Belowground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Soil Water Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Shoot and Root Sample Collection
2.3. Paraffin Sections
2.4. Statistical Analysis
3. Results
3.1. Root and Shoot Biomass
3.2. Traits and Adjustments of Bromus valdivianus and Lolium perenne
3.3. Canonical Variate Analysis of Anatomical Root Structures
| Root Diameter | Stele Diameter | Epidermis Thickness | Cortex Thickness | Tracheary Element | Xylem Diameter | Pericycle Thickness | Endodermis Thickness | Endodermis Wall | Pith Thickness | Pith Wall | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||||
| Bv | 441.25 ± 10.13 | 199.01 ± 2.56 | 15.48 ± 0.96 | 105.45 ± 3.73 | 20.45 ± 0.39 | 6.82 ± 0.19 | 11.99 ± 0.57 | 10.22 ± 0.35 | 2.55 ± 0.14 | 32.03 ± 1.50 | 4.24 ± 0.15 |
| Lp | 325.55 ± 13.01 | 146.54 ± 4.83 | 8.07 ± 0.62 | 69.86 ± 4.05 | 14.76 ± 0.84 | 7.96 ± 0.19 | 7.89 ± 0.46 | 5.56 ± 0.40 | 2.05 ± 0.08 | 53.36 ± 2.89 | 3.72 ± 0.14 |
| Significance | *** | *** | *** | *** | *** | ** | *** | *** | * | *** | *** |
| Soil water content | |||||||||||
| 80–85% FC | 392.03 ± 16.69 | 176.60 ± 7.32 | 12.23 ± 0.71 | 88.25 ± 5.44 | 16.88 ± 0.99 | 6.88 ± 0.22 | 10.63 ± 0.72 | 8.12 ± 0.69 | 2.34 ± 0.11 | 44.96 ± 3.11 | 3.73 ± 0.12 |
| 20–25% FC | 374.76 ± 14.61 | 168.95 ± 4.29 | 11.33 ± 1.42 | 87.06 ± 4.98 | 18.33 ± 0.66 | 7.89 ± 0.18 | 9.26 ± 0.48 | 7.66 ± 0.41 | 2.25 ± 0.13 | 40.43 ± 3.66 | 4.23 ± 0.14 |
| Significance | ns | ns | ns | ns | ns | ** | * | ** | ns | ns | * |
| Species×Soil water content | |||||||||||
| Bv × 80–85% FC | 455.50 ± 13.70 | 209.79 ± 2.34 a | 14.93 ± 0.98 | 108.06 ± 4.69 | 20.83 ± 0.59 a | 5.79 ± 0.16 b | 13.63 ± 0.68 a | 11.30 ± 0.37 a | 2.64 ± 0.16 | 30.36 ± 1.62 c | 3.73 ± 0.17 |
| Bv × 20–25% FC | 427.00 ± 14.39 | 188.23 ± 2.11 b | 16.04 ± 1.55 | 102.84 ± 5.68 | 20.07 ± 0.50 a | 7.86 ± 0.23 a | 10.36 ± 0.66 b | 9.14 ± 0.36 b | 2.46 ± 0.24 | 33.69 ± 2.48 c | 4.75 ± 0.16 |
| Lp × 80–85% FC | 328.57 ± 20.64 | 143.41 ± 8.50 c | 9.52 ± 0.75 | 68.43 ± 6.12 | 12.94 ± 1.20 c | 7.98 ± 0.32 a | 9.63 ± 0.75 c | 4.94 ± 0.61 d | 2.05 ± 0.09 | 59.56 ± 3.71 a | 3.74 ± 0.14 |
| Lp × 20–25% FC | 322.53 ± 15.98 | 149.66 ± 4.91 c | 6.62 ± 0.29 | 71.28 ± 5.50 | 16.58 ± 1.09 b | 7.93 ± 0.24 a | 8.16 ± 0.55 c | 6.19 ± 0.52 c | 2.04 ± 0.12 | 47.16 ± 4.69 b | 3.71 ± 0.19 |
| Significance | ns | ** | ns | ns | * | ** | ** | *** | ns | * | ns |
| Root Diameter | Stele Diameter | Epidermis Thickness | Cortex Thickness | Tracheary Element | Xylem Diameter | Pericycle Thickness | Endodermis Thickness | Endodermis Wall | Pith Thickness | Pith Wall | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | |||||||||||
| Bv | 403.90 ± 12.88 | 158.86 ± 2.72 | 13.13 ± 0.44 | 108.66 ± 4.14 | 18.23 ± 0.27 | 5.49 ± 0.13 | 12.07 ± 0.46 | 9.62 ± 0.37 | 0.89 ± 0.09 | 45.16 ± 2.17 | 5.14 ± 0.10 |
| Lp | 293.86 ± 9.32 | 125.26 ± 6.83 | 11.73 ± 0.52 | 73.48 ± 2.95 | 10.52 ± 1.00 | 7.55 ± 0.28 | 8.59 ± 0.38 | 6.50 ± 0.41 | 1.36 ± 0.13 | 44.65 ± 2.62 | 4.83 ± 0.12 |
| Significance | *** | *** | ns | *** | *** | *** | *** | *** | * | ns | ns |
| Soil water content | |||||||||||
| 80–85% FC | 398.59 ± 14.17 | 159.33 ± 4.54 | 14.13 ± 0.48 | 106.34 ± 4.50 | 15.98 ± 0.75 | 6.60 ± 0.27 | 11.31 ± 0.56 | 9.35 ± 0.45 | 1.21 ± 0.12 | 46.97 ± 2.27 | 4.77 ± 0.10 |
| 20–25% FC | 299.18 ± 8.48 | 124.79 ± 5.13 | 10.73 ± 0.65 | 75.80 ± 3.02 | 12.77 ± 1.02 | 6.44 ± 0.25 | 9.36 ± 0.38 | 6.77 ± 0.38 | 1.04 ± 0.12 | 42.84 ± 2.49 | 5.21 ± 0.11 |
| Significance | *** | *** | ns | *** | *** | ns | ** | *** | ns | ns | * |
| Species × Soil water content | |||||||||||
| Bv × 80–85% FC | 473.7 ± 9.4 a | 168.2 ± 2.41 a | 12.02 ± 0.64 | 130.2 ± 3.62 a | 18.45 ± 0.30 a | 5.91 ± 0.19 b | 13.30 ± 0.63 | 10.87 ± 0.42 | 1.11 ± 0.11 | 51.52 ± 2.91 | 4.64 ± 0.11 b |
| Bv × 20–25% FC | 334.1 ± 7.2 b | 149.5 ± 4.49 b | 14.25 ± 0.45 | 87.1 ± 2.45 b | 18.01 ± 0.54 a | 5.07 ± 0.17 c | 10.85 ± 0.32 | 8.37 ± 0.30 | 0.68 ± 0.15 | 38.79 ± 2.85 | 5.65 ± 0.13 a |
| Lp × 80–85% FC | 323.5 ± 14.8 b | 150.4 ± 11.34 b | 16.25 ± 0.43 | 82.5 ± 3.89 b | 13.51 ± 1.78 b | 7.29 ± 0.51 a | 9.32 ± 0.71 | 7.84 ± 0.66 | 1.30 ± 0.33 | 42.43 ± 3.05 | 4.89 ± 0.17 b |
| Lp × 20–25% FC | 264.28 ± 7.6 c | 100.1 ± 4.02 c | 7.21 ± 0.77 | 64.5 ± 2.86 c | 7.53 ± 0.22 c | 7.81 ± 0.22 a | 7.87 ± 0.37 | 5.16 ± 0.45 | 1.41 ± 0.13 | 46.88 ± 3.71 | 4.77 ± 0.16 b |
| Significance | ** | ** | ns | ** | ** | * | ns | ns | ns | ns | ** |
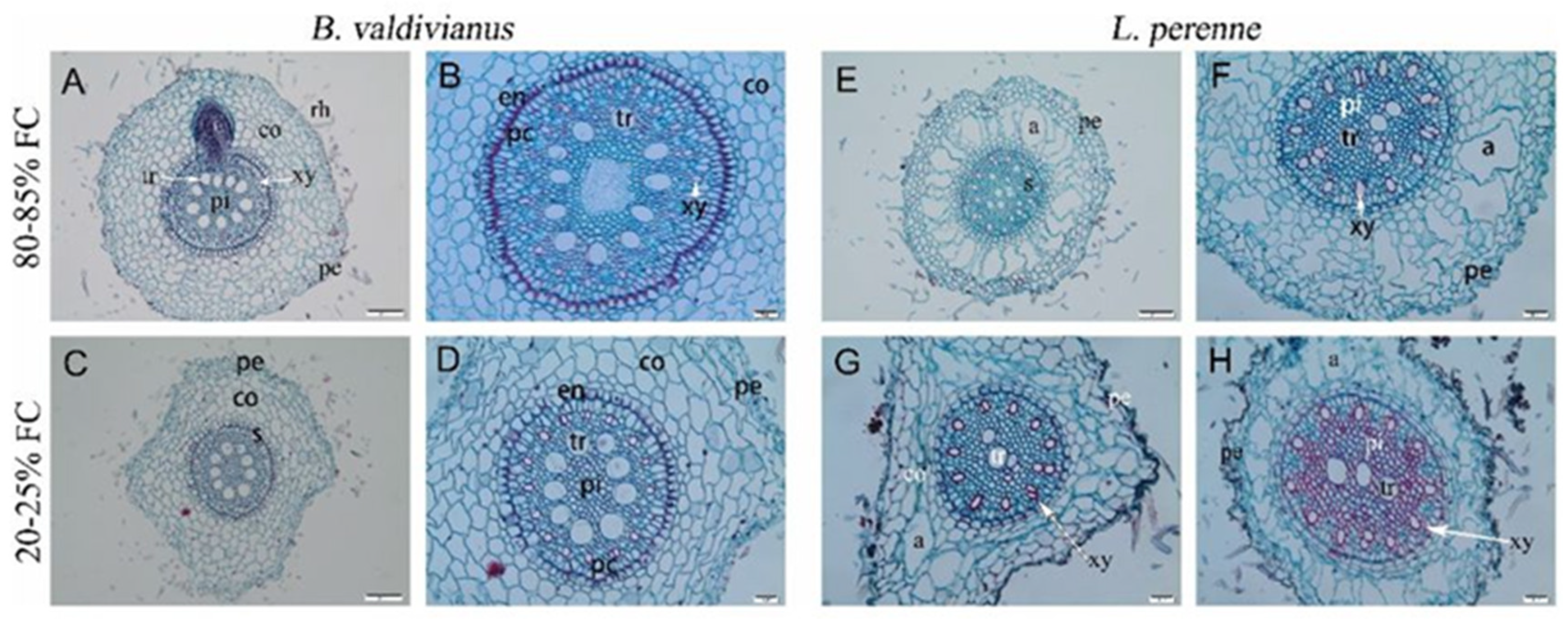
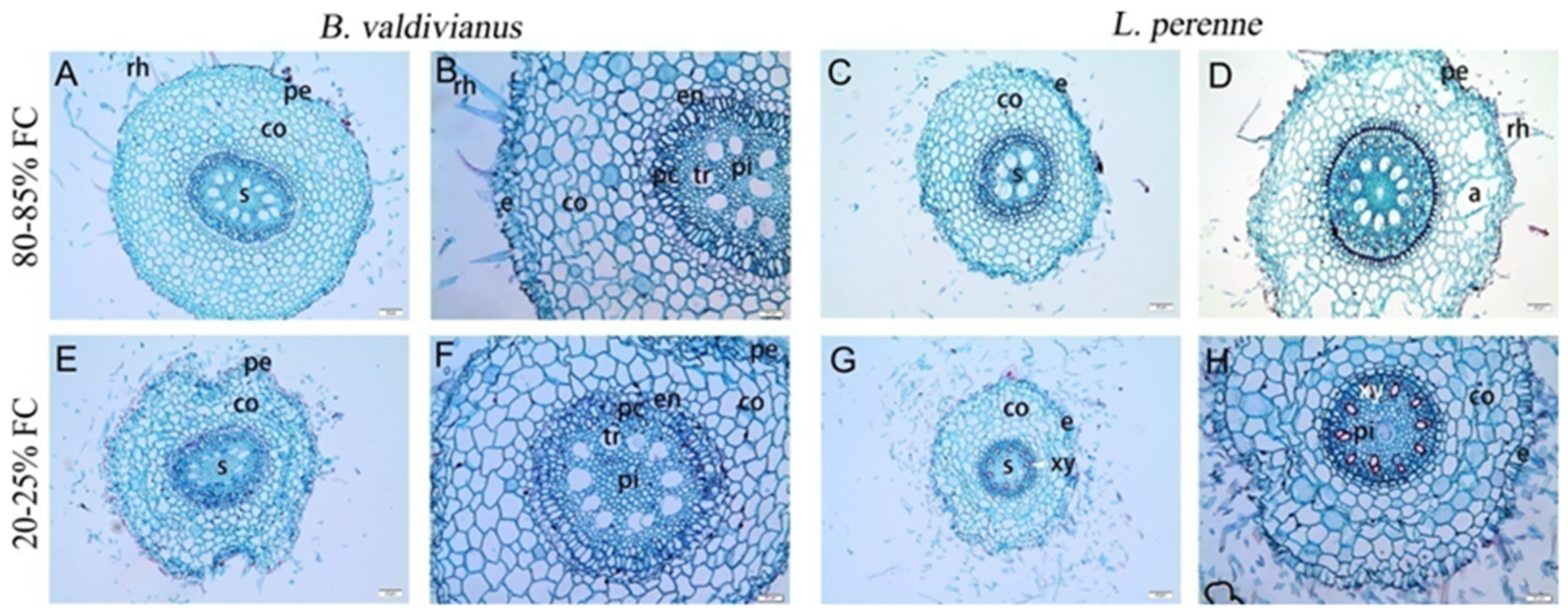


4. Discussion
4.1. Morpho-Anatomical Root Traits and Adjustments to Water
4.2. Modification of Morpho-Anatomical Traits of the Root Relevant to Water Movement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Carbone, G.J.; Grego, J.M. Uncertainty and hotspots in 21st century projections of agricultural drought from CMIP5 models. Sci. Rep. 2019, 9, 4922–4934. [Google Scholar] [CrossRef]
- Cook, B.I.; Mankin, J.S.; Marvel, K.; Williams, A.P.; Smerdon, J.E.; Anchukaitis, K.J. Twenty-first century drought projections in the CMIP6 forcing scenarios. Earth’s Future 2020, 8, e2019EF001461. [Google Scholar] [CrossRef]
- Ukkola, A.M.; Kauwe, M.G.D.; Roderick, M.L.; Abramowitz, G.; Pitman, A.J. Robust future changes in meteorological drought in cmip6 projections despite uncertainty in precipitation. Geophys. Res. Lett. 2020, 47, e2020GL087820. [Google Scholar] [CrossRef]
- Ziervogel, G.; Ericksen, P.J. Adapting to climate change to sustain food security. Wiley Interdiscip. Rev. Clim. Change 2010, 1, 525–540. [Google Scholar] [CrossRef]
- Thornton, P.K.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Glob. Change Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Serrano, S.M.; Peña-Angulo, D.; Beguería, S.; Domínguez-Castro, F.; Tomás-Burguera, M.; Noguera, I.; Gimeno-Sotelo, L.; El Kenawy, A. Global drought trends and future projections. Phil. Trans. R. Soc. A 2022, 380, 20210285–20210308. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Kigel, J.; Pinchak, W.E. Water deficit, heat tolerance, and persistence of summer-dormant grasses in the U.S. Southern plains. Crop Sci. 2009, 49, 2363–2370. [Google Scholar] [CrossRef]
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.E.; Thornton, P.K.; Herrero, M. Impacts of climate change on the livestock food supply chain; a review of the evidence. Glob. Food Secur. 2021, 28, 100488–100505. [Google Scholar] [CrossRef]
- López, I.F.; Kemp, P.D.; Dörner, J.; Descalzi, C.A.; Balocchi, O.A.; García, S. Competitive strategies and growth of neighbouring Bromus valdivianus Phil. and Lolium perenne L. plants under water restriction. J. Agron. Crop Sci. 2013, 199, 449–459. [Google Scholar] [CrossRef]
- Makbul, S.; Güler, N.S.; Durmus, N.; Güven, S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Bot. 2011, 35, 369–377. [Google Scholar] [CrossRef]
- Saha, P.; Sade, N.; Arzani, A.; Wilhelmi, M.M.R.; Coe, K.M.; Li, B.; Blumwald, E. Effects of abiotic stress on physiological plasticity and water use of Setaria viridis (L.) P. Beauv. Plant Sci. 2016, 251, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef]
- Volaire, F. A unified framework of plant adaptive strategies to drought: Crossing scales and disciplines. Glob. Change Biol. 2018, 24, 2929–2938. [Google Scholar] [CrossRef]
- Polania, J.A.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective use of water and increased dry matter partitioned to grain contribute to yield of common bean improved for drought resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef]
- Stone, E.L.; Kalisz, P.J. On the maximum extent of tree roots. For. Ecol. Manag. 1991, 46, 59–102. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. The global biogeography of roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Peterson, R.L. Adaptations of root structure in relation to biotic and abiotic factors. Can. J. Bot. 1992, 70, 661–675. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 33, 215–225. [Google Scholar] [CrossRef] [PubMed]
- García-Favre, J.; López, I.F.; Cranston, L.M.; Donaghy, D.J.; Kemp, P.D. The growth response of pasture brome (Bromus valdivianus Phil.) to defoliation frequency under two soil-water restriction levels. Agronomy 2021, 11, 300. [Google Scholar] [CrossRef]
- Barkaouia, K.; Roumet, C.; Volaire, F. Mean root trait more than root trait diversity determines drought resilience in native and cultivated Mediterranean grass mixtures. Agric. Ecosyst. Environ. 2016, 231, 122–132. [Google Scholar] [CrossRef]
- Zwicke, M.; Picon-Cochard, C.; Morvan-Bertrand, A.; Prud’homme, M.-P.; Volaire, F. What functional strategies drive drought survival and recovery of perennial species from upland grassland? Ann. Bot. 2015, 116, 1001–1015. [Google Scholar] [CrossRef]
- Mostajeran, A.; Rahimi-Eichi, V. Drought stress effects on root anatomical characteristics of rice cultivars (Oryza saliva L.). Pak. J. Agric. Sci. 2008, 11, 2173–2183. [Google Scholar] [CrossRef]
- Labdelli, A.; Adda, A.; Halis, Y.; Soualem, S. Effects of water regime on the structure of roots and stems of durum wheat (Triticum durum Desf.). J. Bot. 2014, 2014, 703874. [Google Scholar] [CrossRef]
- Brent, D.B.; David, K.; Adam, J.L.; James, B. Evaluation of turf-type interspecific hybrids of meadow fescue with perennial ryegrass for improved stress tolerance. Crop Sci. 2014, 54, 355–365. [Google Scholar] [CrossRef]
- García-Favre, J.; Cranston, L.M.; López, I.F.; Poli, C.H.E.C.; Donaghy, D.J.; Caram, N.; Kemp, P.D. Pasture brome and perennial ryegrass characteristics that influence ewe lamb dietary preference during different seasons and periods of the day. Animal 2023, 17, 100865–100874. [Google Scholar] [CrossRef]
- Ordóñez, I.; López, I.F.; Kemp, P.D.; Descalzi, C.A.; Horn, R.; Zúñiga, F.; Dorota, D.; Dörner, J. Effect of pasture improvement managements on physical properties and water content dynamics of a volcanic ash soil in southern Chile. Soil Tillage Res. 2018, 178, 55–64. [Google Scholar] [CrossRef]
- López, I.; Balocchi, O.; Lailhacar, P.; Oyarzún, C. Characterization of the growing sites of six native and naturalized species in the Humid Dominion of Chile. Agro Sur 1997, 25, 62–80. [Google Scholar] [CrossRef]
- García-Favre, J.; López, I.F.; Cranston, L.M.; Donaghy, D.J.; Kemp, P.D.; Ordóñez, I.P. Functional contribution of two perennial grasses to enhance pasture production and drought resistance under a leaf regrowth stage defoliation criterion. J. Agron. Crop Sci. 2023, 209, 144–160. [Google Scholar] [CrossRef]
- Ordóñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J.; Dörner, J.; García-Favre, J.; Zhang, Y. A short-term effect of multi-species pastures and the plant’s physiological response on pasture growth. Eur. J. Agron. 2024, 159, 127232. [Google Scholar] [CrossRef]
- Ordóñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J.; Zhang, Y.; Herrmann, P. Response of Bromus valdivianus (pasture brome) growth and physiology to defoliation frequency based on leaf stage development. Agronomy 2021, 11, 2058. [Google Scholar] [CrossRef]
- Oliveira, B.A.; López, I.F.; Cranston, L.M.; Kemp, P.D.; Donaghy, D.J.; Dörner, J.; López-Villalobos, N.; García-Favre, J.; Ordóñez, I.P.; Van Hale, R. 18O isotopic labelling and soil water content fluctuations validate the hydraulic lift phenomena for C3 grass species in drought conditions. Plant Stress 2024, 11, 100414–100429. [Google Scholar] [CrossRef]
- Zhang, Y.; García-Favre, J.; Hu, H.; López, I.F.; Ordóñez, I.P.; Cartmill, A.D.; Kemp, P.D. Aboveground structural attributes and morpho-anatomical response strategies of Bromus valdivianus Phil. and Lolium perenne L. to severe soil water restriction. Agronomy 2023, 13, 2964. [Google Scholar] [CrossRef]
- Olmos, E.; Sanchez-Blanco, M.J.; Fernandez, T.; Alarcón, J.J. Subcellular effects of drought stress in Rosmarinus officinalis. Plant Biol. 2007, 9, 77–84. [Google Scholar] [CrossRef]
- Descalzi, C.A.; López, I.F.; Kemp, P.D.; Dörner, J.; Ordóñez, I. Pasture restoration improvement methods for temperate degraded pastures and consequences of the climatic seasonality on soil–pasture complex. J. Agron. Crop Sci. 2020, 206, 130–147. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Descalzi, C.; Balocchi, O.; López, I.; Kemp, P.; Dörner, J. Different soil structure and water conditions affect the growing response of Lolium perenne L. and Bromus valdivianus Phil. growing alone or in mixture. J. Soil Sci. Plant Nutr. 2018, 18, 617–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Calderón-Urrea, A.; Tian, C.; Bai, X.; Wei, J. Anatomical changes to protect organelle integrity account for tolerance to alkali and salt stress in Melilotus officinalis. Plant Soil 2016, 406, 327–340. [Google Scholar] [CrossRef]
- Ruizin, S.E. Plant Micro Technique and Microscopy; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Jobson, J.D. Applied Multivariate Data Analysis. Vol. II: Categorical and Multivariate Methods; Springer: New York, NY, USA, 1996. [Google Scholar]
- Shoaib, M.; Banerjee, B.P.; Hayden, M.; Kant, S. Roots’ Drought Adaptive Traits in Crop Improvement. Plants 2022, 11, 2256. [Google Scholar] [CrossRef]
- Dolezal, J.; Klimes, A.; Dvorsky, M.; Riha, P.; Klimesova, J.; Schweingruber, F. Disentangling evolutionary, environmental and morphological drivers of plant anatomical adaptations to drought and cold in Himalayan graminoids. Oikos 2019, 128, 1576–1587. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Burks, J.; Tumber-Dávila, S.J. Rooted in potential: Advances in estimating spatiotemporal root water uptake in situ. New Phytol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Kumar, P.; Gupta, B.N. Root surface area measurements based on adsorption and desorption of nitrite. Plant Soil 1995, 175, 133–137. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, L.; Ren, Y.; Zhang, T.; Zhang, S.; Li, H.; Chen, Y.; Zhang, S. The Higher Water Absorption Capacity of Small Root System Improved the Yield and Water Use Efficiency of Maize. Plants 2022, 11, 2300. [Google Scholar] [CrossRef]
- Lüttge, U.; Laties, G.G. Selective inhibition of absorption and long distance transport in relation to the dual mechanisms of ion absorption in maize seedlings. Plant Physiol. 1967, 42, 181–185. [Google Scholar] [CrossRef]
- Macklon, A.E.S.; Sim, A. Cortical cell fluxes and transport to the stele in excised root segments of Allium cepa L. IV. Calcium as affected by its external concentration. Planta 1981, 152, 381–387. [Google Scholar] [CrossRef]
- Kotula, L.; Clode, P.L.; Striker, G.G.; Pedersen, O.; Läuchli, A.; Shabala, S.; Colmer, T.D. Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare). New Phytol. 2015, 208, 1114–1125. [Google Scholar] [CrossRef]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Parenchyma, collenchyma, and sclerenchyma. In Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants; Springer Nature: Cham, Switzerland, 2018; pp. 182–212. [Google Scholar]
- Hose, E.; Clarkson, D.T.; Steudle, E.; Schreiber, L.; Hartung, W. The exodermis: A variable apoplastic barrier. J. Exp. Bot. 2001, 52, 2245–2264. [Google Scholar] [CrossRef] [PubMed]
- Granse, D.; Titschack, J.; Ainouche, M.; Jensen, K.; Koop-Jakobsen, K. Subsurface aeration of tidal wetland soils: Root-system structure and aerenchyma connectivity in Spartina (Poaceae). Sci. Total Environ. 2022, 802, 149771. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Omori, F. Breeding for flooding tolerant maize using “teosinte” as a germplasm resource. Plant Root 2007, 1, 17–21. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Koop-Jakobsen, K.; Fischer, J.; Wenzhöfer, F. Survey of sediment oxygenation in rhizospheres of the saltmarsh grass—Spartina anglica. Sci. Total Environ. 2017, 589, 191–199. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Zeng, H.; Liu, M.; Miao, Y.; Wu, H.; Kardol, P. The nutrient absorption–transportation hypothesis: Optimizing structural traits in absorptive roots. New Phytol. 2017, 213, 1569–1572. [Google Scholar] [CrossRef]
- Katou, K.; Taura, T.; Furumoto, M. A model for water transport in the stele of plant roots. Protoplasma 1987, 140, 123–132. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9–20. [Google Scholar] [CrossRef]
- Pace, P.F.; Cralle, H.T.; El-Halawany, S.H.M.; Cothren, J.T.; Senseman, S.A. Drought-induced changes in shoot and root growth of young cotton plants. J. Cotton Sci. 1999, 3, 183–187. [Google Scholar]
- Huang, B.R.; Fry, J.D. Root anatomical, physiological, and morphological responses to drought stress for tall fescue cultivars. Crop Sci. 1998, 38, 1017–1022. [Google Scholar] [CrossRef]
- Pirnajmedin, F.; Majidi, M.M.; Saeidi, G.; Gheysari, M.; Volaire, F.; Barre, P.; Osivand, A.H.; Sarfaraz, D. Persistence, recovery and root traits of tall fescue genotypes with different flowering date under prolonged water stress. Euphytica 2017, 213, 269. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, B.; Zhong, S.; Wang, D.; Ma, J.; Sun, W. Aboveground biomass and root/shoot ratio regulated drought susceptibility of ecosystem carbon exchange in a meadow steppe. Plant Soil 2018, 432, 259–272. [Google Scholar] [CrossRef]
- Kozela, C.; Regan, S. How plants make tubes. Trends Plant Sci. 2003, 8, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Barceló, A.R. Xylem parenchyma cells deliver the H2O2 necessary for lignification in differentiating xylem vessels. Planta 2005, 220, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Robert, C.A.M.; Thakur, M.P. Drought intensity and duration effects on morphological root traits vary across trait type and plant functional groups: A meta-analysis. BMC Ecol. Evol. 2024, 24, 92–103. [Google Scholar] [CrossRef]
- Wang, P.; Calvo-Polanco, M.; Reyt, G.; Barberon, M.; Champeyroux, C.; Santoni, V.; Maurel, C.; Franke, R.B.; Ljung, K.; Novak, O.; et al. Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Sci. Rep. 2019, 9, 4227–4238. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The regulation of plant cell wall organisation under salt stress. Front. Plant Sci. 2023, 14, 1118313–1118329. [Google Scholar] [CrossRef]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root endodermis and exodermis: Structure, function, and responses to the environment. J. Plant Growth Regul. 2003, 21, 335–351. [Google Scholar] [CrossRef]
- Viana, W.G.; Scharwies, J.D.; Dinneny, J.R. Deconstructing the root system of grasses through an exploration of development, anatomy and function. Plant Cell Environ. 2022, 45, 602–619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; García-Favre, J.; Hu, H.; López, I.F.; Ordóñez, I.P.; Cartmill, A.D.; Symonds, V.; Kemp, P.D. Belowground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Soil Water Restriction. Agronomy 2025, 15, 1024. https://doi.org/10.3390/agronomy15051024
Zhang Y, García-Favre J, Hu H, López IF, Ordóñez IP, Cartmill AD, Symonds V, Kemp PD. Belowground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Soil Water Restriction. Agronomy. 2025; 15(5):1024. https://doi.org/10.3390/agronomy15051024
Chicago/Turabian StyleZhang, Yongmei, Javier García-Favre, Haiying Hu, Ignacio F. López, Iván P. Ordóñez, Andrew D. Cartmill, Vaughan Symonds, and Peter D. Kemp. 2025. "Belowground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Soil Water Restriction" Agronomy 15, no. 5: 1024. https://doi.org/10.3390/agronomy15051024
APA StyleZhang, Y., García-Favre, J., Hu, H., López, I. F., Ordóñez, I. P., Cartmill, A. D., Symonds, V., & Kemp, P. D. (2025). Belowground Structural Attributes and Morpho-Anatomical Response Strategies of Bromus valdivianus Phil. and Lolium perenne L. to Soil Water Restriction. Agronomy, 15(5), 1024. https://doi.org/10.3390/agronomy15051024






