−1): white (390–760 nm, Tungsram lamps 40 W F33, Lumiqon, Warszawa, Poland), red (647–770 nm, Philips TLD 36 W, Signify, Warszawa, Poland), or blue (400–492 nm, Philips TLD 36 W, Signify, Warszawa, Poland), or in darkness (control), at 20 ± 2 °C for 12 weeks, to induce adventitious bulb formation. Relative humidity (RH) during cultivation amounted to approximately 95%. The cultures were passaged onto fresh medium every four weeks.
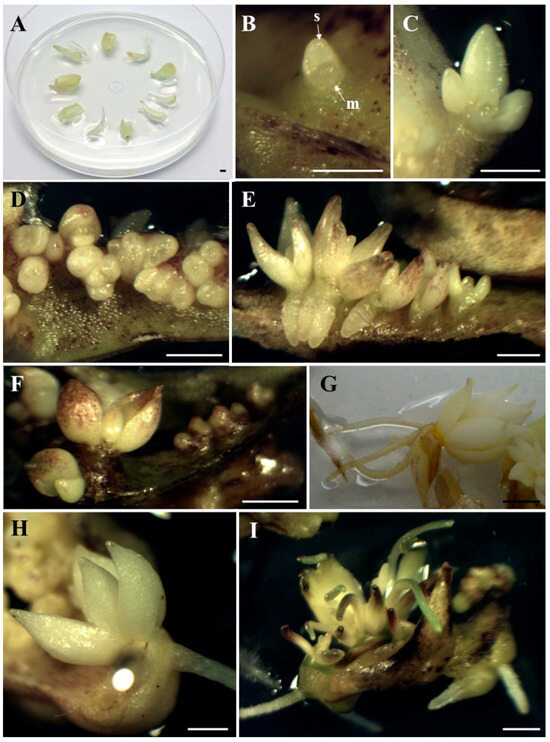
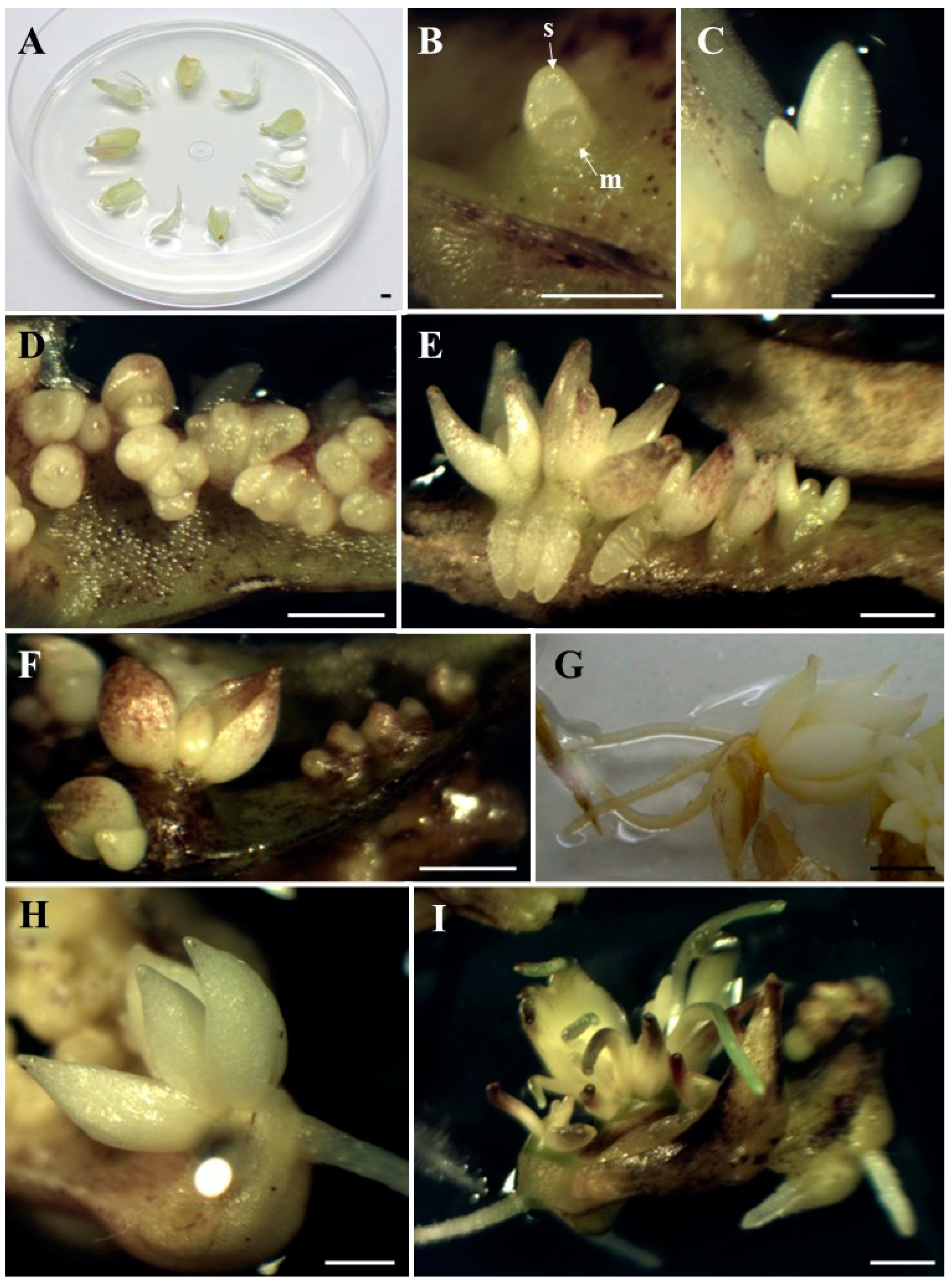
Figure 1.
Bulb scale explants (A). Direct formation of the adventitious bulb with visible apical meristem (m) and first bulb scale (s) under the influence of blue light × 3% sucrose × 0.5 µM BAP, after 4 weeks of cultivation (B); an adventitious bulb forming under the influence of red light × 3% sucrose, after 6 weeks of cultivation (C); group of adventitious bulbs, under the influence of white light × 6% sucrose after 5 weeks of cultivation (D) and 9 weeks of cultivation (E); bulbs formed under influence of blue light × 6% sucrose, after 6 weeks of cultivation (F); well-developed rooted bulb, formed after 12 weeks of cultivation: in darkness × 3% sucrose × 0.5 µM BAP (G) and under the influence of red light × 6% sucrose × 0.5 µM BAP (H); adventitious bulbs with developing roots and leaves, under the influence of white light × 6% sucrose × 0.5 µM BAP, after 12 weeks of cultivation (I). Bar: 2 mm.
The induction of adventitious bulbs (percentage of explants forming bulbs and the number of bulbs per explant) was recorded at four-week intervals. After 12 weeks of cultivation, the percentage of bulbs forming leaves and roots and the number of leaves and roots per bulb were also assessed.
Ten explants were used in each replicate (Petri dish). Ten replicates were exposed to each of the four light and four media combinations. One hundred explants were used for each treatment. All data (previously checked for normality by the Shapiro–Wilk test) were analyzed using Statistica 14 software (StatSoft, Kraków, Poland). The data were arcsine transformed, before subjection to analysis of variance. A two-way ANOVA was conducted, followed by Tukey’s multiple range test at a significance level of p ≤ 0.05.
3. Results and Discussion
In this study, the formation of adventitious bulbs via direct organogenesis of Tulipa tarda under different light and medium treatments was observed. The adventitious bulbs formed directly on the surface of the explants and consisted of well-developed scales (Figure 1B,C). Bulb regeneration is more desirable than shoot regeneration because the bulbs have a more favorable surface-to-volume ratio and are covered with an outer layer that protects them from desiccation [23]. Direct adventitious bulb induction was also observed in a previous experiment with T. tarda [15] and in other ornamental geophytes such as Allium aflatunense, Fritillaria imperialis, Lilium ledebourii, Lilium candidum, or Lachenalia viridiflora [8,41,42,43,44,45]. Thanks to the direct formation of adventitious bulbs, omitting the shoot formation phase, the time of bulb formation is saved. According to Yasemin and Beruto [3], direct bulb induction not only shortens the production period, but also prevents hyperhydricity, eliminates the need for hardening, and increases their survival rate. As stated by Kritskaya et al. [46], direct organogenesis is used as one of the techniques for the in vitro reproduction of tulips. In vitro reproduction of other tulips, which involved indirect regeneration of adventitious bulbs from initial explants, was characterized by low efficiency [29]. Moreover, in indirect organogenesis, the probability of somaclonal variability is higher [12]. At the beginning of the present experiment, higher bulb formation frequencies (40–56%), regardless of the light spectrum, were observed under the influence of 6% sucrose, although red light significantly decreased the percentage of explants forming bulbs to 16%. However, from the 8th week of the experiment, it was darkness that had a positive effect on the percentage of explants that formed bulbs, which ultimately amounted to 70–80%, regardless of the medium composition. Red light combined with 3% sucrose and 0.5 µM BAP completely inhibited adventitious bulb formation (Table 1). Taeb and Alderson [29], Famelaer et al. [30], and Podwyszyńska [23] also used 6% sucrose for the development of Tulipa bulbs in their experiments. However, in previous studies with T. tarda [15,16], 6% sucrose resulted in a smaller number of bulbs, although this also depended on the light and BAP. As for darkness, in some cases, it can enhance growth and morphogenesis [47]. It was in the dark that Lachenalia ‘Ronina’ formed the most adventitious bulbs compared to different light spectra [48]. Despite the above results, taking into account the number of adventitious bulbs formed, it was observed that the best treatment was white light combined with 6% sucrose (Figure 1D,E). Under these conditions, the largest number of adventitious bulbs was formed throughout the entire experimental period, and after 12 weeks of culture, there were 12 adventitious bulbs per explant. The addition of BAP to the medium resulted in the second highest number with an average of 7.6 bulbs per explant (Table 2). So far, in experiments with T. tarda, a maximum of 3.8 [15] or 7.3 [16] bulbs per explant were obtained, in both cases on media with 3% sucrose and 0.5 µM BAP in darkness. However, the highest bulblet formation efficiency in Lilium candidum was also obtained under white light conditions [44,49]. Meanwhile, Lachenalia ‘Rupert’ produced bulbs in all lighting environments (white, red, blue light, darkness) [48]. Regarding this experiment, the results are different when white light interacted with 3% sucrose; then, the addition of BAP significantly increased the number of adventitious bulbs (from 1.1 to 5.6 bulb/explant). BAP, together with 3% sucrose and under light conditions, also increased the number of bulbs regenerated in Tulipa gesneriana cultures [50]. Similarly, in the case of Hyacinthus orientalis cultures, BAP also promoted bulb formation [51]. Considering the effect of darkness on the number of adventitious bulb formed, it was observed that the composition of the media did not significantly affect the formation of bulbs throughout the cultivation period, which ultimately ranged from 2.9 to 4.1 bulbs per explant. This was similar to a previous experiment with T. tarda, where neither the concentration of sucrose nor the presence of BAP significantly influenced the number of adventitious bulbs formed in the dark [15]. In studies with the Tulipa ‘Apeldoorn’, the highest number of bulbs was obtained when the explants were grown in the dark or irradiated with white light [38]. In this study, under blue light, BAP added to the medium-reduced bulb formation, although differences were not significant (Figure 1F). Under red light, the maximum number of adventitious bulbs was obtained on the medium with 3% sucrose (6.7 bulbs per explant). In the study by Maślanka and Prokopiuk [16], under the influence of blue light and 6% sucrose, a lower number of bulbs was also observed after adding BAP to the medium and under red light and 3% sucrose, there were only two bulbs per explant. In an experiment with Hyacinthus sp., blue light also caused fewer bulbs to form compared to red light and darkness [36]. Comparing the current experiment with the previous one performed by Maślanka and Prokopiuk [16], it appears that the explants from bulb scale are more efficient than those from callus tissue. Bakhshaie et al. [18] in Lilium spp. culture, also used bulb scales from aseptic in vitro bulblets as the most responsive explant. Pałka et al. [44] did the same with Lilium candidum.

Table 1.
Effect of light spectrum and medium composition on the induction of adventitious bulbs within 12 weeks of cultivation.

Table 2.
Effect of light spectrum and medium composition on the number of adventitious bulbs per explant within 12 weeks of cultivation.
After 9–12 weeks of cultivation, some adventitious bulbs began to develop roots and then also leaves. As in the case of adventitious bulbs, a higher percentages of bulbs developing roots (49.2 to 66.2 in the dark) (Figure 1G) do not translate into a larger number of roots (obtained in various light and medium conditions, with 2.3–2.9 roots per bulb). Under red light, bulbs only rooted in the medium containing 6% sucrose and 0.5 µM BAP (Figure 1H). Also under white and blue light, the presence of BAP in the medium was necessary for root development, regardless of the sucrose concentration (Table 3). The formation of adventitious roots from Lachenalia ‘Rupert’ and ‘Ronina’ shoots took place on MS medium containing BAP and NAA, in the dark, under blue and red light, but did not occur under white light [52]. However, Fritillaria persica bulblets developed roots on MS medium with the addition of NAA [53]. Meanwhile, in the case of Lilium ‘Casablanca’ [35], red and blue light induced the highest number of roots per bulblet.

Table 3.
Effect of light spectrum and medium composition on the induction and number of root and leaves, formed by the adventitious bulbs, after 12 weeks of cultivation.
In terms of leaf formation, darkness was superior to other light conditions, regardless of the medium composition. A maximum of 42.4% of adventitious bulbs developed leaves (darkness, 6% sucrose, 0.5 µM BAP), with a maximum of 1.3 leaves per bulb (darkness, 6% sucrose). The leaves also developed under the influence of white light on a medium with 6% sucrose and BAP (Figure 1I) and under the influence of blue light on a medium with 6% sucrose and 3% sucrose in combination with BAP. When exposed to red light, the bulbs did not develop any leaves (Table 3). In the experiment with Lilium candidum, the highest number of bulbs forming leaves was observed under white light [44]. However, in a study on Hyacinthus sp., blue light enhanced adventitious bulbs to form leaf-shaped structures [36].
4. Conclusions
The results demonstrate the highest regenerative potential of Tulipa tarda from bulb scale explants, cultured on MS medium with 6% sucrose and exposed to fluorescent white light. Under these conditions, the largest number of adventitious bulbs (12.1 ± 1.3 bulbs/explant) was formed. Red light combined with 3% sucrose and 0.5 µM BAP completely inhibited the formation of adventitious bulbs. The effect of the medium (sucrose concentration and the presence of BAP) depended on the light conditions. The addition of BAP to the medium, under red, white, and blue light conditions, was necessary for rooting of the adventitious bulbs. A maximum of 2.9 ± 0.6 roots per bulb was obtained (white light × 6% sucrose × BAP), but this result was not significantly higher compared to other treatments. Darkness was more effective than other light conditions, regardless of the medium composition, in terms of the number of adventitious bulbs developing leaves. A maximum of 1.3 ± 0.1 leaves per bulb was achieved (darkness × 6% sucrose).
This method can be successfully used to propagate T. tarda as an alternative to conventional methods.
Funding
This research was supported by the Ministry of Science and Higher Education of the Republic of Poland from subvention funds for University of Agriculture in Krakow.
Data Availability Statement
Data are contained within the article—Author’s own Contribution.
Conflicts of Interest
The author declares no conflicts of interest.
References
- van Scheepen, J. (Ed.) Classified List and International Register of Tulip Names; Royal General Bulbgrowers’ Association, KAVB: Hillegom, The Netherlands, 1996. [Google Scholar]
- Botschantzeva, Z.P. Tulips: Taxonomy, Morphology, Cytology, Phytogeography and Physiology; A. A. Balkema: Rotterdam, The Netherlands, 1982. [Google Scholar]
- Yasemin, S.; Beruto, M. A Review on Flower Bulb Micropropagation: Challenges and Opportunities. Horticulturae 2024, 10, 284. [Google Scholar] [CrossRef]
- De Hertogh, A.; Le Nard, M. The Physiology of Flower Bulbs: A Comprehensive Treatise on the Physiology and Utilization of Ornamental Flowering Bulbous and Tuberous Plants; Elsevier Science Publishers: Amsterdam, The Netherlands, 1993; pp. 617–682. [Google Scholar]
- Okubo, H.; Sochacki, D. Botanical and horticultural aspects of major ornamental geophytes. In Ornamental Geophytes: From Basic Science to Sustainable Production; Kamenetsky, R., Okubo, H., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 77–122. [Google Scholar]
- Rees, A.R. Ornamental Bulbs, Corms and Tubers; CAB International: Wallingford, UK, 1992; pp. 93–111. [Google Scholar]
- Rees, A.R. The Growth of Bulbs, Applied Aspects of the Physiology of Ornamental Bulbous Crop Plants, 1st ed.; Academic Press: London, UK, 1972; p. 311. [Google Scholar]
- Kim, K.-W.; De Hertogh, A. Tissue culture of ornamental flowering bulbs (geophytes). Hortic. Rev. 1997, 18, 87–169. [Google Scholar]
- Orlikowska, T.; Podwyszyńska, M.; Marasek-Ciołakowska, A.; Sochacki, D.; Szymański, R. Tulip. In Ornamental Crops; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; pp. 769–802. [Google Scholar]
- Green, P.B. Organogenesis-a biophysical view. Annu. Rev. Plant Physiol. 1980, 31, 51–82. [Google Scholar] [CrossRef]
- Ziv, M. The Contribution of Biotechnology to Breeding, Propagation and Disease Resistance in Geophytes. In Proceedings of the VII International Symposium on Flowerbulbs, Herzliya, Israel, 1 December 1997. [Google Scholar]
- Schwarz, O.J.; Beaty, R.M. Organogenesis. In Plant Tissue Culture Concepts and Laboratory Exercises; Routledge: Abingdon, UK, 2018; pp. 125–138. [Google Scholar]
- Podwyszyńska, M.; Marasek, A. Effects of thidiazuron and paclobutrazol on regeneration potential of tulip flower stalk explants in vitro and subsequent shoot multiplication. Acta Soc. Bot. Pol. 2003, 72, 181–190. [Google Scholar] [CrossRef]
- Ghaffor, A.; Maqbool, I.; Waseem, K.; Quraishi, A. In vitro response of tulips (Tulipa gesnerina L.) to various growth regulators. Short communication. Int. J. Agr. Biol. 2004, 6, 1168–1169. [Google Scholar]
- Maślanka, M.; Bach, A. Induction of bulb organogenesis in in vitro cultures of tarda tulip (Tulipa tarda Stapf.) from seed-derived explants. Vitr. Cell. Dev. Biol. Plant 2014, 50, 712–721. [Google Scholar] [CrossRef]
- Maślanka, M.; Prokopiuk, B. Bulb organogenesis of Tulipa tarda in vitro cultures in relation to light environment. Acta Agric. Scand. Sect. B 2019, 69, 398–404. [Google Scholar] [CrossRef]
- Hicks, G.S. Patterns of organ development in plant tissue culture and the problem of organ determination. Bot. Rev. 1980, 46, 1–23. [Google Scholar] [CrossRef]
- Bakhshaie, M.; Khosravi, S.; Azadi, P.; Bagheri, H.; van Tuyl, J.M. Biotechnological advances in Lilium. Plant. Cell Rep. 2016, 35, 1799–1826. [Google Scholar] [CrossRef]
- Koster, J. In Vitro Propagation of Tulip. Ph.D. Thesis, University of Leiden, Leiden, The Netherlands, 1993; pp. 1–128. [Google Scholar]
- van Rossum, M.W. Role of Physiological Factors in Tulip Bulb Scale Micropropagation. Ph.D. Thesis, Landbouwuniversiteit Wageningen, Wageningen, The Netherlands, 1997. [Google Scholar]
- Minas, G.J. In vitro propagation of Akama tulip via adventitious organogenesis from bulb slices. In Proceedings of the International Conference on Quality Management in Supply Chains of Ornamentals, Bangkok, Thailand, 3–6 December 2007; pp. 313–316. [Google Scholar]
- Bach, A.; Sochacki, D. Propagation of Ornamental Geophytes: Physiology and Management Systems. In Ornamental Geophytes: From Basic Science to Sustainable Production; Kamenetsky, R., Okubo, H., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 261–286. [Google Scholar]
- Podwyszyńska, M. The mechanisms of in vitro storage organ formation in ornamental geophytes. Floric. Ornam. Biotechnol. 2012, 6, 9–23. [Google Scholar]
- Ascough, G.D.; van Staden, J.; Erwin, J.E. In vitro Storage Organ Formation of Ornamental Geophytes. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 2008; pp. 417–445. [Google Scholar] [CrossRef]
- Kumar, S.; Awasthi, V.; Kanwar, J.K. Influence of growth regulators and nitrogenous compounds on in vitro bulblet formation and growth in oriental lily. Hortic. Sci. 2007, 34, 77–83. [Google Scholar] [CrossRef]
- Malik, M.; Bach, A. Morphogenetic pathways from Narcissus L. ‘Carlton’ in vitro cultures of PC stage flower bud explants according to cytokinin and auxin rations. Acta Sci. Pol. Hortorum Cultus 2016, 15, 101–111. [Google Scholar]
- Prokopiuk, B.; Cioć, M.; Maślanka, M.; Pawłowska, B. Effects of light spectra and benzyl adenine on in vitro adventitious bulb and shoot formation of Lilium regale E.H. Wilson. Propag. Ornam. Plants 2018, 18, 12–18. [Google Scholar]
- Lagram, K.; El Merzougui, S.; Boudadi, I.; Ben El Caid, M.; El Boullani, R.; El Mousadik, A.; Serghini, M.A. In vitro shoot formation and enrooted mini-corm production by direct organogenesis in saffron (Crocus sativus L.). Vegetos 2023, 37, 1–6. [Google Scholar] [CrossRef]
- Taeb, A.G.; Alderson, P.G. Effect of low temperature and sucrose on bulb development and on the carbohydrate status of bulbing shoots of tulip in vitro. J. Hort. Sci. 1990, 65, 193–197. [Google Scholar] [CrossRef]
- Famelaer, I.; Ennik, E.; Eikelboom, W.; Van Tuyl, J.M.; Creemers-Molenaar, J. The initiation of callus and regeneration from callus culture of Tulipa gesneriana. Plant Cell Tiss. Org. Cult. 1996, 47, 51–58. [Google Scholar] [CrossRef]
- de Klerk, G.J.M. Micropropagation of bulbous crops: Technology and present state. Floric. Ornam. Biotechnol. 2012, 6, 1–8. [Google Scholar]
- Paek, K.Y.; Murthy, H.N. High frequency of bulblet regeneration from bulb scale sections of Fritillaria thunbergii. Plant Cell Tiss. Org. Cult. 2002, 68, 247–252. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Mehbub, H.; Akter, A.; Akter, M.A.; Mandal, M.S.H.; Hoque, M.A.; Tuleja, M.; Mehraj, H. Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application. Plants 2022, 11, 3208. [Google Scholar] [CrossRef]
- Lian, M.; Chakrabarty, D.; Paek, K. Growth and uptake of sucrose and mineral ions by bulblets of Lilium oriental hybrid ‘Casablanca’ during bioreactor culture. J. Hortic. Sci. Biotechnol. 2002, 77, 253–257. [Google Scholar] [CrossRef]
- Bach, A.; Malik, M.; Ptak, A.; Kędra, M. Light effects on ornamental microplant shoots and bulbs quality. Acta Hort. 2000, 530, 173–180. [Google Scholar] [CrossRef]
- Bach, A.; Świderski, A. The effect of light quality on organogenesis of Hyacinthus orientalis L. in vitro. Acta Biol. Cracoviensia Ser. Bot. 2000, 42, 115–120. [Google Scholar]
- Bach, A.; Pawłowska, B. Effect of light qualities on cultured in vitro ornamental bulbous plants. In Floriculture, Ornamental and Plant Biotechnology; Advances and Topical Issues; Global Science Books: Hong Kong, 2006; pp. 271–276. [Google Scholar]
- Jasenovska, L.; Brestic, M.; Barboricova, M.; Ferencova, J.; Filacek, A.; Zivcak, M. Analysis of the effects of various light spectra on microgreen species. Folia Hort. 2024, 36, 197–209. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Evenor, D.; Levi-Nissim, A.; Afgin, L.; Lilien-Kipnis, H.; Watad, A.A. Regeneration of plantlets and bulblets from explants and callus of Allium aflatunense cultivars and selection from indigenous Israeli Allium ampeloprasum. Acta Hortic. 1997, 430, 325–330. [Google Scholar] [CrossRef]
- Mohammadi-Dehcheshmeh, M.; Khalighi, A.; Naderi, R.; Sardarii, M.; Ebrahimie, E. Petal a reliable explant for direct bulblet regeneration of endangered wild populations of Fritillaria imperialis L. Acta Physiol. Plant. 2008, 30, 395–399. [Google Scholar] [CrossRef]
- Bakhshaie, M.; Babalar, M.; Mirmasoumi, M.; Khalighi, A. Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell, Tiss. Org. Cult. 2010, 102, 229–235. [Google Scholar] [CrossRef]
- Pałka, P.; Cioć, M.; Hura, K.; Szewczyk-Taranek, B.; Pawłowska, B. Adventitious organogenesis and phytochemical compositionof Madonna lily (Lilium candidum L.) in vitro modeled by different light quality. Plant Cell Tiss. Org. Cult. 2023, 152, 99–114. [Google Scholar] [CrossRef]
- Maślanka, M.; Mazur, J.; Kapczyńska, A. In Vitro Organogenesis of Critically Endangered Lachenalia viridiflora. Agronomy 2022, 12, 475. [Google Scholar] [CrossRef]
- Kritskaya, T.A.; Kashin, A.S.; Kasatkin, M.Y. Micropropagation and somaclonal variation of Tulipa suaveolens (Liliaceaae) in vitro. Russ. J. Dev. Biol. 2019, 50, 209–215. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant Propagation by Tissue Culture: The Background, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia ‘Ronina’ and ‘Rupert’ in vitro cultures under different lighting environments. Scientia Hort. 2015, 188, 23–29. [Google Scholar] [CrossRef]
- Pałka, P.; Muszyńska, B.; Szewczyk, A.; Pawłowska, B. Elicitation and enhancement of phenolics synthesis with zinc oxide nanoparticles and LED light in Lilium candidum L. cultures in vitro. Agronomy 2023, 13, 1437. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Draaj, I.A. The effect of explant source and cytokinin concentration on the direct bulb formation of tulip (Tulipa gesnerina L.) by plant tissue culture technique. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 111–119. [Google Scholar]
- Kizil, S.; Sesiz, U.; Khawar, K.M. Improved in vitro propagation of Hyacinthus orientalis L. using fruits containing immature zygotic embryos and tender leaf sheath as explants. Acta Sci. Pol. Hortorum Cultus 2016, 15, 15–30. [Google Scholar]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. The importance of applied light quality on the process of shoot organogenesis and production of phenolics and carbohydrates in Lachenalia sp. cultures in vitro. S. Afr. J. Bot. 2018, 114, 14–19. [Google Scholar] [CrossRef]
- Kizil, S.; Khawar, K.M. The effects of plant growth regulators and incubation temperatures on germination and bulb formation of Fritillaria persica L. Propag. Ornam. Plants 2014, 14, 133–138. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).