Pollen–Pistil Interactions in Autochthonous Balkan Sweet Cherry Cultivars—The Impact of Genotype and Flowering Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experiment Design
2.2. S-Allele Identification
2.2.1. Extraction of Genomic DNA
2.2.2. PCR Amplification of S-RNase and SFB Alleles
2.2.3. Fragment Analyses of S-Locus Genes
2.2.4. Sequencing of S-RNase Fragment
2.3. Pollination Experiment
2.3.1. Air Temperature
2.3.2. Pollination Procedure
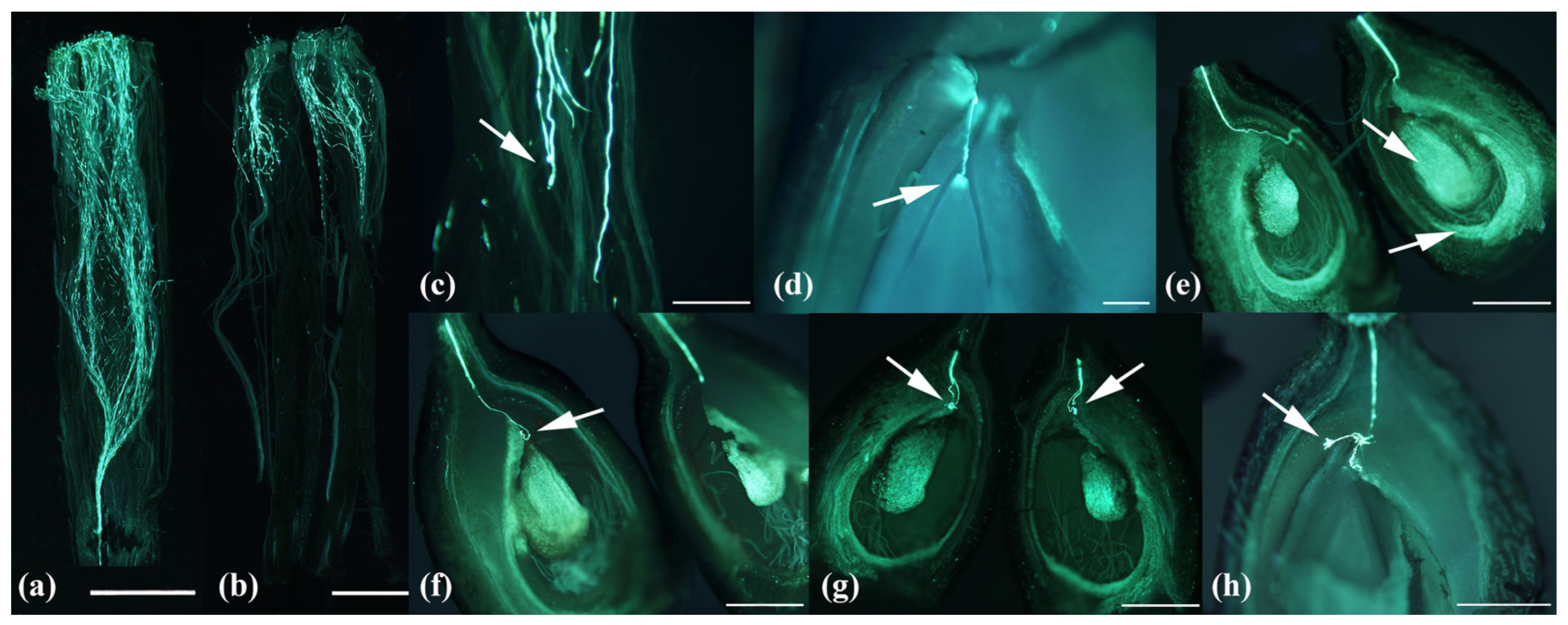
2.3.3. Microscopic Observation of Pollen Performance In Vivo and Ovule Fluorescence
2.3.4. Fruit Set
2.3.5. Statistical Analysis
3. Results
3.1. Identification of S-Haplotypes in Autochthonous Cultivars and Pollenizers
3.2. Sequencing and Characterization of the Novel S40-RNase
3.3. Flowering Temperatures
3.4. Pollen Tube Growth Efficiency
3.4.1. Pollen Tube Number
3.4.2. Pollen Tube Growth Rate
3.4.3. Ovule Vitality and Unusual Pollen Tube Growth
3.4.4. Fruit Set
3.4.5. Correlations Among the Reproductive Parameters
4. Discussion
4.1. S-Haplotype Identification
4.2. Fertilization Efficacy and Impact of Pollenizer
4.3. Fertilization Efficacy and Impact of Pollinated Cultivar
4.4. Fertilization Efficacy and Impact of Flowering Temperatures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quero-García, J.; Schuster, M.; Lopez-Ortega, G.; Charlot, G. Sweet cherry varieties and improvement. In Cherries: Botany, Production and Uses; Quero-Garcia, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CAB International: Wallingford, UK, 2017; pp. 60–94. [Google Scholar] [CrossRef]
- Mariette, S.; Tavaud, M.; Arunyawat, U.; Capdeville, G.; Millan, M.; Salin, F. Population structure and genetic bottleneck in sweet cherry estimated with SSRs and the gametophytic self-incompatibility locus. BMC Genet. 2010, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Pinosio, S.; Marroni, F.; Zuccolo, A.; Vitulo, N.; Mariette, S.; Sonnante, G.; Aravanopoulos, F.A.; Ganopoulos, I.; Palasciano, M.; Vidotto, M.; et al. A draft genome of sweet cherry (Prunus avium L.) reveals genome-wide and local effects of domestication. Plant J. 2020, 103, 1420–1432. [Google Scholar] [CrossRef]
- Wenden, B.; Campoy, J.A.; Lecourt, J.; López Ortega, G.; Blanke, M.; Radičević, S.; Schüller, E.; Spornberger, A.; Christen, D.; Magein, H.; et al. A collection of European sweet cherry phenology data for assessing climate change. Sci. Data 2016, 3, 160108. [Google Scholar] [CrossRef] [PubMed]
- De Nettancourt, D. Incompatibility. In Cellular Interactions; Linskens, H.F., Heslop-Harrison, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 17, pp. 624–639. [Google Scholar] [CrossRef]
- Bošković, R.; Tobutt, K.R. Correlation of stylar ribonuclease zymograms with incompatibility alleles in sweet cherry. Euphytica 1996, 90, 245–250. [Google Scholar] [CrossRef]
- Yamane, H.; Ikeda, K.; Ushijama, K.; Sassa, H.; Tao, R. A pollen-expressed gene for a novel protein with an F-box motif that is very tightly linked to a gene for S-RNase in two species of cherry, Prunus cerasus and P. avium. Plant Cell Physiol. 2003, 44, 764–769. [Google Scholar] [CrossRef]
- Tao, R.; Iezzoni, A.F. The S-RNase-based gametophytic self-incompatibility system in Prunus exhibits distinct genetic and molecular features. Sci. Hortic. 2010, 124, 423–433. [Google Scholar] [CrossRef]
- Sonnenveld, T.; Tobutt, K.R.; Robbins, T.P. Allele-specific PCR detection of sweet cherry self-incompatibiliy (S) alleles S1 to S16 using consensus and allele-specific primers. Theor. Appl. Genet. 2003, 107, 1059–1070. [Google Scholar] [CrossRef]
- Ushijima, K.; Sassa, H.; Tao, R.; Yamane, H.; Dandekar, A.M.; Gradziel, T.M.; Hirano, H. Cloning and characterization of cDNAs encoding S-RNases from almond (Prunus dulcis): Primary structural features and sequence diversity of the S-RNases in Rosaceae. Mol. Gen. Genet. 1998, 260, 261–268. [Google Scholar] [CrossRef]
- Ikeda, K.; Igic, B.; Ushijima, K.; Yamane, H.; Hauck, N.R.; Nakano, R.; Sassa, H.; Iezzoni, A.F.; Kohn, J.R.; Tao, R. Primary structural features of the S haplotype-specific F-box protein, SFB, in Prunus. Sex. Plant Reprod. 2004, 16, 235–243. [Google Scholar] [CrossRef]
- Vaughan, S.P.; Russell, K.; Sargent, D.J.; Tobutt, K.R. Isolation of S-locus F-box alleles in Prunus avium and their application in a novel method to determine self-incompatibility genotype. Theor. Appl. Genet. 2006, 112, 856–866. [Google Scholar] [CrossRef]
- Schuster, M.; Schröpfer, S.; Flachowsky, H. An overview of the self-incompatibility (S) genotypes of cultivated sweet cherries. Acta Hortic. 2024, 1408, 105–112. [Google Scholar] [CrossRef]
- Sonneveld, T.; Robbins, T.P.; Bošković, R.; Tobutt, K.R. Cloning of six cherry self-incompatibility alleles and development of allele-specific PCR detection. Theor. Appl. Genet. 2001, 102, 1046–1055. [Google Scholar] [CrossRef]
- Sonneveld, T.; Tobutt, K.R.; Vaughan, S.P.; Robbins, T.P. Loss of pollen-S function in two self-compatible selections of Prunus avium is associated with deletion/mutation of an S haplotype–specific F-box gene. Plant Cell 2005, 17, 37–51. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Zhang, K.; Jiang, L.; Zhang, L. Development of a simple molecular marker specific for detecting the self-compatible S4′ haplotype in sweet cherry (Prunus avium L.). Plant Mol. Biol. Rep. 2004, 22, 387–398. [Google Scholar] [CrossRef]
- De Cuyper, D.; Sonneveld, T.; Tobutt, K.R. Determining self-incompatibility genotypes in Belgian wild cherries. Mol. Ecol. 2005, 14, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, A.; Hormaza, J.I. Cloning and characterization of genomic DNA sequences of four self-incompatibility alleles in sweet cherry (Prunus avium L.). Theor. Appl. Genet. 2004, 108, 299–305. [Google Scholar] [CrossRef]
- Marchese, A.; Bošković, R.I.; Caruso, T.; Raimondo, A.; Cutuli, M.; Tobutt, K.R. A new self-compatibility haplotype in the sweet cherry ‘Kronio’, S5’, attributable to a pollen-part mutation in the SFB gene. J. Exp. Bot. 2007, 58, 4347–4356. [Google Scholar] [CrossRef]
- Vaughan, S.P.; Bošković, R.I.; Gisbert-Climent, A.; Russell, K.; Tobutt, K.R. Characterisation of novel S-alleles from cherry (Prunus avium L.). Tree Genet. Genomes 2008, 4, 531–541. [Google Scholar] [CrossRef]
- Szikriszt, B.; Doğan, A.; Ercisli, S.; Akcay, M.E.; Hegedűs, A.; Halász, J. Molecular typing of the self-incompatibility locus of Turkish sweet cherry genotypes reflects phylogenetic relationships among cherries and other Prunus species. Tree Genet. Genomes 2013, 9, 155–165. [Google Scholar] [CrossRef]
- Kivistik, A.; Jakobson, L.; Kahu, K.; Laanemets, K. Wild and rare self-incompatibility allele S17 found in 24 sweet cherry (Prunus avium L.) cultivars. Plant Mol. Biol. Rep. 2022, 40, 376–388. [Google Scholar] [CrossRef]
- Sebolt, A.M.; Iezzoni, A.F.; Tsukamoto, T. S-genotyping of cultivars and breeding selections of sour cherry (Prunus cerasus L.) in the Michigan State University sour cherry breeding program. Acta Hortic. 2017, 1161, 31–40. [Google Scholar] [CrossRef]
- Schuster, M.M.; Schröpfer, S. Self-Incompatibility (S) Genotypes of Cultivated Sweet Cherries—An Overview 2023. Update; Open Agrar Repository: Quedlinburg, Germany, 2023; pp. 1–53. [Google Scholar] [CrossRef]
- Granger, A.R. Gene flow in cherry orchards. Theor. Appl. Genet. 2004, 108, 497–500. [Google Scholar] [CrossRef]
- Schuster, M.; Flachowski, H.; Köhler, D. Determination of self-incompatible genotypes in sweet cherry (Prunus avium L.) accessions and cultivars of the German Fruit Gene Bank and from private collections. Plant Breeding 2007, 126, 533–540. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Influence of genotype-temperature interaction on pollen performance. J. Evol. Biol. 2005, 18, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, F.M.; Müller, A.; Bruns, E. Climate changes and trends in phenology of fruit trees and field crops in Germany, 1961–2000. Agr. Forest Meteorol. 2004, 121, 69–78. [Google Scholar] [CrossRef]
- Drkenda, P.; Musić, O.; Marić, S.; Jevremović, D.; Radičević, S.; Hudina, M.; Hodžić, S.; Kunz, A.; Blanke, M. Comparison of climate change effects on pome and stone fruit phenology between Balkan countries and Bonn/Germany. Erwerbs-Obstbau 2018, 60, 295–304. [Google Scholar] [CrossRef]
- Djurdjević, V.; Vuković, A.; Vujadinović Mandić, M. Observed Climate Change in Serbia and Projections of Future Climate Based on Different Scenarios of Future Emissions; United Nation’s Development Program: Belgrade, Serbia, 2018; pp. 1–24. Available online: https://www.klimatskepromene.rs/wp-content/uploads/2021/08/Observed-Climate-Change-and-Projections.pdf (accessed on 5 January 2025).
- Vuković, A.J.; Vujadinović, M.P.; Rendulić, S.M.; Djurdjević, V.S.; Ruml, M.M.; Babić, V.P.; Popović, D.P. Global warming impact on climate change in Serbia for the period 1961–2100. Therm. Sci. 2018, 22, 2267–2280. Available online: https://doiserbia.nb.rs/img/doi/0354-9836/2018/0354-98361800168V.pdf (accessed on 5 January 2025). [CrossRef]
- Vujadinović Mandić, M.; Vuković Vimić, A.; Ranković-Vasić, Z.; Đurović, D.; Ćosić, M.; Sotonica, D.; Nikolić, D.; Đurđević, V. Observed changes in climate conditions and weather-related risks in fruit and grape production in Serbia. Atmosphere 2022, 13, 948. [Google Scholar] [CrossRef]
- Luedeling, E. Climate change impacts on winter chill for temperate fruit and nut production: A review. Sci. Hortic. 2012, 144, 218–229. [Google Scholar] [CrossRef]
- Wenden, B.; Barreneche, T.; Meland, M.; Blanke, M.M. Harmonisation of phenology stages and selected cherry cultivars as bioindicators for climate change. Acta Hortic. 2017, 1162, 9–12. [Google Scholar] [CrossRef]
- Branchereau, C.; Hardner, C.; Dirlewanger, E.; Wenden, B.; Le Dantec, L.; Alletru, D.; Parmentier, J.; Ivančič, A.; Giovannini, D.; Brandi, F.; et al. Genotype-by-environment and QTL-by-environment interactions in sweet cherry (Prunus avium L.) for flowering date. Front. Plant Sci. 2023, 14, 1142974. [Google Scholar] [CrossRef] [PubMed]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Radičević, S.; Cerović, R.; Đorđević, M. Ovule senescence and unusual pollen tube growth in the ovary of sweet cherry as affected by pistilar genotype and temperature. Span. J. Agric. Res. 2018, 16, e0704. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. The effect of temperature on stigmatic receptivity in sweet cherry (Prunus avium L.). Plant Cell Environ. 2003, 26, 1673–1680. [Google Scholar] [CrossRef]
- Quero-Garcia, J.; Iezzoni, A.; Lopez-Ortega, G.; Peace, C.; Fouche, M.; Dirlewanger, E. Advances and challenges in cherry breeding. In Achieving Sustainable Cultivation of Temperate Zone Tree Fruits and Berries; Lang, G., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; Volume 2, pp. 1–34. [Google Scholar] [CrossRef]
- Fotirić-Akšić, M.; Nikolić, T. Analysis of pomological traits in new promising sweet cherry genotypes. Genetika 2013, 45, 873–880. [Google Scholar] [CrossRef]
- Fotirić-Aksić, M.; Nikolić, T.; Zec, G.; Cerović, R.; Nikolić, M.; Rakonjac, V.; Nikolić, D. ’Canetova’, a new sweet cherry cultivar from Serbia. Acta Hortic. 2016, 1139, 91–94. [Google Scholar] [CrossRef]
- Marić, S.; Radičević, S.; Milošević, N.; Glišić, I.; Đorđević, M.; Banović Đeri, B. An overview of S-genotype diversity in sweet cherry landraces grown in the central region of the Republic of Serbia. J. Pomol. 2023, 57, 93–103. [Google Scholar] [CrossRef]
- Radunić, M.; Jazbec, A.; Ercisli, S.; Čmelik, Z.; Goreta Ban, S. Pollen-pistil interaction influence on the fruit set of sweet cherry. Sci. Hortic. 2017, 224, 358–366. [Google Scholar] [CrossRef]
- Vokurka, A.; Židovec, V.; Jeran, N.; Karlović, K.; Li, H.; Duralija, B.; Wang, J.; Dujmović Purgar, D.; Bolarić, S. Native cultivars of sour and sweet cherries in Croatia: Main characteristics and potential of production in marginal areas. Acta Hortic. 2021, 1315, 77–84. [Google Scholar] [CrossRef]
- Gjamovski, V.; Kiprijanovski, M.; Arsov, T. Evaluation of some cherry varieties grafted on Gisela 5 rootstock. Turk. J. Agric. For. 2016, 40, 737–745. [Google Scholar] [CrossRef]
- Gjamovski, V.; Kiprijanovski, M.; Arsov, T. Morphological and pomological characteristics of some autochthonous sweet cherry cultivars in the Republic of Macedonia. Acta Hortic. 2016, 1139, 147–152. [Google Scholar] [CrossRef]
- Marić, S.; Radičević, S.; Popovska, M.; Gjamovski, V.; Milošević, N. Identification of S-alleles in some indigenous sweet cherry genotypes grown in Ohrid region. In Book of Proceedings of X International Scientific Agriculture Symposium ‘Agrosym 2019’, Jahorina, Republic of Srpska, Bosnia and Herzegovina, 3–6 October 2019; Kovačević, D., Ed.; University of East Sarajevo, Faculty of Agriculture: East Sarajevo, Republic of Srpska, Bosnia and Herzegovina, 2019; pp. 493–499. [Google Scholar] [CrossRef]
- Selamovska, A.; Gjamovski, V.; Taseska-Gjorgjijevski, M.; Nedelkovski, D.; Bandjo Oreshkovikj, K.; Korunovska, B.; Djoljevska-Milenkovska, R. Comparative studies of the content of antioxidants in fruits of some autochthonous cherry varieties. J. Agric. Plant Sci. 2022, 20, 33–40. [Google Scholar] [CrossRef]
- Vokurka, A.; Radoš, L.; Krmpot, T.; Krpan, P.; Bolarić, S. S-allele constitution of some local sweet cherry varieties from Bosnia and Herzegovina. In Proceedings of the 54th Croatian & 14th International Symposium on Agriculture, Vodice, Croatia, 17–22 February 2019. [Google Scholar] [CrossRef]
- Đurić, G.; Mićić, N. The conservation of fruit germplasm in Bosnia and Herzegovina. Acta Hortic. 2024, 1412, 17–24. [Google Scholar] [CrossRef]
- Milatović, D.; Nikolić, M.; Miletić, N. Sweet and Sour Cherry, 2nd ed.; Scientific Pomological Society of Serbia: Čačak, Republic of Serbia, 2015; pp. 163–200. [Google Scholar]
- Radičević, S.; Marić, S.; Cerović, R.; Milošević, N.; Mitrović, O. Cultivar composition and fruit quality of introduced sweet cherry (Prunus avium L.) cultivars. J. Pomol. 2016, 50, 101–109. Available online: https://www.casopisnvd.rs/pdf-dow/50_195-196/4.pdf (accessed on 5 January 2025).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Marić, S.; Radičević, S.; Lukić, M.; Cerović, R.; Paunović, S.A. Determination of S-genotype in apple and sweet cherry cultivars released at Fruit Research Institute, Čačak. Genetika 2017, 49, 127–138. [Google Scholar] [CrossRef]
- Tao, R.; Yamane, H.; Sugiura, A.; Murayama, H.; Sassa, H.; Mori, H. Molecular typing of S-alleles through identification, characterization and cDNA cloning for S-RNases in sweet cherry. J. Amer. Soc. Hort. Sci. 1999, 124, 224–233. [Google Scholar] [CrossRef]
- Yamane, H.; Tao, R.; Sugiura, A.; Hauck, N.R.; Iezzoni, A.F. Identification and characterization of S-RNases in tetraploid sour cherry (Prunus cerasus). J. Am. Soc. Hortic. Sci. 2001, 126, 661–667. [Google Scholar] [CrossRef]
- Marić, S.; Radičević, S.; Sirbu, S.; Zhivondov, A.; Cerović, R.; Milošević, N. S-genotyping of some sweet cherry cultivars released within breeding programmes in the Balkan region. J. Agric. Food Environ. Sci. 2018, 72, 103–108. Available online: https://journals.ukim.mk/index.php/jafes/article/view/1190/1013 (accessed on 5 January 2025).
- Ikeda, K.; Ushijima, K.; Yamane, H.; Tao, R.; Hauck, N.R.; Sebolt, A.M.; Iezzoni, A.F. Linkage and physical distances between the S-haplotype S-RNase and SFB genes in sweet cherry. Sex. Plant Reprod. 2005, 17, 289–296. [Google Scholar] [CrossRef]
- Sonneveld, T.; Robbins, T.P.; Tobutt, K.R. Improved discrimination of self-incompatibility S-RNase alleles in cherry and high throughput genotyping by automated sizing of first intron polymerase chain reaction products. Plant Breed. 2006, 125, 305–307. [Google Scholar] [CrossRef]
- Cachi, A.M.; Wünsch, A. S-genotyping of sweet cherry varieties from Spain and S-locus diversity in Europe. Euphytica 2014, 197, 229–236. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018; pp. 1–204. [Google Scholar] [CrossRef]
- Winsor, J.A.; Stephenson, A.G. Demographics of pollen tube growth in Cucurbita pepo. Can. J. Bot. 1995, 73, 583–589. [Google Scholar] [CrossRef]
- Preil, W. Observing of pollen tube in pistil and ovarian tissue by means of fluorescence microscopy. Zeiss Info. 1970, 75, 24–25. [Google Scholar]
- Kho, Y.O.; Baër, J. Fluorescence microscopy in botanical research. Zeiss Info. 1971, 76, 54–57. [Google Scholar]
- Cerović, R.; Ružić, Ð. Pollen tube growth in sour cherry (Prunus cerasus) at different temperatures. J. Hortic. Sci. Biotech. 1992, 67, 333–340. [Google Scholar] [CrossRef]
- Ercisli, S.; Radunic, M.; Gadze, J.; Ipek, A.; Skaljac, M.; Cmelik, Z. S-RNase based S-genotyping of Croatian sweet cherry (Prunus avium L.) genotypes. Sci. Hortic. 2012, 139, 21–24. [Google Scholar] [CrossRef]
- Lisek, A.; Rozpara, E.; Głowacka, A.; Kucharska, D.; Zawadzka, M. Identification of S-genotypes of sweet cherry cultivars from Central and Eastern Europe. Hortic. Sci. 2015, 42, 13–21. [Google Scholar] [CrossRef]
- Ipek, A.; Gulen, H.; Akcay, M.E.; Ipek, M.; Ergin, S.; Eris, A. Determination of self-incompatibility groups of sweet cherry genotypes from Turkey. Genet. Mol. Res. 2011, 10, 253–260. [Google Scholar] [CrossRef]
- Marchese, A.; Giovannini, D.; Leone, A.; Mafrica, R.; Palasciano, M.; Cantini, C.; Di Vaio, C.; De Salvador, F.R.; Giacalone, G.; Caruso, T.; et al. S-genotype identification, genetic diversity and structure analysis of Italian sweet cherry germplasm. Tree Genet. Genomes 2017, 13, 93. [Google Scholar] [CrossRef]
- Radičević, S.; Cerović, R.; Marić, S.; Đorđević, M.; Fotirić-Akšić, M. Investigations of reproductive biology and S-incompatibility in cherries at Fruit Research Institute-Čačak. In Scientific Program and Book of Abstracts of Final Conference of COST Action FA1104 ‘Sustainable Production of High-Quality Cherries for the European Market’, Naoussa, Greece, 4–8 April 2016; COST Association: Brussels, Belgium, 2016; pp. 54–55. [Google Scholar]
- Marić, S.; Radičević, S.; Milošević, N.; Popovska, M.; Malchev, S.; Glišić, I.; Đorđević, M. An overview of self-incompatibility (S) genotypes of autochthonous sweet cherries grown in Balkan region. J. Mt. Agric. Balk. 2020, 23, 168–181. [Google Scholar]
- Kato, S.; Mukai, Y. Allelic diversity of S-RNase at the self-incompatibility locus in natural flowering cherry populations (Prunus lannesiana var. speciosa). Heredity 2004, 92, 249–256. [Google Scholar] [CrossRef]
- Hormaza, J.I.; Herrero, M. Pollen performance as affected by the pistilar genotype in sweet cherry (Prunus avium L.). Protoplasma 1999, 208, 129–135. [Google Scholar] [CrossRef]
- Radičević, S.; Cerović, R.; Nikolić, D.; Đorđević, M. The effect of genotype and temperature on pollen tube growth and fertilization in sweet cherry (Prunus avium L.). Euphytica 2016, 209, 121–136. [Google Scholar] [CrossRef]
- Alburquerque, N.; Burgos, L.; Sedgley, M.; Egea, J. Contributing to the knowledge of the fertilisation process in four apricot cultivars. Sci. Hortic. 2004, 102, 387–396. [Google Scholar] [CrossRef]
- Ðorđević, M.; Cerović, R.; Nikolić, D.; Radičević, S.; Lukić, M. Pollen tubes growth in the plum pistils in relations to initial fruit set. J. Mt. Agric. Balk. 2012, 15, 726–733. [Google Scholar]
- Radičević, S.; Ognjanov, V.; Marić, S.; Barać, G. The effect of genotype and temperature interaction on pollen performance in the pistils of autochthonous sour cherry cultivar ‘Feketićka’. Zemdirbyste 2021, 108, 271–278. Available online: https://zemdirbyste-agriculture.lt/1083_str-35/ (accessed on 5 January 2025). [CrossRef]
- Choi, C.; Tao, R.; Andersen, R.L. Identification of self-incompatibility alleles and pollen incompatibility groups in sweet cherry by PCR based S-allele typing and controlled pollination. Euphytica 2002, 123, 9–20. [Google Scholar] [CrossRef]
- Choi, C.; Andersen, R.L. A preliminary study on physiological and S-allele specific breakdown of self-incompatibility in sweet cherry. Acta Hortic. 2005, 667, 83–88. [Google Scholar] [CrossRef]
- Cerović, R.; Ružić, Đ.; Mićić, N. Viability of plum ovules at different temperatures. Ann. Appl. Biol. 2000, 137, 53–59. [Google Scholar] [CrossRef]
- Egea, J.; Burgos, L. Ovule differences between single-kernelled and double-kernelled fruits in almond (Prunus dulcis). Ann. Appl. Biol. 2000, 136, 291–295. [Google Scholar] [CrossRef]
- Alburquerque, N.; Burgos, L.; Egea, J. Variability in the developmental stage of apricot ovules at anthesis and its relationship with fruit set. Ann. Appl. Biol. 2002, 141, 147–152. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Flower emasculation accelerates ovule degeneration and reduces fruit set in sweet cherry. Sci. Hortic. 2009, 119, 455–457. [Google Scholar] [CrossRef]
- Herrero, M. Male and female synchrony and the regulation of mating in flowering plants. Philos. Trans. R. Soc. B, Biol. Sci. 2003, 358, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rosaceae). Am. J. Bot. 2004, 91, 558–564. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. The effect of temperature on pollen germination, pollen tube growth and stigmatic receptivity in peach (Prunus persica L. Batsch.). Plant Biol. 2005, 7, 476–483. [Google Scholar] [CrossRef]
- Iovane, M.; Izzo, L.G.; Cirillo, A.; Romano, L.E.; Di Vaio, C.; Aronne, G. Flowering and pollen resilience to high temperature of apricot cultivars. Sci. Hortic. 2022, 304, 111261. [Google Scholar] [CrossRef]
- Milošević, N.; Glišić, I.; Đorđević, M.; Cerović, R.; Radičević, S.; Marić, S.; Milošević, T.; Nikolić, D. Influence of pollination treatments and temperature regimes on progamic phase and fruit set in three European plum (Prunus domestica L.) cultivars tollerant/resistant to Sharka virus. Eur. J. Agron. 2023, 149, 126909. [Google Scholar] [CrossRef]
- Meland, M.; Frøynes, O.; Fotiric Akšić, M.; Pojskić, N.; Kalamujić Stroil, B.; Lasic, L.; Gasi, F. Identifying pollen donors and success rate of individual pollinizers in European plum (Prunus domestica L.) using microsatellite markers. Agronomy 2020, 10, 264. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Warm temperatures at bloom reduce fruit set in sweet cherry. J. Appl. Bot. Food Qual. 2007, 81, 158–164. Available online: https://digital.csic.es/handle/10261/4386 (accessed on 5 January 2025).
- Erez, A.; Yablowitz, Z.; Korcinski, R. Greenhouse peach growing. Acta Hortic. 1998, 465, 593–600. [Google Scholar] [CrossRef]
- Erez, A.; Yablowitz, Z.; Korcinski, R.; Zilberstaine, M.; Fokkema, N.J. Grenhouse-growing of stone fruits: Effect of temperature on competing sinks. Acta Hortic. 2000, 513, 417–425. [Google Scholar] [CrossRef]
- Kozai, N.; Beppu, K.; Mochioka, R.; Boonprakob, U.; Subhadrabandhu, S.; Lillecrapp, A.M.; Wallwork, M.A.; Sedgley, M. Female and male sterility cause low fruit set in a clone of the ‘Trevatt’ variety of apricot (Prunus armeniaca L.). Sci. Hortic. 1999, 82, 255–263. [Google Scholar] [CrossRef]
- Nava, G.A.; Dalmago, G.A.; Bergamaschi, H.; Paniz, R.; Santos, R.P.; Marodin, G.A. Effect of high temperatures in the preblooming and blooming periods on ovule formation, pollen grains and yield of ‘Granada’ peach. Sci. Hortic. 2009, 122, 37–44. [Google Scholar] [CrossRef]
- Rodrigo, J.; Herrero, M. Effects of pre-blossom temperatures on flower development and fruit set in apricot. Sci. Hortic. 2002, 92, 125–135. [Google Scholar] [CrossRef]
- Kozai, N.; Beppu, K.; Mochioka, R.; Boonprakob, U.; Subhadrabandhu, S.; Kataoka, I. Adverse effects of high temperature on the development of reproductive organs in ‘Hakuho’ peach trees. J. Hortic. Sci. Biotech. 2004, 79, 533–537. [Google Scholar] [CrossRef]
- Carpenedo, S.; Bassols, M.C.; Franzon, R.C.; Byrne, D.H.; Silva, J.B. Stigmatic receptivity of peach flowers submitted to heat stress. Acta Sci. Agron. 2020, 42, 42450. Available online: http://www.redalyc.org/articulo.oa?id=303062597021 (accessed on 5 January 2025). [CrossRef]
- Fadón, E.; Fernandez, E.; Luedeling, E.; Rodrigo, J. Agroclimatic requirements and adaptation potential to global warming of Spanish cultivars of sweet cherry (Prunus avium L.). Eur. J. Agron. 2023, 145, 126774. [Google Scholar] [CrossRef]
- Fadón, E.; Herrera, S.; Guerrero, B.I.; Guerra, M.E.; Rodrigo, J. Chilling and heat requirements of temperate stone fruit trees (Prunus sp.). Agronomy 2020, 10, 409. [Google Scholar] [CrossRef]
- Egea, J.A.; Caro, M.; García-Brunton, J.; Gambín, J.; Egea, J.; Ruiz, D. Agroclimatic metrics for the main stone fruit producing areas in Spain in current and future climate change scenarios: Implications from an adaptive point of view. Front. Plant Sci. 2022, 13, 842628. [Google Scholar] [CrossRef]
- Herrero, M. Flower biology and fruit set in cherry. Acta Hortic. 2017, 1161, 345–351. [Google Scholar] [CrossRef]
- Samdarashi, S.; Jha, S. Climate-Smart Agriculture: Integrating Genetics, Breeding and Management. In Genetics and Plant Breeding: Roles in Climate Resilience, Nutritional Security, and Sustainable Development Goals; Stella International Publisher: Haryana, India, 2024; Available online: https://www.researchgate.net/publication/387682981_Chapter_Climate-Smart_Agriculture_Integrating_Genetics_27_Breeding_and_Management (accessed on 21 February 2025).
- Yan, J.; Wang, X. Machine learning bridges omics sciences and plant breeding. Trends Plant Sci. 2023, 28, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, Y.; Li, D.; Xu, S.; Huang, Y. Smart horticulture as an emerging interdisciplinary field combining novel solutions: Past development, current challenges, and future perspectives. Hortic. Plant J. 2024, 10, 1257–1273. [Google Scholar] [CrossRef]

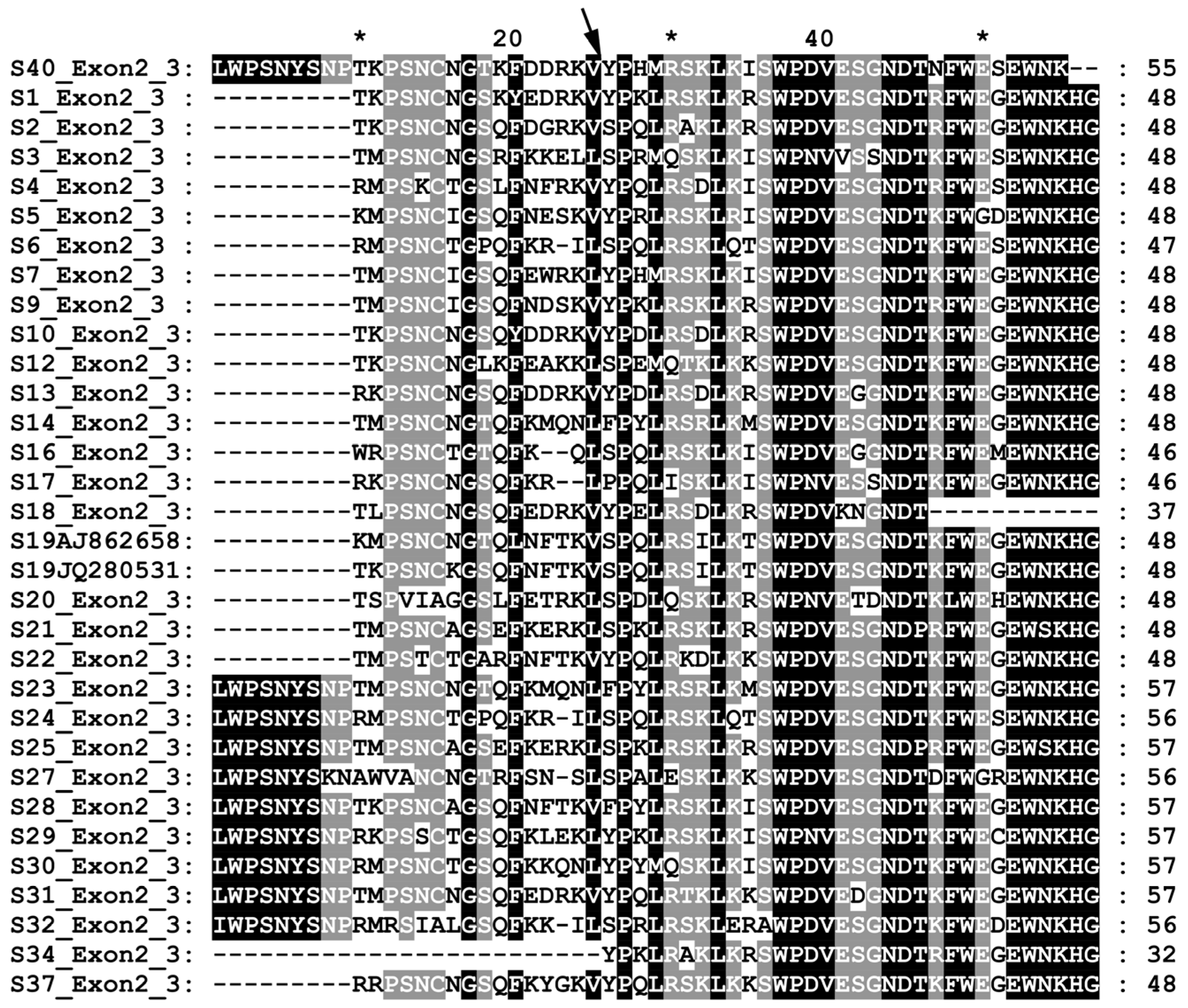
| Cultivar | S-Haplotype | Incompatibility Group |
|---|---|---|
| ‘Canetova’ | S5S6 | XV |
| ‘Dolga Šiška’ | S3S12 | XXII |
| ‘G-2’ | S2S3 | IV |
| ‘Ohridska Crna’ | S4S40 | 0 a |
| Pollenizer | ||
| ‘Burlat’ | S3S9 | XVI |
| ‘Kordia’ | S3S6 | VI |
| ‘Lapins’ | S1S4’ | SC b |
| ‘Rita’ | S5S22 | 0 |
| ‘Summit’ | S1S2 | I |
| ‘Sunburst’ | S3S4’ | SC |
| Cultivar | Flowering Season | Period | Average Mean Temperature (°C) | Average Max. Temperature (°C) | Average Min. Temperature (°C) |
|---|---|---|---|---|---|
| ‘Canetova’ | I | BFF a | 10.23 | 18.34 | 2.67 |
| FF b | 6.28 | 11.29 | 2.01 | ||
| II | BFF | 10.26 | 18.74 | 1.91 | |
| FF | 15.98 | 26.39 | 6.90 | ||
| ‘G-2’ | I | BFF | 12.00 | 17.00 | 6.80 |
| FF | 11.00 | 16.60 | 5.20 | ||
| II | BFF | 10.80 | 17.60 | 4.40 | |
| FF | 7.10 | 11.40 | 3.30 | ||
| ‘Dolga Šiška’ | I | BFF | 10.59 | 18.55 | 3.70 |
| FF | 12.55 | 23.10 | 5.30 | ||
| II | BFF | 10.34 | 18.74 | 2.49 | |
| FF | 13.49 | 20.17 | 8.03 | ||
| ‘Ohridska Crna’ | I | BFF | 12.74 | 23.48 | 4.41 |
| FF | 13.09 | 22.87 | 5.83 | ||
| II | BFF | 10.69 | 17.22 | 3.00 | |
| FF | 12.99 | 20.55 | 6.37 |
| Pollination Variant (A) | STU s | STM t | BS u | OVR v | FP w (%) | FS x (%) | |
|---|---|---|---|---|---|---|---|
| ‘Canetova’ × ‘Burlat’ | 33.78 ± 8.55 a | 19.98 ± 3.21 a | 10.94 ± 1.12 a | 2.76 ± 1.20 b | 51.51 ± 16.63 a | 11.05 ± 1.91 d | |
| ‘Canetova’ × ‘Lapins’ | 30.87 ± 9.36 b | 18.12 ± 1.65 b | 8.38 ± 0.45 c | 2.34 ± 0.18 c | 54.61 ± 16.04 a | 35.80 ± 13.09 a | |
| ‘Canetova’ × ‘Rita’ | 30.51 ± 9.86 b | 19.62 ± 2.85 ab | 9.92 ± 1.57 b | 3.18 ± 0.48 a | 53.57 ± 3.97 a | 31.67 ± 16.56 b | |
| ‘Canetova’ SP y | 24.69 ± 13.30 c | 3.48 ± 3.19 d | 0.67 ± 0.48 e | 0.15 ± 0.02 e | 0.00 ± 0.00 c | 0.00 ± 0.00 e | |
| ‘Canetova’ OP z | 23.13 ± 12.31 c | 13.11 ± 9.82 c | 4.56 ± 2.55 d | 1.15 ± 1.09 d | 16.65 ± 18.27 b | 24.04 ± 4.43 c | |
| Flowering season (B) | |||||||
| I | 33.85 ± 11.39 a | 16.93 ± 7.80 a | 6.45 ± 4.25 b | 2.01 ± 1.67 a | 31.33 ± 27.92 b | 14.54 ± 9.29 b | |
| II | 23.34 ± 7.49 b | 14.89 ± 7.81 a | 7.34 ± 4.02 a | 1.82 ± 0.93 b | 39.21 ± 24.52 a | 26.50 ± 19.54 a | |
| A × B | |||||||
| ‘Canetova’ × ‘Burlat’ | I | 41.39 ± 0.70 a | 22.05 ± 0.34 a | 11.79 ± 0.67 a | 3.84 ± 0.33 a | 66.67 ± 0.96 b | 10.69 ± 0.97 e |
| II | 26.17 ± 2.89 d | 17.92 ± 3.59 b | 10.08 ± 0.72 b | 1.69 ± 0.03 d | 36.36 ± 1.08 f | 11.41 ± 2.65 e | |
| ‘Canetova’ × ‘Lapins’ | I | 39.40 ± 0.91 ab | 19.36 ± 0.24 b | 8.41 ± 0.43 c | 2.43 ± 0.01 bc | 40.00 ± 1.32 e | 24.35 ± 5.71 bc |
| II | 22.34 ± 0.62 d | 16.87 ± 1.45 b | 8.34 ± 0.57 c | 2.25 ± 0.25 c | 69.23 ± 0.87 a | 47.27 ± 1.20 a | |
| ‘Canetova’ × ‘Rita’ | I | 39.55 ± 1.10 ab | 22.16 ± 0.78 a | 8.69 ± 0.70 c | 3.56 ± 0.38 a | 50.00 ± 1.04 d | 16.86 ± 4.46 d |
| II | 21.47 ± 1.77 d | 17.07 ± 0.43 b | 11.17 ± 1.04 ab | 2.81 ± 0.10 b | 57.14 ± 0.37 c | 46.48 ± 2.70 a | |
| ‘Canetova’ SP | I | 36.83 ± 0.82 bc | 6.39 ± 0.42 c | 1.07 ± 0.17 f | 0.11 ± 0.19 e | 0.00 ± 0.00 h | 0.00 ± 0.00 f |
| II | 12.53 ± 0.15 e | 0.58 ± 0.14 e | 0.27 ± 0.25 g | 0.20 ± 0.26 e | 0.00 ± 0.00 h | 0.00 ± 0.00 f | |
| ‘Canetova’ OP | I | 12.08 ± 0.67 e | 4.16 ± 0.07 d | 2.30 ± 0.50 e | 0.17 ± 0.30 e | 0.00 ± 0.00 h | 20.74 ± 0.97 cd |
| II | 34.19 ± 3.46 c | 22.06 ± 0.91 a | 6.81 ± 0.86 d | 2.14 ± 0.12 c | 33.33 ± 1.23 g | 27.35 ± 3.92 b | |
| ANOVA | |||||||
| A | * | * | * | * | * | * | |
| B | * | ns | * | * | * | * | |
| A × B | * | * | * | * | * | * | |
| Pollination Variant (A) | STU s | STM t | BS u | OVR v | FP w (%) | FS x (%) | |
|---|---|---|---|---|---|---|---|
| ‘G-2’ × ‘Burlat’ | 14.01 ± 1.07 d | 8.21 ± 1.09 e | 4.22 ± 0.53 d | 1.93 ± 0.48 b | 41.46 ± 16.23 c | 8.93 ± 8.34 b | |
| ‘G-2’ × ‘Lapins’ | 38.80 ± 16.09 c | 23.97 ± 12.18 b | 8.68 ± 2.11 b | 3.51 ± 0.73 a | 40.20 ± 2.44 c | 11.97 ± 7.57 b | |
| ‘G-2’ × ‘Rita’ | 40.04 ± 15.46 c | 30.17 ± 10.48 a | 9.71 ± 0.99 a | 3.16 ± 0.36 a | 53.12 ± 3.66 a | 8.12 ± 4.64 b | |
| ‘G-2’ SP y | 42.35 ± 13.13 b | 11.80 ± 1.59 d | 2.05 ± 0.32 e | 0.33 ± 0.37 c | 3.35 ± 3.65 d | 0.27 ± 0.42 c | |
| ‘G-2’ OP z | 47.42 ± 7.33 a | 19.06 ± 4.20 c | 6.19 ± 1.06 c | 1.94 ± 0.76 b | 44.16 ± 10.09 b | 22.44 ± 15.47 a | |
| Flowering season (B) | |||||||
| I | 40.44 ± 17.92 a | 19.75 ± 9.8 a | 6.33 ± 3.22 a | 2.20 ± 1.07 a | 36.71 ± 20.42 a | 11.95 ± 7.06 a | |
| II | 32.80 ± 14.04 b | 17.53 ± 11.70 b | 6.01 ± 3.01 a | 2.57 ± 1.26 a | 36.20 ± 18.08 a | 8.74 ± 13.91 a | |
| A × B | |||||||
| ‘G-2’ × ‘Burlat’ | I | 13.72 ± 0.99 f | 7.47 ± 1.01 g | 4.15 ± 0.76 d | 2.28 ± 0.38 d | 56.25 ± 0.67 a | 16.43 ± 1.13 bc |
| II | 15.30 ± 0.17 f | 8.94 ± 0.63 fg | 4.25 ± 0.33 d | 1.58 ± 0.27 e | 26.67 ± 1.33 g | 1.43 ± 1.96 e | |
| ‘G-2’ × ‘Lapins’ | I | 54.24 ± 0.94 a | 35.04 ± 1.75 b | 10.54 ± 0.70 a | 2.85 ± 0.09 bc | 42.31 ± 1.07 d | 17.51 ± 4.95 b |
| II | 23.34 ± 0.32 e | 12.92 ± 0.93 e | 6.83 ± 0.58 c | 4.17 ± 0.11 a | 38.09 ± 0.64 e | 6.04 ± 3.62 bcd | |
| ‘G-2’ × ‘Rita’ | I | 26.02 ± 2.48 d | 20.67 ± 1.38 c | 8.84 ± 0.32 b | 3.06 ± 0.41 bc | 50.00 ± 1.99 c | 11.41 ± 3.39 bcd |
| II | 54.07 ± 1.11 a | 39.67 ± 1.44 a | 10.59 ± 0.29 a | 3.25 ± 0.38 b | 56.25 ± 0.54 a | 4.82 ± 3.14 cde | |
| ‘G-2’ SP | I | 54.23 ± 2.32 a | 12.99 ± 1.44 ef | 2.15 ± 0.46 e | 0.33 ± 0.57 f | 0.00 ± 0.00 i | 0.54 ± 0.00 e |
| II | 30.54 ± 1.04 c | 10.63 ± 0.22 f | 1.95 ± 0.05 e | 0.30 ± 0.12 f | 6.67 ± 0.15 h | 0.00 ± 0.00 e | |
| ‘G-2’ OP | I | 53.98 ± 2.67 a | 22.58 ± 2.43 c | 5.99 ± 1.26 c | 2.59 ± 0.41 cd | 35.00 ± 1,57 f | 13.46 ± 5.38 bc |
| II | 40.86 ± 0.36 b | 15.53 ± 0.98 d | 6.39 ± 1.06 c | 1.28 ± 0.07 e | 53.33 ± 0.65 b | 31.42 ± 18.10 a | |
| ANOVA | |||||||
| A | * | * | * | * | * | * | |
| B | * | * | ns | ns | ns | ns | |
| A × B | * | * | * | * | * | * | |
| Pollination Variant (A) | STU s | STM t | BS u | OVR v | FP w (%) | FS x (%) | |
|---|---|---|---|---|---|---|---|
| ‘Dolga Šiška’ × ‘Kordia’ | 14.75 ± 4.87 c | 6.58 ± 2.82 c | 3.81 ± 1.82 b | 1.68 ± 0.40 ab | 11.73 ± 4.49 c | 6.94 ± 3.80 c | |
| ‘Dolga Šiška’ × ‘Summit’ | 23.18 ± 6.70 a | 11.59 ± 2.69 a | 5.41 ± 1.01 a | 1.87 ± 0.48 a | 9.71 ± 2.30 b | 9.39 ± 3.49 b | |
| ‘Dolga Šiška’ × ‘Sunburst’ | 21.54 ± 4.05 b | 7.83 ± 1.11 b | 3.84 ± 1.35 b | 1.62 ± 0.57 b | 27.50 ± 2.89 a | 0.19 ± 0.47 d | |
| ‘Dolga Šiška’ SP y | 15.10 ± 2.36 c | 0.00 ± 0.00 e | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | |
| ‘Dolga Šiška’ OP z | 22.39 ± 10.76 ab | 3.22 ± 1.21 d | 1.04 ± 0.66 c | 0.35 ± 0.19 c | 11.85 ± 3.9 c | 17.43 ± 8.80 a | |
| Flowering season (B) | |||||||
| I | 18.97 ± 7.49 b | 4.49 ± 3.49 b | 1.98 ± 1.75 b | 1.02 ± 0.83 b | 10.55 ± 8.47 b | 4.31 ± 4.11 b | |
| II | 19.81 ± 6.79 a | 7.19 ± 4.89 a | 3.65 ± 2.51 a | 1.19 ± 0.92 a | 13.77 ± 10.34 a | 9.27 ± 9.85 a | |
| A × B | |||||||
| ‘Dolga Šiška’ × ‘Kordia’ | I | 10.32 ± 0.22 g | 4.03 ± 0.54 d | 2.16 ± 0.18 de | 1.33 ± 0.17 bc | 7.69 ± 0.78 e | 3.64 ± 0.36 c |
| II | 19.19 ± 0.54 d | 9.13 ± 0.14 b | 5.46 ± 0.26 ab | 2.02 ± 0.08 a | 15.78 ± 0.92 c | 10.24 ± 1.80 b | |
| ‘Dolga Šiška’ × ‘Summit’ | I | 17.23 ± 2.43 e | 9.28 ± 1.35 b | 4.67 ± 0.57 c | 2.27 ± 0.27 a | 11.73 ± 0.71 d | 7.98 ± 2.10 b |
| II | 29.13 ± 0.40 b | 13.89 ± 0.60 a | 6.15 ± 0.74 a | 1.48 ± 0.16 b | 7.69 ± 0.94 e | 10.79 ± 4.50 b | |
| ‘Dolga Šiška’ × ‘Sunburst’ | I | 17.91 ± 1.13 de | 7.00 ± 0.56 c | 2.66 ± 0.35 d | 1.11 ± 0.05 bc | 25.00 ± 0.44 b | 0.38 ± 0.67 d |
| II | 25.17 ± 0.48 c | 8.67 ± 0.83 b | 5.02 ± 0.48 bc | 2.14 ± 0.18 a | 30.00 ± 1.42 a | 0.00 ± 0.00 d | |
| ‘Dolga Šiška’ SP | I | 17.20 ± 0.35 e | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 e | 0.00 ± 0.00 f | 0.00 ± 0.00 d |
| II | 13.00 ± 0.77 f | 0.00 ± 0.00 f | 0.00 ± 0.00 f | 0.00 ± 0.00 e | 0.00 ± 0.00 f | 0.00 ± 0.00 d | |
| ‘Dolga Šiška’ OP | I | 32.21 ± 0.25 a | 2.17 ± 0.45 e | 0.45 ± 0.12 f | 0.33 ± 0.15 d | 8.33 ± 0.41 e | 9.54 ± 0.40 b |
| II | 12.58 ± 0.34 f | 4.28 ± 0.35 d | 1.63 ± 0.22 e | 0.31 ± 0.25 d | 15.38 ± 0.84 c | 25.32 ± 2.61 a | |
| ANOVA | |||||||
| A | * | * | * | * | * | * | |
| B | * | * | * | * | * | * | |
| A × B | * | * | * | * | * | * | |
| Pollination Variant (A) | STU s | STM t | BS u | OVR v | FP w (%) | FS x (%) | |
|---|---|---|---|---|---|---|---|
| ‘Ohridska Crna’ × ‘Kordia’ | 12.21 ± 4.23 c | 8.24 ± 4.73 b | 4.59 ± 3.65 b | 1.85 ± 0.73 a | 43.16 ± 7.59 a | 24.73 ± 14.71 a | |
| ‘Ohridska Crna’ × ‘Summit’ | 12.02 ± 6.25 c | 6.05 ± 4.63 c | 3.19 ± 2.13 c | 1.76 ± 0.56 a | 28.04 ± 2.21 d | 14.19 ± 64.48 c | |
| ‘Ohridska Crna’ × ‘Sunburst’ | 24.32 ± 0.40 a | 12.83 ± 4.63 a | 5.89 ± 0.58 a | 1.91 ± 0.52 a | 33.33 ± 0.49 c | 18.17 ± 13.02 b | |
| ‘Ohridska Crna’ SP y | 9.97 ± 10.53 d | 4.3 ± 4.76 e | 0.96 ± 1.05 e | 0.00 ± 0.00 b | 0.00 ± 0.00 e | 0.17 ± 0.41 d | |
| ‘Ohridska Crna’ OP z | 22.66 ± 5.09 b | 5.23 ± 2.95 d | 2.29 ± 1.49 d | 1.75 ± 0.99 a | 34.75 ± 7.65 b | 25.91 ± 14.71 a | |
| Flowering season (B) | |||||||
| I | 17.17 ± 8.73 a | 6.85 ± 3.72 b | 2.76 ± 1.81 b | 1.32 ± 0.88 b | 27.15 ± 14.70 b | 10.48 ± 7.94 b | |
| II | 15.31 ± 8.26 b | 7.81 ± 5.72 a | 4.09 ± 3.15 a | 1.59 ± 1.04 a | 28.55 ± 18.37 a | 22.89 ± 14.01 a | |
| A × B | |||||||
| ‘Ohridska Crna’ × ‘Kordia’ | I | 6.33 ± 0.34 g | 1.83 ± 0.26 f | 1.28 ± 0.25 f | 1.25 ± 0.10 c | 30.77 ± 0.74 d | 22.98 ± 3.99 cd |
| II | 17.72 ± 0.16 d | 10.26 ± 0.47 c | 5.11 ± 0.49 c | 2.26 ± 0.08 b | 55.56 ± 0.88 a | 26.47 ± 2.54 bc | |
| ‘Ohridska Crna’ × ‘Summit’ | I | 8.35 ± 0.97 f | 3.95 ± 0.43 e | 1.27 ± 0.12 f | 1.22 ± 0.06 c | 30.00 ± 0.75 d | 9.16 ± 1.00 ef |
| II | 16.04 ± 0.33 e | 12.55 ± 0.35 b | 7.91 ± 0.31 a | 2.50 ± 0.28 ab | 26.08 ± 0.28 f | 19.22 ± 5.31 d | |
| ‘Ohridska Crna’ × ‘Sunburst’ | I | 24.33 ± 0.58 b | 11.94 ± 0.42 b | 5.72 ± 0.83 bc | 1.46 ± 0.16 c | 33.33 ± 0.58 c | 6.64 ± 0.91 f |
| II | 24.31 ± 0.27 b | 13.71 ± 0.68 a | 6.07 ± 0.23 b | 2.35 ± 0.26 b | 33.33 ± 0.63 c | 29.69 ± 4.94 b | |
| ‘Ohridska Crna’ SP | I | 19.57 ± 0.94 c | 8.66 ± 0.73 d | 1.92 ± 0.15 f | 0.00 ± 0.00 d | 0.00 ± 0.00 g | 0.33 ± 0.58 g |
| II | 0.37 ± 0.05 h | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 d | 0.00 ± 0.00 g | 0.00 ± 0.00 g | |
| ‘Ohridska Crna’ OP | I | 27.25 ± 0.98 a | 7.90 ± 0.14 d | 3.36 ± 0.36 d | 2.64 ± 0.27 a | 41.67 ± 1.04 b | 12.92 ± 0.68 e |
| II | 18.08 ± 0.59 d | 2.56 ± 0.59 f | 0.95 ± 0.13 e | 0.86 ± 0.16 d | 27.78 ± 0.86 e | 39.07 ± 5.28 a | |
| ANOVA | |||||||
| A | * | * | * | * | * | * | |
| B | * | * | * | * | * | * | |
| A × B | * | * | * | * | * | * | |
| Parameter | STU a | STM b | BS c | OVR d | OF e | UB f | FP g | FS h |
|---|---|---|---|---|---|---|---|---|
| Cross-pollination variant | ||||||||
| STU | / | |||||||
| STM | 0.94 * | / | ||||||
| BS | 0.77 * | 0.84 * | / | |||||
| OVR | 0.60 * | 0.63 * | 0.71 * | / | ||||
| OF | 0.07 | −0.14 | −0.21 | −0.13 | / | |||
| UB | −0.28 | −0.32 | −0.39 | −0.20 | 0.46 * | / | ||
| FP | 0.40 | 0.51 * | 0.59 * | 0.58 * | −0.40 | −0.37 | / | |
| FS | −0.01 | 0.12 | 0.33 | 0.15 | −0.46 * | −0.18 | 0.52 * | / |
| Open-pollination variant | ||||||||
| STU | / | |||||||
| STM | 0.79 * | / | ||||||
| BS | 0.70 | 0.95 * | / | |||||
| OVR | 0.67 | 0.74 * | 0.70 | / | ||||
| OF | 0.14 | −0.16 | −0.30 | −0.36 | / | |||
| UB | 0.15 | 0.56 | 0.52 | 0.33 | −0.52 | / | ||
| FP | 0.61 | 0.61 | 0.70 | 0.73 * | −0.30 | 0.15 | / | |
| FS | −0.31 | −0.02 | 0.08 | −0.22 | −0.80 * | 0.19 | 0.25 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radičević, S.; Marić, S.; Glišić, I.; Cerović, R.; Đorđević, M.; Milošević, N.; Rakonjac, V.; Čolić, S.; Popovska, M.; Gjamovski, V.; et al. Pollen–Pistil Interactions in Autochthonous Balkan Sweet Cherry Cultivars—The Impact of Genotype and Flowering Temperature. Agronomy 2025, 15, 646. https://doi.org/10.3390/agronomy15030646
Radičević S, Marić S, Glišić I, Cerović R, Đorđević M, Milošević N, Rakonjac V, Čolić S, Popovska M, Gjamovski V, et al. Pollen–Pistil Interactions in Autochthonous Balkan Sweet Cherry Cultivars—The Impact of Genotype and Flowering Temperature. Agronomy. 2025; 15(3):646. https://doi.org/10.3390/agronomy15030646
Chicago/Turabian StyleRadičević, Sanja, Slađana Marić, Ivana Glišić, Radosav Cerović, Milena Đorđević, Nebojša Milošević, Vera Rakonjac, Slavica Čolić, Melpomena Popovska, Viktor Gjamovski, and et al. 2025. "Pollen–Pistil Interactions in Autochthonous Balkan Sweet Cherry Cultivars—The Impact of Genotype and Flowering Temperature" Agronomy 15, no. 3: 646. https://doi.org/10.3390/agronomy15030646
APA StyleRadičević, S., Marić, S., Glišić, I., Cerović, R., Đorđević, M., Milošević, N., Rakonjac, V., Čolić, S., Popovska, M., Gjamovski, V., & Banović Đeri, B. (2025). Pollen–Pistil Interactions in Autochthonous Balkan Sweet Cherry Cultivars—The Impact of Genotype and Flowering Temperature. Agronomy, 15(3), 646. https://doi.org/10.3390/agronomy15030646






