The Relationship Between the Germination of Silky Bent Grass (Apera spica-venti (L.) Beauv.) Diaspores and Their Age, Place of Occurrence, and Action of Stimulating Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Weed Diaspores
2.1.1. A. spica-venti Collection Region
2.1.2. Global and National Distribution of the Weed
2.1.3. Taxonomy of the Weed
2.1.4. Diaspores Description of A. spica-venti
2.2. In Vitro Experiment in Petri Dishes
2.3. In the Study Determined Indicators
2.4. Statistical Analyses
3. Results
3.1. Germination of A. spica-vention
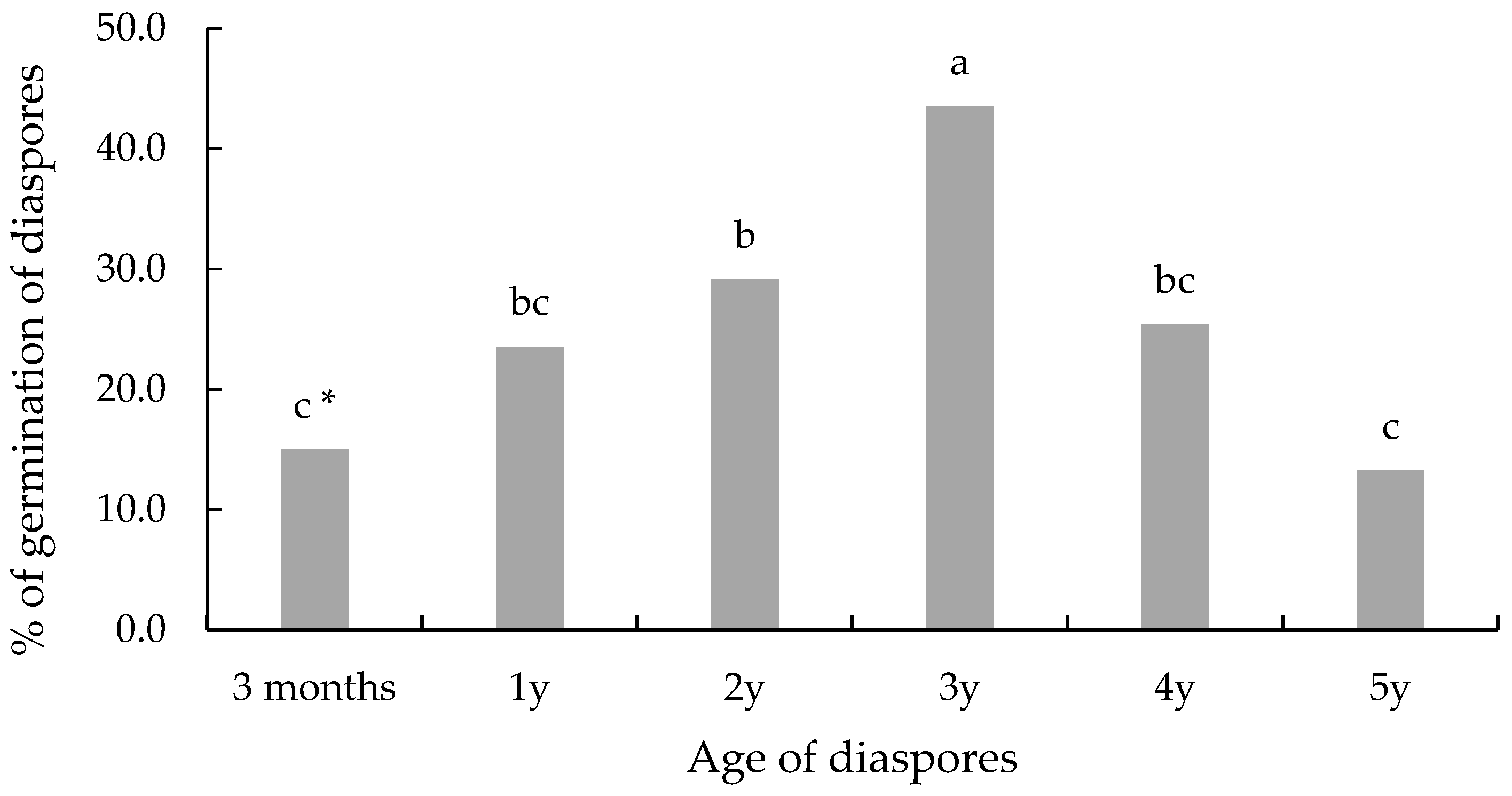
3.2. Age of Diaspores and Germination
3.3. Dynamics of Germination
3.4. Correlations of Germination with Macronutrients
3.5. Effect of Biostimulants on Germination
3.5.1. Effect of Biostimulants on Germination—Alge
3.5.2. Effect of Biostimulants on Germination—Silicon
3.6. Correlations of Germination with Different Solution—Alge
3.7. Correlations of Germination with Different Solution—Silicon
3.8. Leaf Length
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Soukup, J.; Nováková, K.; Hamouz, P.; Náměstek, J. Ecology of Silky Bent Grass (Apera spica-venti (L.) Beauv.), Its Importance and Control in the Czech Republic. J. Plant Dis. Prot. 2006, 20, 73–80. [Google Scholar]
- United States Department of Agriculture. Weed Risk Assessment for Apera spica-venti; United States Department of Agriculture: Washington, DC, USA, 2016.
- Weed Fact Sheet Apera spica-venti. HRAC Europe 2021. Available online: https://hracglobal.com/europe/files/docs/EHRAC-Weed-Fact-Sheet-APESV.pdf (accessed on 3 March 2025).
- van der Pijl, L. Principles of Dispersal in Higher Plants, 3rd ed.; Springer: Berlin, Germany, 1982. [Google Scholar]
- Hintze, C.; Heydel, F.; Hoppe, C.; Cunze, S.; König, A.; Tackenberg, O. D3: The dispersal and diaspore database—Baseline data and statistics on seed dispersal. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 180–192. [Google Scholar] [CrossRef]
- Seed Information Database (SID). Society for Ecological Restoration, International Network for Seed Based Restoration and Royal Botanic Gardens Kew. 2023. Available online: https://ser-sid.org/ (accessed on 3 February 2023).
- Małecka-Jankowiak, I.; Blecharczyk, A.; Sawinska, Z.; Piechota, T.; Waniorek, B. Impact of crop sequence and tillage system on weed infestation of winter wheat. Fragm. Agron. 2015, 32, 54–63. [Google Scholar]
- Nautiyal, P.C.; Sivasubramaniam, K.; Dadlani, M. Seed Dormancy and Regulation of Germination. In Seed Science and Technology; Dadlani, M., Yadava, D.K., Eds.; Springer: Singapore, 2023; pp. 39–66. [Google Scholar] [CrossRef]
- Eshan, S.; Manoj, M. Seed germination variability: Why do genetically identical seeds not germinate at the same time? J. Exp. Bot. 2023, 74, 3462–3475. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, G.; Zhang, J.; Guanqun, W. Analysis of gene expression in early seed germination of rice: Landscape and genetic regulation. BMC Plant Biol. 2022, 22, 70. [Google Scholar] [CrossRef]
- Li, R.; Chen, L.; Wu, Y.; Zhang, R.; Baskin, C.C.; Baskin, J.M.; Hu, X. Effects of Cultivar and Maternal Environment on Seed Quality in Vicia sativa. Front. Plant Sci. 2017, 8, 1411. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Keizer, P.; van Eeuwijk, F.; Smeekens, S.; Bentsink, L. Natural variation for seed longevity and seed dormancy are negatively correlated in Arabidopsis. Plant Physiol. 2012, 160, 2083–2092. [Google Scholar] [CrossRef]
- Kucewicz, M.; Holdynski, C. Germination of Matricaria maritima subsp. inodora (L.) dostal (scentless mayweed) achenes depending on their maturity and position on the mother plant. J. Plant Prot. Res. 2003, 43, 143–153. [Google Scholar]
- Saric-Krsmanovic, M.; Umiljendic, G.; Santric, L.; Radivojevic, L. Impact of storage conditions on seed germination and seedling growth of wild oat (Avena fatua L.) at different temperatures. Pestic. I Fitomed. 2015, 30, 243–248. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Dixon, K.W.; Silveira, F.A.O.; Chapman, T.; Di Sacco, A.; Mondoni, A.; Turner, S.; Cross, A. Dormancy and germination: Making every seed count in restoration. Restor. Ecol. 2020, 28, 256–265. [Google Scholar] [CrossRef]
- Gniazdowska, A.; Budnicka, K.; Krasuska, U. Regulation of Seed Dormancy and Germination—Endogenous Factors and Environmental Interactions. In Biodiversity-From Cell to Ecosystem; Publishing Agency EkoPress: Białystok, Poland, 2013; ISBN 9788362069378. [Google Scholar]
- Bochenek, A. Ecophysiological determinants of the Dynamics of Weed Seed Soil Bank. Post. Nauk Rol. 1998, 6, 83–100. [Google Scholar]
- Bochenek, A.; Gołaszewski, J.; Giełwanowska, I. Current Views on the Concept of Seed Dormancy. Post. Nauk Rol. 2009, 3–4, 127–136. [Google Scholar]
- Grzesiuk, S.; Łuczyńska, J. Physiological and biochemical transformations in seeds during the rest and storage. Zesz. Probl. Postęp. Nauk Rol. 1971, 113, 69–96. [Google Scholar]
- Baskin, C.; Baskin, J.M. Seed Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014; pp. 150–162. [Google Scholar]
- Zemanek, J. The Control of Silky Bent and Dicotyledonous Weeds in Cereal Crops. In Wheat Documenta; CIBA-GEIGY: Basle, Switzerland, 1980; pp. 46–49. [Google Scholar]
- Holzner, W.; Hayashi, I.; Glauninger, J. Reproductive Strategy of Annual Agrestals. In Biology and Ecology of Weeds; Springer: Dordrecht, The Netherlands, 1982; pp. 111–121. [Google Scholar]
- Warwick, S.I.; Blacki, L.D.; Zilkey, B.F. Biology of Canadian Weeds. 72. Apera spica-venti. Can. J. Plant Sci. 1985, 65, 711–721. [Google Scholar] [CrossRef]
- Kukowski, T. Investigations on the Ecology and Control of Loose Silky-Bent (Apera spica-venti (L.) P.B.). In Winter Wheat; Prace Opolskiego Towarzystwa Przyjaciół Nauk, Ed.; PWN: Warszawa, Poland, 1978. [Google Scholar]
- Hanelt, V.P. Ökologische Und Systematische Aspekte Der Lebensdauer von Samen. Biol. Rundsch. 1977, 15, 81–91. [Google Scholar]
- Auškalnienė, O.; Kadžienė, G.; Stefanovičienė, R.; Jomantaitė, B. Development of Herbicides Resistance in Apera spica-venti in Lithuania. Zemdirbyste-Agriculture 2020, 107, 99–104. [Google Scholar] [CrossRef]
- Adamczewski, K.; Matysiak, K.; Kierzek, R.; Kaczmarek, S. Significant Increase of Weed Resistance to Herbicides in Poland. J. Plant Prot. Res. 2019, 59, 139–150. [Google Scholar] [CrossRef]
- Babineau, M.; Mathiassen, S.K.; Kristensen, M.; Kudsk, P. Fitness of ALS-Inhibitors Herbicide Resistant Population of Loose Silky Bentgrass (Apera spica-venti). Front. Plant Sci. 2017, 8, 1660. [Google Scholar] [CrossRef]
- Massa, D.; Kaiser, Y.; Andújar-Sánchez, D.; Carmona-Alférez, R.; Mehrtens, J.; Gerhards, R. Development of a Geo-Referenced Database for Weed Mapping and Analysis of Agronomic Factors Affecting Herbicide Resistance in Apera spica-venti L. Beauv. (Silky Windgrass). Agronomy 2013, 3, 13–27. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of Seed Dormancy and Germination Mechanisms in a Changing Environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Knapowski, T.; Barczak, B.; Kozera, W.; Wszelaczynska, E.; Poberezny, J. Crop Stimulants as a Factor Determining the Yield and Quality of Winter Wheat Grown in Notec Valley, Poland. Curr. Sci. 2019, 116, 1009–1015. [Google Scholar] [CrossRef]
- Sikorska, A.; Gugała, M.; Zarzecka, K.; Domański, Ł. The Impact of Foliar Application of Biostimulators on the Morphological Characteristics of the Leaf Rosette of Winter Rape Plants. Agron. Sci. 2022, 77, 5–14. [Google Scholar] [CrossRef]
- Sikorska, A.; Gugała, M.; Zarzecka, K.; Domański, Ł.; Mystkowska, I. Morphological Features of Winter Rape Cultivars Depending on the Applied Growth Stimulators. Agriculture 2022, 12, 1747. [Google Scholar] [CrossRef]
- Kozak, M.; Wondołowska-Grabowska, A.; Serafin-Andrzejewska, M.; Gniadzik, M.; Kozak, M.K. Biostimulants—Yesterday, Today and Tomorrow. In Agriculture of the 21st Century—Problems and Challenges; Idea Knowledge Future: Wrocław, Poland, 2016; pp. 114–122. [Google Scholar]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of Non-Microbial Biostimulants in Regulation of Seed Germination and Seedling Establishment. Plant Growth Regul. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj/eng (accessed on 3 March 2025).
- Al-Juthery, H.W.A.; Abbas Drebee, H.; Al-Khafaji, B.M.K.; Hadi, R.F. Plant Biostimulants, Seaweeds Extract as a Model (Article Review). IOP Conf. Ser. Earth Environ. Sci. 2020, 553, 012015. [Google Scholar] [CrossRef]
- Mystkowska, I.T. Biostimulators as a Factor Affecting the Yield of Edible Potato. Acta Agrophys. 2018, 25, 307–315. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of Seaweeds on Agricultural Crop Production as Biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Durand, N.; Briand, X.; Meyer, C. The Effect of Marine Bioactive Substances (N PRO) and Exogenous Cytokinins on Nitrate Reductase Activity in Arabidopsis Thaliana. Physiol. Plant. 2003, 119, 489–493. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Isolation and Identification of Cytokinins in a New Commercial Seaweed Product Made from Fucus serratus L. J. Appl. Phycol. 1997, 9, 327–330. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Plant Growth Regulators Can Enhance the Recovery of Kentucky Bluegrass Sod from Heat Injury. Crop Sci. 2003, 43, 952. [Google Scholar] [CrossRef]
- Mansour, H.A.; Nofal, O.A.; Gaballah, M.S.; El-Nasharty, A.B. Impact of Algae Extract Foliar Application on Two Wheat Varieties with Using Two Irrigation Systems. Biosci. Res. 2019, 16, 356–366. [Google Scholar]
- Matysiak, K.; Miziniak, W. Assessment of the Biostimulative Effect of Lithovit and Kelpak on Selected Biometric and Qualitative Features of Winter Oilseed Rape. Prog. Plant Prot. 2019, 59, 188–195. [Google Scholar] [CrossRef]
- Deepak, R.; Sangita, Y.; Bhanu, V. Effect of silicon on plants under abiotic stress condition—A review. Scientist. Off. J. Sci. R Acad. 2022, 1, 412–426. [Google Scholar]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Bacchus, G.L. An Evaluation of the of Biodynamic Practices Foliar-Applied Silica Spray on Nutrient Quality of Organic and Conventionally Fertilized Lettuce (Lactuca sativa L.). J. Org. Syst. 2010, 5, 4–13. [Google Scholar]
- Brogowski, Z. Silicon in Soil and Its Role in Plant Nutrition. Post. Nauk. Rol. 2000, 6, 9–16. [Google Scholar]
- Kulkarni, M.G.; Gupta, S.; Ngoroyemoto, N.; Stirk, W.A.; Van Staden, J. Smoke, Seaweed Extracts, and Vermicompost Leachates—Classical Natural Plant Biostimulants. In Biostimulants for Crops from Seed Germination to Plant Development; Elsevier: Amsterdam, The Netherlands, 2021; pp. 73–85. [Google Scholar]
- Layek, J.; Das, A.; Idapuganti, R.G.; Sarkar, D.; Ghosh, A.; Zodape, S.T.; Lal, R.; Yadav, G.S.; Panwar, A.S.; Ngachan, S.; et al. Seaweed Extract as Organic Bio-Stimulant Improves Productivity and Quality of Rice in Eastern Himalayas. J. Appl. Phycol. 2018, 30, 547–558. [Google Scholar] [CrossRef]
- Lejman, A.; Ogórek, R.; Parylak, D. The Influence of the Habitat on the Chemical Composition and Morphology of Silky Bent Grass (Apera spica-venti (L.) Beauv.) Occurring in Arable Fields (Lower Silesia, Poland). Agronomy 2022, 12, 1883. [Google Scholar] [CrossRef]
- FNA Editorial Committee. Flora of North America North of Mexico; 24 Poaceae (part 1); Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- ISTA Purity Committee. ISTA Universal List. Description by Deborah Meyer California Department of Food & Agriculture. 2014. Available online: https://www.seedtest.org/en/services-header/tools/purity-committee/universal-list-species.html (accessed on 3 March 2025).
- Morales, W.R.; Vanbellinghen, C.; Bodson, B.; Henriet, F. Study of factors influencing the germination of Apera spica-venti (L.) P. Beauv. in controlled conditions. Commun. Agric. Appl. Biol. Sci. 2013, 78, 701–706. [Google Scholar] [PubMed]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Bliss, C.I. The method of probits. Science 1934, 79, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.K. Effect of Light Environment during Soil Disturbance on Germination and Emergence Pattern of Weeds. Ann. Appl. Biol. 1995, 127, 561–571. [Google Scholar] [CrossRef]
- Bochenek, A. Impact of Biotic Factors and Cultivation Treatment on Weed Seed Bank in the Soil. Post. Nauk Rol. 2000, 2, 19–29. [Google Scholar]
- Tester, M.; Morris, C. The Penetration of Light through Soil. Plant Cell Environ. 1987, 10, 281–286. [Google Scholar] [CrossRef]
- Gutterman, Y. Maternal Effects on Seeds during Development. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 59–84. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Chen, J.; Clark, D.; Perez, H.; Huo, H. Effects of Maternal Environment on Seed Germination and Seedling Vigor of Petunia × Hybrida under Different Abiotic Stresses. Plants 2021, 10, 581. [Google Scholar] [CrossRef]
- Chala, B.; Bekana, G. Review on seed process and storage condition in relation to seed moisture and ecological factor. J. Nat. Sci. Res. 2017, 7, 84–90. [Google Scholar]
- Gebeyehu, B. Review on: Effect of Seed Storage Period and Storage Environment on Seed Quality. Int. J. Appl. Agric. Sci. 2020, 6, 185–190. [Google Scholar] [CrossRef]
- Holm, R.E. Volatile Metabolites Controlling Germination in Buried Weed Seeds. Plant Physiol. 1972, 50, 293–297. [Google Scholar] [CrossRef]
- Górnik, K.; Grzesik, M. Genetic, Environmental and Maternal Factors Affecting the Quality of Seeds. Post. Nauk Rol. 1998, 5, 37–48. [Google Scholar]
- Hejcman, M.; Křišťálová, V.; Červená, K.; Hrdličková, J.; Pavlů, V. Effect of Nitrogen, Phosphorus and Potassium Availability on Mother Plant Size, Seed Production and Germination Ability of Rumex crispus. Weed Res. 2012, 52, 260–268. [Google Scholar] [CrossRef]
- Sánchez, J.; Albornoz, F.; Contreras, S. High Nitrogen Fertilization Decreases Seed Weight but Increases Longevity in Tomato Seeds. Horticulturae 2022, 8, 942. [Google Scholar] [CrossRef]
- Kristó, I.; Vályi-Nagy, M.; Rácz, A.; Irmes, K.; Szentpéteri, L.; Jolánkai, M.; Kovács, G.P.; Fodor, M.Á.; Ujj, A.; Valentinyi, K.V.; et al. Effects of Nutrient Supply and Seed Size on Germination Parameters and Yield in the Next Crop Year of Winter Wheat (Triticum aestivum L.). Agriculture 2023, 13, 419. [Google Scholar] [CrossRef]
- Contreras, S.; Molina, J.; García, M.; Sánchez, J.; Chorbadjian, R.A.; Fuentes, F.; Albornoz, F. Seeds yield and quality of quinoa (Chenopodium quinoa Willd.) plants grown under different nitrogen fertilization doses. Int. J. Agric. Nat. Resour. 2024, 51, 68–74. [Google Scholar] [CrossRef]
- Kapczyńska, A. Effect of seed age and fertilization on the growth and decorative quality of selected ornamental grasses. Folia Hortic. 2012, 24, 73–80. [Google Scholar] [CrossRef]
- Gasque, M.; García-Fayos, P. Seed dormancy and longevity in Stipa tenacissima L. (Poaceae). Plant Ecol. 2003, 168, 279–290. [Google Scholar] [CrossRef]
- Austin, R.B. The Growth of Watercress (Rorippa nasturtium aquaticum (L) Hayek) from Seed as Affected by the Phosphorus Nutrition of the Parent Plant. Plant Soil 1966, 24, 113–120. [Google Scholar] [CrossRef]
- Porter, M.A.; Paulsen, G.M. Grain Protein Response to Phosphorus Nutrition of Wheat. Agron. J. 1983, 75, 303–305. [Google Scholar] [CrossRef]
- Harrington, J.F. Germination of Seeds from Carrot, Lettuce, and Pepper Plants Grown under Severe Nutrient Deficiencies. Hilgardia 1960, 30, 219–235. [Google Scholar] [CrossRef]
- Zhu, Y. Influence of Nitrogen and Phosphorus Fertilization on Quality and Germination Potential of Smooth Bromegrass Seed. Int. J. Agric. Biol. 2018, 20, 361–368. [Google Scholar] [CrossRef]
- Mutum, L.; Janda, T.; Darkó, É.; Szalai, G.; Hamow, K.Á.; Molnár, Z. Outcome of Microalgae Biomass Application on Seed Germination and Hormonal Activity in Winter Wheat Leaves. Agronomy 2023, 13, 1088. [Google Scholar] [CrossRef]
- Karbarz, M.; Piziak, M.; Żuczek, J.; Duda, M. Influence of Microalgae Planktochlorella Nurekis Clones on Seed Germination. Agronomy 2022, 13, 9. [Google Scholar] [CrossRef]
- Rupawalla, Z.; Shaw, L.; Ross, I.L.; Schmidt, S.; Hankamer, B.; Wolf, J. Germination Screen for Microalgae-Generated Plant Growth Biostimulants. Algal Res. 2022, 66, 102784. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Moncada, A. Influence of Ecklonia Maxima Extracts on Growth, Yield, and Postharvest Quality of Hydroponic Leaf Lettuce. Horticulturae 2021, 7, 440. [Google Scholar] [CrossRef]
- Lewandowska, S.; Michalak, I.; Niemczyk, K.; Detyna, J.; Bujak, H.; Arik, P. Influence of the Static Magnetic Field and Algal Extract on the Germination of Soybean Seeds. Open Chem. 2019, 17, 516–525. [Google Scholar] [CrossRef]
- Di Stasio, E.; Rouphael, Y.; Colla, G.; Raimondi, G.; Giordano, M.; Pannico, A.; El-Nakhel, C.; De Pascale, S. The Influence of Ecklonia Maxima Seaweed Extract on Growth, Photosynthetic Activity and Mineral Composition of Brassica rapa L. Subsp. sylvestris under Nutrient Stress Conditions. Eur. J. Hortic. Sci. 2018, 82, 286–293. [Google Scholar] [CrossRef]
- Tuchy, Ł.; Chowańska, J.; Chojnacka, K. Seaweed extracts as biostimulants of plant growth: Review. Chemik 2013, 67, 636–641. [Google Scholar]
- Sawicka, B.; Barbaś, P.; Dąbek-Gad, M. The problem of weed infestation in conditions of applying the growth bioregulators and foliar fertilization in potato cultivation. Nauka Przyr. Technol. 2011, 5, 9. [Google Scholar]
- Maziarek, A.; Parylak, D.; Wacławowicz, R. The Effect of Biostymulants and Stubble Crop on Weed Infestation of Short-Term Spring Wheat Monoculture. Prog. Plant Prot. 2015, 55, 170–176. [Google Scholar] [CrossRef]
- Wawrzyniak, M.K.; Michalak, M.; Chmielarz, P. Effect of Different Conditions of Storage on Seed Viability and Seedling Growth of Six European Wild Fruit Woody Plants. Ann. For. Sci. 2020, 77, 58. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Effects of NAA and Ecklonia maxima Extracts on Lettuce and Tomato Transplant Production. Agronomy 2022, 12, 329. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; González-González, M.F.; Velasco-Ramírez, A.P.; Velasco-Ramírez, S.F.; Santacruz-Ruvalcaba, F.; Zamora-Natera, J.F. Seaweed Extract Components Are Correlated with the Seeds Germination and Growth of Tomato Seedlings. Seeds 2023, 2, 436–448. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, J.; Li, M.; Li, Y.; Zhou, P.; Wang, Q.; Sun, Y.; Zhu, G.; Wang, Q.; Zhang, P.; et al. Effect of Silica-Based Nanomaterials on Seed Germination and Seedling Growth of Rice (Oryza sativa L.). Nanomaterials 2022, 12, 4160. [Google Scholar] [CrossRef]
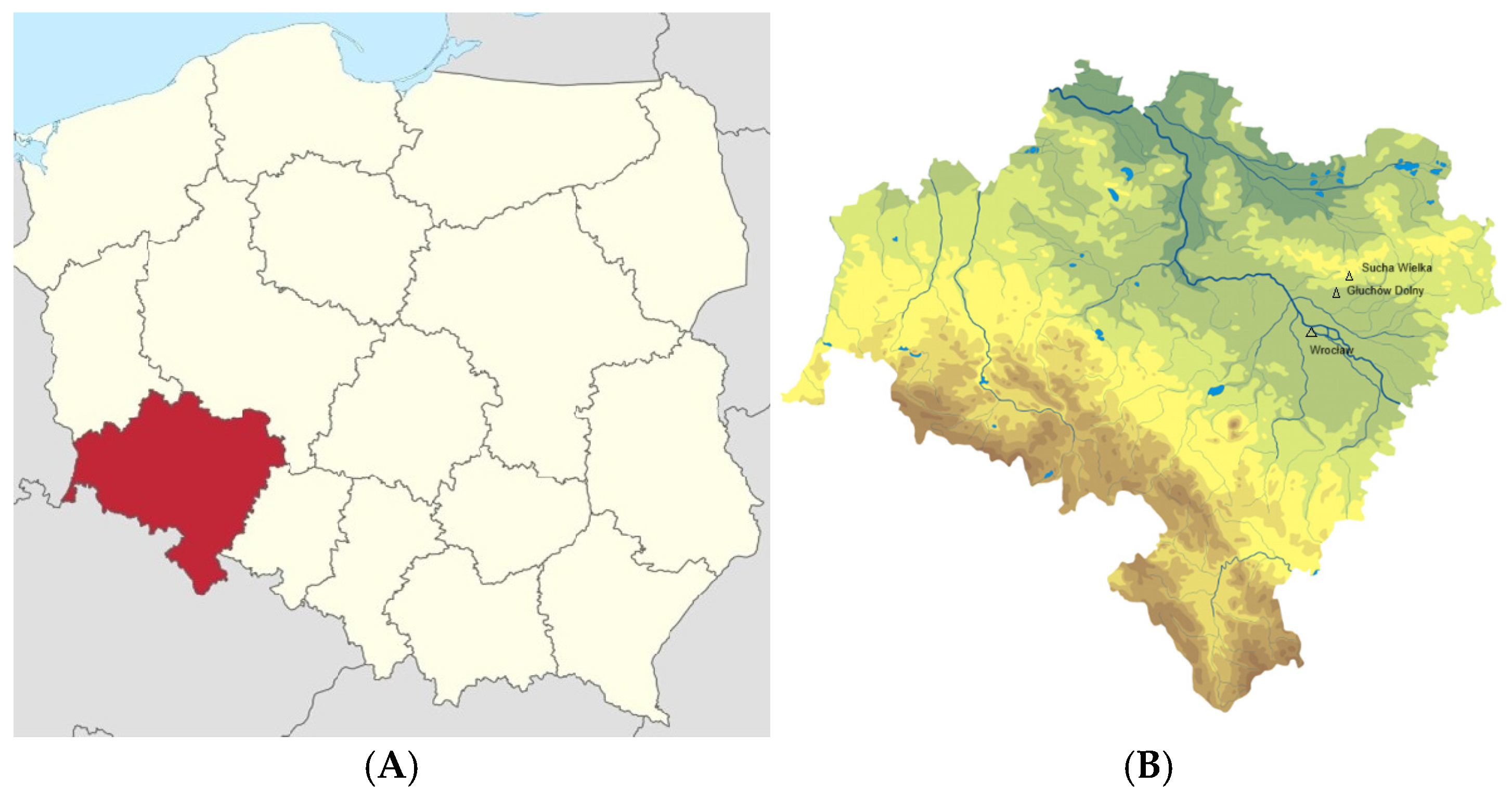
| Study Sites | Locality | Geographical Latitudes | Year of Study | ||
|---|---|---|---|---|---|
| 2016 | 2017 | 2019 | |||
| I | Wrocław | 51.11, 17.14 | x winter wheat | x winter wheat | |
| II | Wrocław | 51.11, 17.14 | x winter wheat | x winter wheat | |
| III | Sucha Wielka | 51.32, 17,16 | x winter wheat | x winter wheat | |
| IV | Wrocław | 51.11, 17.14 | x rye | x rye | |
| V | Głuchów Dolny | 51.28, 17.12 | x winter wheat | x winter wheat | |
| Variants | Diaspores Age | Type of Substance | Night/Day | Humidity |
|---|---|---|---|---|
| Variant 1 | diaspores 3 months after harvest annual and multi-year diaspores | Distilled water | Night 14 h/10 °C Day 10 h/15 °C | 70% humidity |
| Variant 2 | diaspores 3 months after harvest annual and multi-year diaspores | Biostimulants | Night 14 h/10 °C Day 10 h/15 °C | 70% humidity |
| Lp. | Study Sites 1 | 2016 | 2017 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|
| I 1 | A 2016 | 20.00 BC3 a4 | - | 39.75 A b | 34.50 AB a | 12.75 C a |
| V | B 2016 | 8.75 B a | - | 55.75 A a | 51.75 A a | 13.75 B a |
| III | C 2017 | - 2 | 5.75 B a | 31.75 A b | 32.50 A b | 9.25 B a |
| IV | D 2017 | - | 15.25 B a | 45.00 A a | 39.50 A ab | 11.00 B a |
| II | E 2017 | - | 16.00 B a | 56.25 A a | 50.25 A a | 20.50 B a |
| I | A 2019 | - | - | 15.25 B b | 26.50 A a | 18.50 AB abc |
| V | B 2019 | - | - | 25.00 B a | 44.00 A a | 26.50 B ab |
| III | C 2019 | - | - | 9.50 A bc | 12.50 A a | 10.00 A c |
| IV | D 2019 | - | - | 28.25 A a | 22.25 A a | 31.50 A a |
| II | E 2019 | - | - | 6.00 A c | 12.00 A a | 13.50 A bc |
| Pearson (r) | Diaspores | |||||
|---|---|---|---|---|---|---|
| Harvest Year | 1-Year | 2-Year | 3-Year | 4-Year | ||
| Soil | N | −0.25 | 0.28 | −0.42 | 0.69 | 0.72 |
| P | 0.26 | −0.28 | 0.05 | 0.41 | 0.44 | |
| K | −0.15 | −0.23 | 0.20 | 0.67 | 0.95 | |
| pH | 0.17 | −0.34 | −0.36 | −0.57 | −0.09 | |
| Study Sites | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Control | E. maxima | Control | E. maxima | Control | E. maxima | |
| A 2019 | 15.25 B1 a2 | 20.00 B a | 26.50 A a | 13.50 ABC b | 18.50 ABC b | 29.00 B a |
| B 2019 | 25.00 A b | 34.25 A a | 44.00 A a | 17.75 AB b | 26.50 AB b | 48.75 A a |
| C 2019 | 9.50 BC a | 11.50 C a | 12.50 A a | 8.75 BC a | 10.00 C b | 31.50 B a |
| D 2019 | 28.25 A b | 44.25 A a | 22.25 A a | 25.50 A a | 31.50 A a | 37.50 AB a |
| E 2019 | 6.00 C b | 16.50 BC a | 12.00 A a | 4.50 C b | 13.50 BC a | 7.50 C a |
| Study Sites | 2019 | 2020 | 2021 | |||
|---|---|---|---|---|---|---|
| Control | Silicon | Control | Silicon | Control | Silicon | |
| A 2019 | 15.25 B1 b2 | 24.50 B a | 26.50 A a | 2.50 CD b | 18.50 ABC b | 36.25 B a |
| B 2019 | 25.00 A b | 46.00 A a | 44.00 A a | 0.00 D b | 26.50 AB b | 54.00 A a |
| C 2019 | 9.50 BC a | 12.50 C a | 12.50 A b | 20.50 B a | 10.00 C b | 41.00 B a |
| D 2019 | 28.25 A b | 52.00 A a | 22.25 A a | 37.00 A a | 31.50 A b | 55.50 A a |
| E 2019 | 6.00 C b | 21.75 BC a | 12.00 A a | 7.50 C a | 13.50 BC a | 11.00 C a |
| Pearson (r) | Diaspores | |||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | ||
| Soil | N | −0.45 | −0.61 | −0.29 |
| P | −0.13 | −0.18 | −0.64 | |
| K | −0.56 | −0.62 | −0.70 | |
| pH | −0.15 | −0.14 | −0.15 | |
| Pearson (r) | Diaspores | |||
|---|---|---|---|---|
| 2019 | 2020 | 2021 | ||
| Soil | N | −0.32 | −0.97 | −0.55 |
| P | −0.15 | −0.17 | −0.58 | |
| K | −0.52 | −0.62 | −0.79 | |
| pH | −0.08 | −0.71 | −0.28 | |
| Study Sites | 2019 | 2020 | 2021 |
|---|---|---|---|
| A 2019 | 12.90 A1 a2 | 11.88 A ab | 8.49 AB b |
| B 2019 | 10.87 A a | 11.39 A a | 7.25 BC b |
| C 2019 | 9.59 A a | 10.63 A a | 6.55 C a |
| D 2019 | 12.09 A a | 12.40 A a | 9.18 A a |
| E 2019 | 11.75 A a | 12.01 A a | 8.11 AB b |
| Study Sites | 2019 | 2020 | 2021 |
|---|---|---|---|
| A 2019 | 11.07 B1 a2 | 7.15 C c | 9.59 AB b |
| B 2019 | 10.59 B a | 0.00 D c | 9.03 B b |
| C 2019 | 9.08 C a | 9.47 B a | 9.33 AB a |
| D 2019 | 12.80 A a | 9.33 B b | 10.10 A b |
| E 2019 | 13.56 A a | 13.49 A a | 10.02 A b |
| Study Sites | 2019 | 2020 | 2021 |
|---|---|---|---|
| A 2019 | 10.91 AB1 a2 | 8.75 A b | 10.69 AB a |
| B 2019 | 9.80 B a | 6.68 B b | 9.43 C a |
| C 2019 | 9.66 B a | 7.87 AB b | 9.32 C ab |
| D 2019 | 11.84 AB a | 9.34 A b | 10.28 B ab |
| E 2019 | 12.78 A a | 8.11 AB b | 11.36 A a |
| Study Sites | 2019 | 2020 | 2021 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Silicon | E. maxima | Control | Silicon | E. maxima | Control | Silicon | E. maxima | |
| A 2019 | 12.90 a1 | 11.07 a | 10.91 a | 11.39 a | 0.00 c | 6.68 b | 8.49 c | 9.59 b | 10.64 a |
| B 2019 | 10.87 a | 10.59 a | 9.80 a | 12.40 a | 9.34 b | 9.35 b | 7.25 b | 9.03 a | 9.43 a |
| C 2019 | 9.59 a | 9.08 a | 9.66 a | 10.63 a | 9.47 a | 7.87 b | 6.55 b | 9.32 a | 9.38 a |
| D 2019 | 12.10 a | 12.80 a | 11.43 a | 11.88 a | 7.15 c | 8.75 b | 9.18 b | 10.10 a | 10.28 a |
| E 2019 | 11.75 b | 13.56 a | 12.78 ab | 12.01 a | 13.49 a | 8.11 b | 8.10 b | 10.03 a | 11.36 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejman, A. The Relationship Between the Germination of Silky Bent Grass (Apera spica-venti (L.) Beauv.) Diaspores and Their Age, Place of Occurrence, and Action of Stimulating Substances. Agronomy 2025, 15, 715. https://doi.org/10.3390/agronomy15030715
Lejman A. The Relationship Between the Germination of Silky Bent Grass (Apera spica-venti (L.) Beauv.) Diaspores and Their Age, Place of Occurrence, and Action of Stimulating Substances. Agronomy. 2025; 15(3):715. https://doi.org/10.3390/agronomy15030715
Chicago/Turabian StyleLejman, Agnieszka. 2025. "The Relationship Between the Germination of Silky Bent Grass (Apera spica-venti (L.) Beauv.) Diaspores and Their Age, Place of Occurrence, and Action of Stimulating Substances" Agronomy 15, no. 3: 715. https://doi.org/10.3390/agronomy15030715
APA StyleLejman, A. (2025). The Relationship Between the Germination of Silky Bent Grass (Apera spica-venti (L.) Beauv.) Diaspores and Their Age, Place of Occurrence, and Action of Stimulating Substances. Agronomy, 15(3), 715. https://doi.org/10.3390/agronomy15030715






