Abstract
Rhodococcus erythropolis is an environmental Gram-positive Actinobacterium with a versatile metabolism involved in various bioconversions and degradations. Rhodococci are best known for their great potential in numerous decontamination and industrial processes. However, they can also prevent plant disease by disrupting quorum sensing-based communication of Gram-negative soft-rot bacteria, by degrading N-acyl-homoserine lactone signaling molecules. Such biocontrol activity results partly from the action of the γ-lactone catabolic pathway. This pathway is responsible for cleaving the lactone bond of a wide range of compounds comprising a γ-butyrolactone ring coupled to an alkyl or acyl chain. The aliphatic products of this hydrolysis are then activated and enter fatty acid metabolism. This short pathway is controlled by the presence of the γ-lactone, presumably sensed by a TetR-like transcriptional regulator, rather than the presence of the pathogen or the plant-host in the environment of the Rhodococci. Both the density and biocontrol activity of R. erythropolis may be boosted in crop systems. Treatment with a cheap γ-lactone stimulator, for example, the food flavoring γ-caprolactone, induces the activity in the biocontrol agent, R. erythropolis, of the pathway degrading signaling molecules; such treatments thus promote plant protection.
1. Introduction
Virulence functions in a dozen plant-associated bacterial genera have now been described to be regulated by autoinducer-1-based quorum sensing [1,2]. This mechanism involves both the synthesis and perception of N-acyl-homoserine lactone (NAHSL) signaling molecules leading coordinated gene expression in a bacterial population. The communication used by these plant pathogenic bacteria is a potentially useful target for the development of novel biocontrol methods [3,4,5]. These biocontrol methods are not aimed at eradicating the pathogen, but rather at exploiting its mechanisms of cellular communication to reduce the expression of virulence systems.
The quorum sensing system is central to inducing the synthesis of cell wall lytic enzymes and harpins by the soft-rot bacteria, Dickeya and Pectobacterium spp. These species have thus been used as the major plant pathogen models for studying quorum sensing regulation and for testing biocontrol strategies [6,7,8,9,10,11,12]. The principle of “quorum quenching” has been broadened to include the use of rhizospheric NAHSL-degrading bacteria, which can protect the plant against P. atrosepticum [13], P. carotovorum [14,15,16] or Dickeya spp. [17] maceration symptoms. Antagonistic bacteria degrading NAHSL signaling molecules can be found both in soil and hydroponic systems, particularly in the vicinity of both the aerial (phyllosphere) and underground (rhizosphere) parts of plants. Many of these antagonistic bacteria are members of the genera, Acinetobacter, Agrobacterium, Bacillus, Delftia, Ochrobactrum, Pseudomonas, Rhodococcus and Serratia [18,19,20,21]. In particular, the physiological and biocontrol traits of Rhodococcus erythropolis isolates have attracted the attention of phytopathologists (for a review, see [9]).
Rhodococcus erythropolis is an environmental Gram-positive bacterium with a high G + C content in DNA, belonging to the nocardioform actinomycetes. Members of the species have been isolated from diverse aquatic and telluric media, including seawater, alpine soil or coastal sediments from the Arctic to the Antarctic, illustrating the ability of the species to adapt to cold temperatures (psychrotolerant strains) [22,23,24]. However, they are strictly aerobic and can only colonize superficial environments not deprived of O2. R. erythropolis cells are non-motile and do not form spores. The composition of the cell envelope is unusual, with a high mycolic acid content, contributing to the high cell surface hydrophobicity [25,26]. This typical hydrophobic surface favors adhesion to various media; it also contributes to determining the location of the cells and their survival between polar and non-polar media, for example, between water and oil [22,23]. In addition, Rhodococci have a wide catabolic diversity and specific enzymatic capabilities [23,27,28,29,30,31]. This metabolic diversity is partly due to the presence and mobilization of large linear plasmids. There are also multiple homologs of many enzymes encoded by the genome, and these homologs may be involved in similar, but not identical, catabolic pathways contributing to Rhodococcus versatility [30]. Some strains produce biofilms [26] and biosurfactants, predominantly composed of glycolipids with a significant emulsifying ability; these products facilitate the utilization of hydrophobic compounds [26,32,33,34]. Other strains produce enzymes involved in various bioconversions and degradations (including oxidation, dehydrogenation, epoxidation, hydrolysis, hydroxylation, dehalogenation and desulfurization) [23,29,31]. Some of these enzymes are currently being used as biocatalysts in biotechnological and industrial processes, including at low temperatures [24,35,36,37,38]. Numerous Rhodococcus strains are also resistant to a large variety of recalcitrant compounds and present an exceptional ability to degrade hydrophobic and xenobiotic compounds, including lignin, petroleum and various pesticides [23,39,40,41,42,43]. R. erythropolis has understandably been referred to as a “master” of metabolism [27] and “remarkable” [23], in particular because its robustness to many environmental stresses [44] is such that it is currently being used in numerous biological decontamination processes [23,40,45,46,47,48,49,50,51]. However, it now appears also to be a potential biological control agent, expressing a catabolic mechanism protecting plants.
2. Biocontrol Activity: Rhodococcal Quorum Quenching vs. Pectobacterial Quorum Sensing
The principle of quorum quenching control is based on the interaction between protective agents and pathogens near the plant-host or on the plant surface (i.e., at the site of plant wounds). This interaction can be a result of inoculation with the biocontrol agent or of stimulation of the relevant indigenous microflora. The biocontrol agent degrades signaling molecules, thereby disrupting pathogen communication and leading to a decrease in virulence. The potato is an important crop worldwide [52,53], and there are epidemiological reports of devastating potato diseases, indicating the emergence of new pectinolytic agents [11,54], and therefore, this biocontrol strategy has primarily been applied to the Solanum tuberosum-Pectobacterium atrosepticum model [9,17,55]. In the psychrotolerant pathogen, P. atrosepticum, NAHSL based-quorum sensing controls virulence factors contributing to the typical potato blackleg infection and tuber maceration [56]. Almost one third of the genes in P. atrosepticum are under quorum sensing control, and these genes include those coding for plant cell-wall degrading enzymes and their secretion systems [57,58]. NAHSL based-quorum sensing can also regulate mechanisms involved in the manipulation of plant cell defenses, like harpin synthesis, leading to the hypersensitive response (HR) in non-host plant [13]. Generating signaling molecules is therefore central to plant-P. atrosepticum interactions (Figure 1A). Indeed, a single mutation of the NAHSL synthase gene or the degradation of signaling molecules before their release into the microenvironment is sufficient to prevent both the emergence of any symptoms and an HR by the plant [13,59]. One anti-virulence approach is based on the selective stimulation of NAHSL-degrading bacteria. R. erythropolis strain R138 is a biocontrol agent that has been isolated from potato in a hydroponic system; in vitro, it efficiently degrades diverse γ-lactones, including NAHSL, within a few hours [18]. This catabolism appears to occur only inside rhodococcal cells, because the enzymes involved are intracellular or associated with the membrane [60,61,62]. The activity of strain R138 for the biocontrol of pathogens has been also revealed: its presence significantly reduces the maceration of tubers inoculated with the pathogen [9] (Figure 1B).
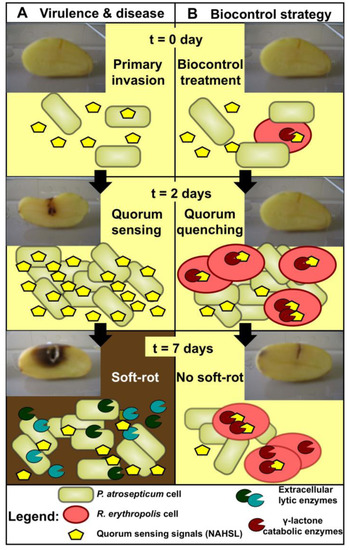
Figure 1.
Involvement of the R. erythropolis catabolism in Pectobacterium quorum quenching and control of tuber soft-rot. (A) The key role of pathogen communication in the chronology of events leading to tuber soft-rot. During the primary invasion stage, Pectobacterium cells multiply and constitutively produce small diffusible signaling molecules (N-acyl-homoserine lactones, NAHSL); The confinement of pathogens in a tuber wound can increase the concentration of signals; It is only when the bacterial population reaches a quorum that the effective concentration of NAHSL is obtained and, consequently, the release of lytic enzymes responsible for the maceration of the tuber is triggered; (B) The presence of a biocontrol agent strongly degrading γ-lactones (i.e., Rhodococcus erythropolis R138) near the pathogen in the plant organs to be protected prevents the NAHSL concentration from reaching the critical threshold, thereby preventing the release of lytic enzymes and tuber soft-rot. Deprived of plant nutrients, the density of the pathogen declines after a few days.
Figure 1.
Involvement of the R. erythropolis catabolism in Pectobacterium quorum quenching and control of tuber soft-rot. (A) The key role of pathogen communication in the chronology of events leading to tuber soft-rot. During the primary invasion stage, Pectobacterium cells multiply and constitutively produce small diffusible signaling molecules (N-acyl-homoserine lactones, NAHSL); The confinement of pathogens in a tuber wound can increase the concentration of signals; It is only when the bacterial population reaches a quorum that the effective concentration of NAHSL is obtained and, consequently, the release of lytic enzymes responsible for the maceration of the tuber is triggered; (B) The presence of a biocontrol agent strongly degrading γ-lactones (i.e., Rhodococcus erythropolis R138) near the pathogen in the plant organs to be protected prevents the NAHSL concentration from reaching the critical threshold, thereby preventing the release of lytic enzymes and tuber soft-rot. Deprived of plant nutrients, the density of the pathogen declines after a few days.
Rhodococcal quenching in plant tissue was fully demonstrated only in 2013 [63]. The involvement of R. erythropolis catabolism in biocontrol activity was characterized by cloning the qsdA lactonase master gene into a heterologous host (Escherichia coli) and by creating a qsdA deletion in R. erythropolis. The effects of these strains on NAHSL breakdown in tuber were quantified. qsdA transcription in R. erythropolis carrying a plasmid-borne qsdA::gfp transcriptional fusion has been also studied in situ by confocal laser scanning microscopy (CLSM). In these experiments, a virulent P. atrosepticum isolate and a recombinant of this strain producing negligible amounts of NAHSL were used to generate the quorum sensing process and tuber soft-rot. These studies showed that (i) the biocontrol activity of R. erythropolis is induced in potato tuber by P. atrosepticum N-3-oxo-octanoyl-L-HSL signaling molecules and (ii) the rhodococcal γ-lactone catabolic pathway is involved both in quorum quenching and the control of tuber soft-rot.
3. Catabolic Pathways of R. erythropolis Involved in Biocontrol
3.1. The γ-Lactone Catabolic Pathway
A lactone is an ester resulting from the spontaneous condensation of an alcohol group and a carboxylic group carried by the same molecule. It is a closed ring made from two or more carbon atoms, including an endocyclic oxygen. γ-lactones are lactones in which the ring, the γ-butyrolactone core, is composed of one oxygen and four carbons. R. erythropolis can assimilate various γ-lactones, and, indeed, use them as the sole source of both carbon and energy: indeed, in minimal media containing γ-lactones, the mean doubling time is four hours, two hours less than that on the uncyclizable structural analog hexanoate [64]. However, even though R. erythropolis can hydrolyze the γ-butyrolactone [65,66], only γ-lactone molecules with an aliphatic branched chain on the lactone ring can be fully assimilated; this structure is essential for the biosynthesis of all the molecules necessary for cell multiplication on minimal media.
The pathway of γ-lactone assimilation by R. erythropolis has been investigated through a proteomic approach involving two-dimensional gel electrophoresis coupled to Matrix-Assisted Laser Desorption Ionization (MALDI) Mass Spectrometry (MS) analysis. The proteins synthesized by R. erythropolis grown in minimal medium with a γ-lactone (γ-caprolactone) or its aliphatic structural analog (hexanoate) were compared. This work revealed a new pathway, not previously described in either prokaryotic or eukaryotic cells, and identified most of the enzymes involved [64]. In this short pathway, the lactonase QsdA (quorum sensing signal degradation A) hydrolyzes the lactone bond of a wide range of flavoring compounds containing a γ-butyrolactone ring coupled to an alkyl or acyl chain. The resulting open-chain forms are activated by CoA thioester linkage by the long-chain fatty acid-CoA ligase FadD. The activated aliphatic acids obtained can be assimilated or enter a dissimilative pathway involving β-oxidation-like enzymes or a ω-oxidation step coupled with the β-ketoadipate pathway and, thereby, enter the amphibolic Krebs cycle (Figure 2).
The lactonase QsdA in this pathway is of particular interest, because it catalyzes the opening of the γ-lactone ring, a selective and limiting first step of the pathway. QsdA (formerly acyl homoserine lactonase A, or AhlA) was first described as a phosphotriesterase-like lactonase that exhibits a very low phosphotriesterase activity [65]. Because phosphotriesters are not found in nature, this promiscuous activity is suspected to have emerged recently in evolution, following the use of compounds, such as paraoxon insecticide [65,67]. QsdA is, more accurately, a true lactonase, degrading various compounds with a preference for relatively hydrophobic lactones, including fragrance molecules used in the agro-food industry and the plant aromatic, dihydrocoumarin [64,65]. It also degrades a wide range of NAHSL: those with an acyl chain of between six and 14 carbons long with or without substitution at carbon 3. It is this property that gives the enzyme its name and is the reason for the growing interest [62,63,64,65]. QsdA can open various δ-lactone rings, but these activities are weaker and of less relevance to biocontrol than the opening of γ-forms [18,65].
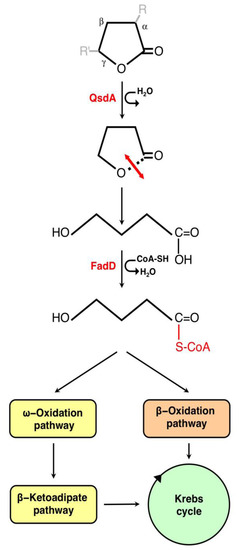
Figure 2.
Gamma-lactone catabolic pathway of R. erythropolis. The lactonase QsdA can hydrolyze the lactone bond of various γ-butyrolactone rings coupled to an N-acyl chain (R) linked to the Cα or an alkyl chain (R’) linked to the Cγ. The open-chain form of the resulting molecule is then activated by a coenzyme A thioester linkage by the fatty acid degradation D-CoA ligase (FadD). The resulting products can enter the common pathways of fatty acid metabolism.
Figure 2.
Gamma-lactone catabolic pathway of R. erythropolis. The lactonase QsdA can hydrolyze the lactone bond of various γ-butyrolactone rings coupled to an N-acyl chain (R) linked to the Cα or an alkyl chain (R’) linked to the Cγ. The open-chain form of the resulting molecule is then activated by a coenzyme A thioester linkage by the fatty acid degradation D-CoA ligase (FadD). The resulting products can enter the common pathways of fatty acid metabolism.
3.2. Regulation of the γ-Lactone Catabolic Pathway
The expression of the γ-lactone catabolic pathway is induced not by the invasion step or the presence of the soft-rot pathogen, but by its quorum sensing-based communication: thus, the synthesis of QsdA enzyme is activated only by a source of lactone or a pathogen that can produce NAHSL [63]. This suggests that there is a mechanism involved in signal transduction from the detection of γ-lactone substrates/NAHSL signals to the transcription of the qsdA operon in R. erythropolis. The qsdA operon has been found in all R. erythropolis isolates studied, including rhizosphere and mycorrhizosphere isolates, and “soil decontaminating” reference strains, like strains DCL14 and PR4 [64]. The whole genomic sequence of strain PR4 was made available in 2005 [68]. Analysis of this genome sequence in the region upstream from the qsdA operon reveals a putative TetR family transcriptional regulator gene, which we have named qsdR, and its appropriate DNA binding sites (Figure 3). TetR family proteins are found in diverse bacteria, including both Gram-positive and -negative [69,70]. They generally act as repressors of transcription and regulate various cellular activities, including, in R. erythropolis, metabolic activities [71]. The TetR regulator was first identified in E. coli, where it controls the expression of genes encoding a tetracycline efflux pump. Without tetracycline in the cell, the TetR protein binds to the operator region and inhibits the biosynthesis of the resistance protein, TetA. When tetracycline is present, it binds to TetR, changing the conformation, causing TetR detachment from the operator region, resulting in the expression of the gene encoding the resistance protein, TetA [69,70]. A similar mechanism is presumed to control the qsdA operon with γ-lactone in the role of tetracycline (Figure 3). This regulation mechanism is a classical and economic system for the induction of the pathway by its substrate, which appears well suited to the roles played by the γ-lactone catabolic pathway in rhodococcal metabolism and fitness.
3.3. Roles of the γ-Lactone Catabolic Pathway and Regulation of Rhodococcal Communication
The γ-lactone pathway of R. erythropolis differs from other biocontrol mechanisms in that it is based on a catabolic principle. It differs from approaches involving antibiosis, competition for iron or plant-induced systemic resistance based on the synthesis of secondary metabolic compounds. It is an example of a catabolic pathway in which the broad spectrum of the substrates allows it to contribute to bacterial nutrition, the control of communication and detoxification. Indeed, at least three roles can be assigned to the qsdA operon: the γ-lactone catabolic pathway is a pathway used to assimilate various lactones encountered in the environment as nutrients; it serves to disrupt bacterial communication and the functions under control of NAHSL-based quorum sensing employed by some microorganisms and, thereby, obtaining a competitive advantage over them; or it is associated with cellular detoxification. Related to this last point, we note that R. erythropolis can degrade a wide range of recalcitrant molecules, including molecules derived from pesticides or petroleum [23,41,42,46,72]. The steps in the degradation of these compounds, which can alter membrane integrity, are often associated with an intermediate lactonase activity (i.e., enol-lactone-hydrolyzing activities) responsible for linearization of the target molecules, such that they enter the β-ketoadipate or β-oxidation pathways [66,73,74]. Furthermore, 3-oxo substituted-HSL molecules are bactericidal to Gram-positive bacteria [75]. Indeed, Gram-positive bacteria may have developed NAHSL-degrading enzymes to protect themselves against the antibacterial activity of NAHSL, thereby favoring their survival in the natural environment [75,76].
There is another possibility for the role of this pathway: it may be involved in the control of signaling molecules used by Rhodococcus and/or Streptomyces cells. Even if it is known that Gram-positive bacteria do not produce NAHSL [77], the presence of a γ-butyrolactone quorum sensing system is suspected in Rhodococcus genus. This communication system, only identified and characterized in the closely related actinobacterial genus, Streptomyces, typically consists of a γ-butyrolactone synthase (AfsA in Streptomyces griseus) and a cognate receptor (ArpA in S. griseus) [78]. However, the γ-butyrolactone signal identified in the S. griseus species, 2-isocapryloyl-3R-hydroxymethyl-γ-butyrolactone, commonly named factor A, is a more complex molecule than NAHSL. It carries not one, but two aliphatic chains on α and β carbons of the lactone ring [79]. In silico analysis of the R. erythropolis PR4 genome revealed the presence of genes encoding AfsA and ArpA homologs with 31% and 36% amino acid sequence identity, respectively, suggesting the possible presence of a functional γ-butyrolactone quorum sensing system in Rhodococcus. Lactone degradation by R. erythropolis may be a metabolic pathway involved in the regulation of signal turn over, as observed in Agrobacterium tumefaciens, which can both produce and degrade its own signals [80].
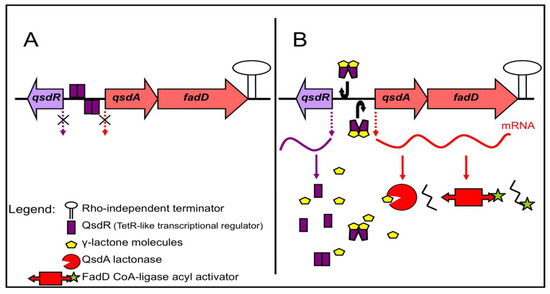
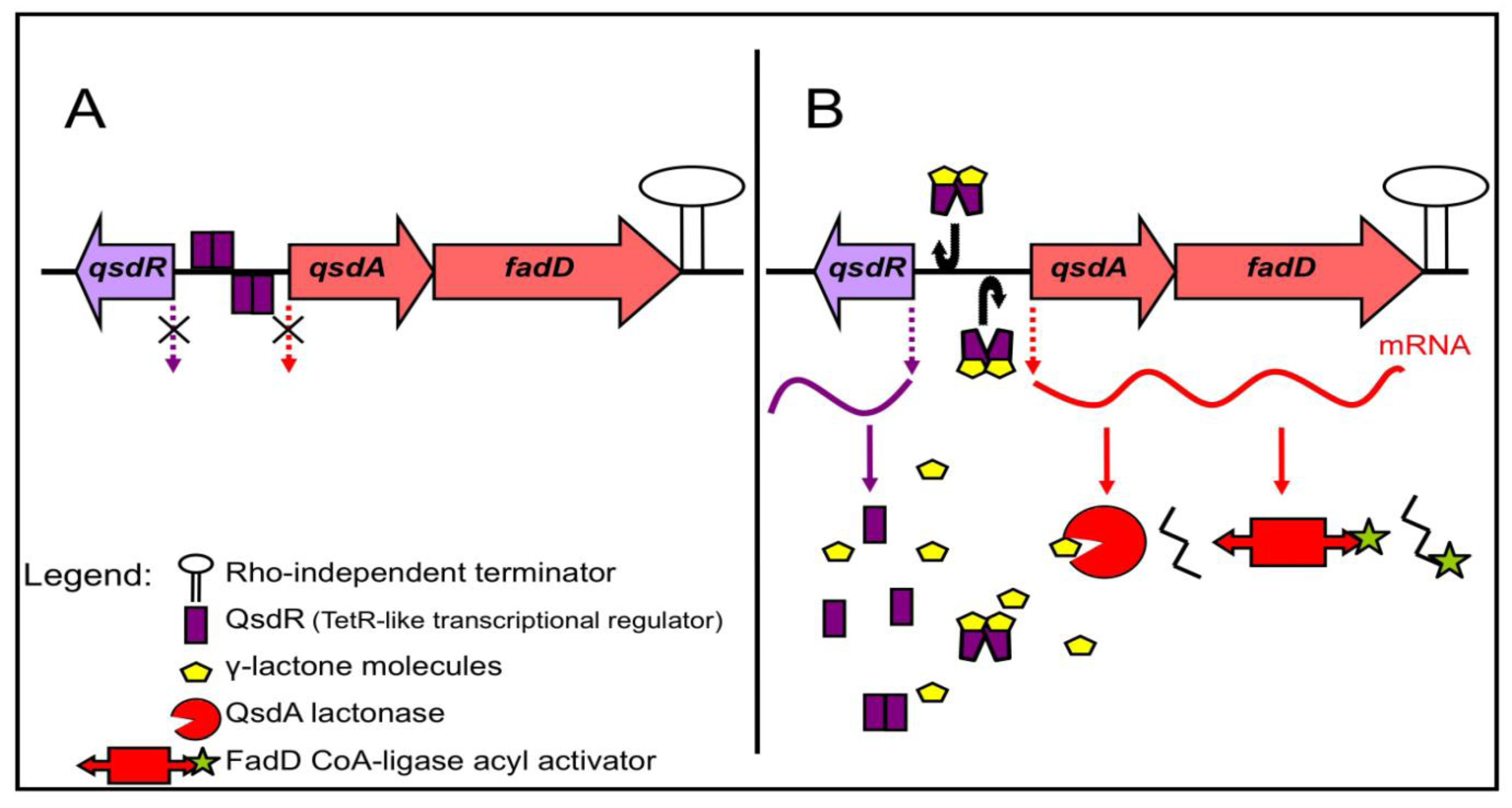
Figure 3.
The qsdA operon of R. erythropolis and its putative mechanism of regulation. The γ-lactone catabolic pathway is induced by various γ-lactone molecules, including N-acyl homoserine lactone signaling molecules. (A) In the absence of a γ-lactone source, the QsdR (quorum sensing signal degradation) regulator protein form dimers that bind to the operator region, switching off the biosynthesis of catabolic enzymes; (B) When γ-lactones are present, they bind to QsdR, thereby changing the conformation, such that QsdR dimers cannot bind to the operator region. The result is the expression of the genes encoding catabolic enzymes.
Figure 3.
The qsdA operon of R. erythropolis and its putative mechanism of regulation. The γ-lactone catabolic pathway is induced by various γ-lactone molecules, including N-acyl homoserine lactone signaling molecules. (A) In the absence of a γ-lactone source, the QsdR (quorum sensing signal degradation) regulator protein form dimers that bind to the operator region, switching off the biosynthesis of catabolic enzymes; (B) When γ-lactones are present, they bind to QsdR, thereby changing the conformation, such that QsdR dimers cannot bind to the operator region. The result is the expression of the genes encoding catabolic enzymes.
3.4. Other Biocontrol Pathways in R. erythropolis
Recent work suggests that the γ-lactone catabolic pathway may well not be the only process involved in the control of soft-rot in planta [63]. Deletion of the qsdA gene from the biocontrol agent, R. erythropolis (resulting in strain R138-ΔqsdA), did not completely abolish the biocontrol activity. This suggests that R138 strain possesses alternative mechanisms of NAHSL inactivation in addition to, or compensating for the loss of, the γ-lactone catabolic pathway. R. erythropolis produces multiple homologs of various catabolic enzymes, enhancing the species’ metabolic versatility [27,30]. Therefore, there may be one or more alternative enzymes for lactone catabolism other than QsdA. These putative alternative metabolic routes may also involve an acylase, which liberates a free homoserine lactone and a fatty acid, or an oxido-reductase, whose activity results in hydroxyl derivative production; the degradation products of such activities cannot act as signaling molecules [81]. In support of this possibility, traces of these three activities have been detected in vitro in various R. erythropolis strains [60,61,62]. Proteomic analysis has identified an amidase and an oxido-reductase produced by strain R138 during growth in minimal medium, but only in the presence, as a sole carbon source, of N-octanoyl-L-HSL or N-3-oxo-octanoyl-L-HSL, respectively [82].
4. Improving Biocontrol Activity
4.1. Stimulation of Lactonase Activity
In the soil and rhizosphere, NAHSL-degrading bacteria may make up 5%–15% of the total cultivable bacteria and are thus a small, but non-negligible, resource for developing biocontrol formulations [3,19,83]. However, the strength of signal degradation activities is often insufficient for bacteria to prevent extensive damage to crops when conditions are favorable for pathogens. Work on lactone metabolism and mechanisms of its regulation reveal that NAHSL catabolism in ecosystems may be boosted by introducing various NAHSL structural analogs to act as stimulators of NAHSL-degrading activity (Figure 4). For this approach to work, these structural analogs would have to be degraded in a non-specific manner and induce catabolic pathways that degrade signaling molecules. Thus, it may be possible to stimulate these catabolic pathways by releasing inducing substrates into the environment without adverse effects, due to the release of pathogen signals. To assess this possibility, a collection of compounds, each with structural similarities or metabolic associations with the conserved core of NAHSL, have been used individually as sole carbon sources in a synthetic medium inoculated with soil samples. After two cycles of enrichment, the resulting bacterial consortia were compared for their capacity to inactivate NAHSL. All consortia obtained from lactone enrichments exhibited a better NAHSL-degrading activity than that of consortia obtained from enrichments performed using mannitol, an easily catabolized polyol without a lactone bond. These consortia included bacteria obtained from rhizospheric soil samples, cultivated in the presence of either γ-caprolactone, ε-caprolactone or γ-heptalactone with enhanced NAHSL degradation capability. Interestingly, only the γ-caprolactone and γ-heptalactone consortia showed biocontrol activity against P. atrosepticum in tuber assays, identifying these molecules as possible biostimulator and biocontrol compounds. This is presumably a consequence of their γ-butyrolactone rings linked to an aliphatic chain, both traits common to NAHSL signals and not present in ε-caprolactone [9,18].
4.2. Stimulation of R. erythropolis Fitness
Biocontrol strategy success is also dependent on biotic and abiotic factors, which condition the installation, development and persistence of a large enough population of the protecting agent and expression of sufficient activity to control the disease [52,84]. Work using stimulated quorum quenching approaches suggests that some γ-lactones act also as chemicals promoting the growth of biocontrol bacteria. For example, in the presence of γ-lactones, rather than of the only plant-host, R. erythropolis seems to have a considerable advantage over other bacterial genera, only a small number of which can use this source of carbon in the vicinity of the plant [18,61,85] (Figure 4).
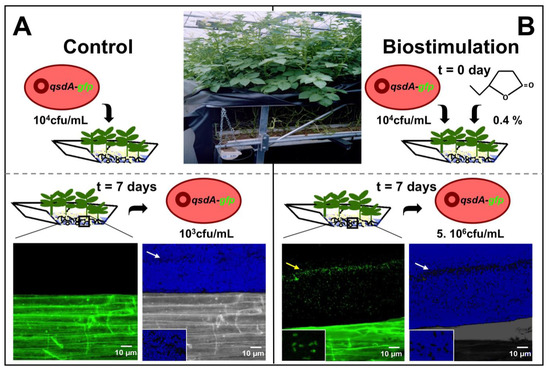
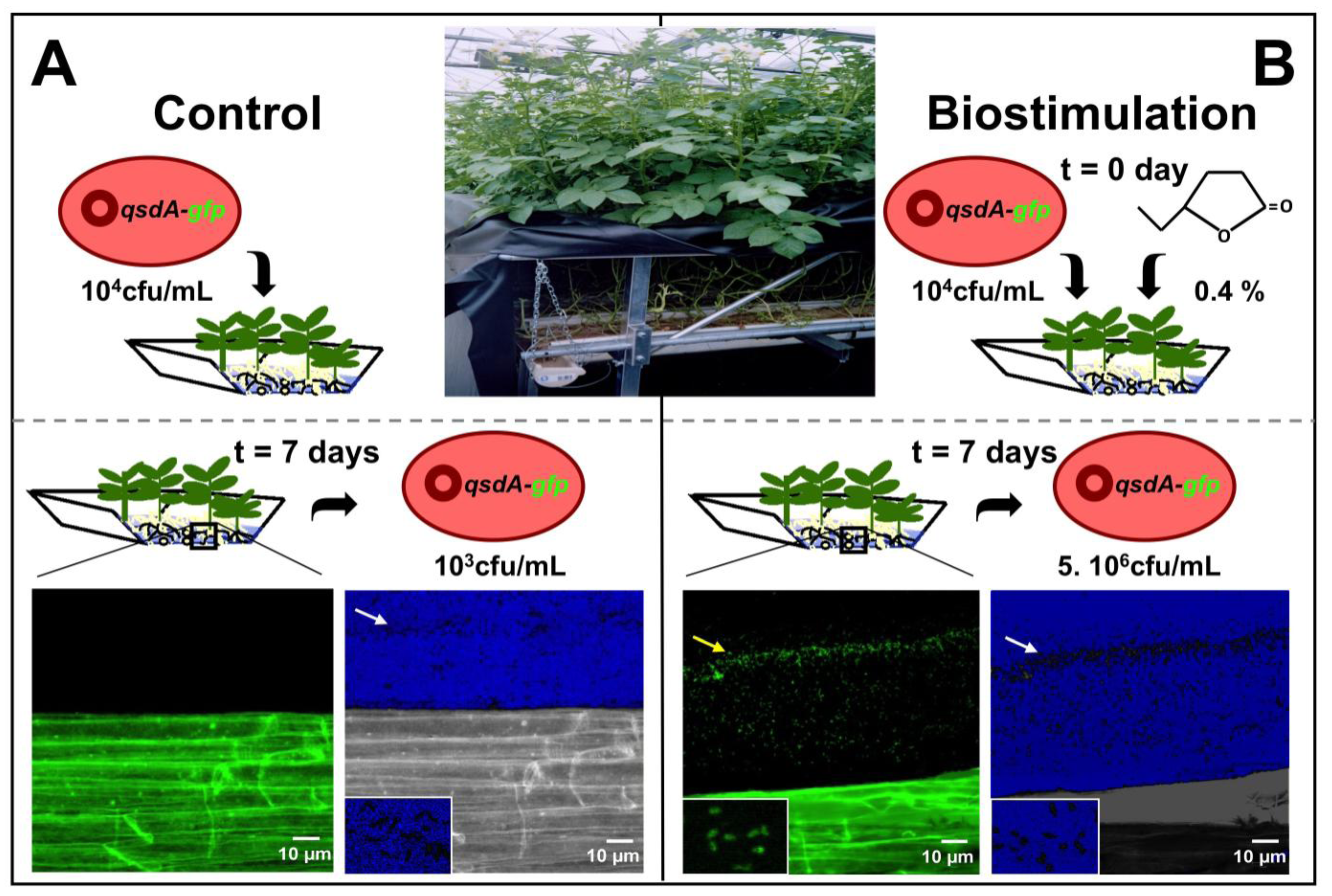
Figure 4.
Stimulation of R. erythropolis fitness and γ-lactone catabolic activity in a hydroponic system. (A) The inoculation of the biocontrol agent, Rhodococcus erythropolis R138, in a potato hydroponic system triggers a weak root colonization by the rhodococcal population, essentially embedded in root mucilage (white arrow on the picture with shifted colors); (B) The addition of the stimulator γ-caprolactone (0.4 g/L) to the plant nutrient solution significantly increases the cell density of the biocontrol agent (white arrow). The breakdown of lactones associated with this growth is revealed by confocal laser scanning microscopy using an R138 strain containing a plasmid-borne qsdA::gfp transcriptional fusion. The green fluorescent protein and the QsdA lactonase are synthesized by rhodococcal cells (yellow arrow) only in the presence of biostimulating molecules.
Figure 4.
Stimulation of R. erythropolis fitness and γ-lactone catabolic activity in a hydroponic system. (A) The inoculation of the biocontrol agent, Rhodococcus erythropolis R138, in a potato hydroponic system triggers a weak root colonization by the rhodococcal population, essentially embedded in root mucilage (white arrow on the picture with shifted colors); (B) The addition of the stimulator γ-caprolactone (0.4 g/L) to the plant nutrient solution significantly increases the cell density of the biocontrol agent (white arrow). The breakdown of lactones associated with this growth is revealed by confocal laser scanning microscopy using an R138 strain containing a plasmid-borne qsdA::gfp transcriptional fusion. The green fluorescent protein and the QsdA lactonase are synthesized by rhodococcal cells (yellow arrow) only in the presence of biostimulating molecules.
Various γ-lactones are fragrance molecules used in the agro-food, perfume and cosmetic industries: γ-caprolactone and γ-heptalactone are best known as food additives, for example, in beverages, ice cream, candy and tobacco. They are colorless, oily liquids with a nutty, coconutty or malty odor, which are generally prepared industrially by oxidation of phenyl or cyclohexanone groups [64,86,87,88]. They are both authorized as flavoring agents by the European Food Safety Authority. This status of non-toxic products intended for human consumption and their reasonable cost of production facilitate their use in a large-scale tests, as they have no human or environmental toxicity. Added to the plant growth substrate in hydroponic or soil systems, γ-caprolactone and γ-heptalactone promote the growth of bacteria capable of degrading both these substrates and also NAHSL [9,76,89]. For example, γ-caprolactone-mediated biostimulation of native NAHSL-degrading bacteria has been evaluated in an industrial-scale plant hydroponic system, used for the production in greenhouses of the certified tubers from potato plants [85]. In these systems, bacterial populations on the surface of S. tuberosum roots and tubers reach up to 1010 colony forming units per gram of fresh weight [90,91]. After γ-caprolactone-treatment, NAHSL degraders made up to 70% of the total culturable bacteria, and most of these strains were related to the R. erythropolis species. This demonstrated strong biostimulation of the native quorum quenching populations by γ-caprolactone. Most of the Rhodococcus isolates recovered from this type of biostimulated hydroponic system exhibit the three relevant properties: use of the biostimulator as a sole source of carbon, rapid degradation of NAHSL signaling molecules and effective protection of potato tubers against maceration caused by P. atrosepticum [85,92]. However, it is important to note that such biostimulation is transient: chemical analysis reveals a rapid degradation of γ-caprolactone added to the hydroponic culture medium and in the tissue of potato plants, and this correlates with rhodococcal stimulation [18,85]. R. erythropolis populations (indigenous and inoculated) have recently been successfully stimulated in larger scale trials in hydroponic culture in greenhouses. Various γ-lactone-containing molecules were tested, and the best activity was observed with molecules carrying a lateral chain shorter than four carbons, such as γ-caprolactone and γ-heptalactone. In these experiments, the addition of 0.4 g/L of γ-heptalactone increased the numbers of NAHSL degraders up to 60% of the bacteria cultured, with the R. erythropolis population being the major culturable quorum quenching population. Most of the Rhodococcus isolates were able to reduce maceration symptoms to 20% of those in the unprotected controls [85,89].
4.3. Formulations Incorporating R. erythropolis strains
A critical step for the application of this type of biocontrol strategy is the application phase and, thus, the establishment of microbial formulations. Indeed, inoculated bacteria must resist the phenomena of dilution, predation and competition with the native microflora, as well as the nutritional stress and abiotic features of their novel environment. The success of the application depends on the bacteria remaining viable in soils and also on their catabolic capacity, which will determine their fitness. It is therefore necessary to develop bacterial formulations that optimize these traits.
The established interest in R. erythropolis for biotechnology and bioremediation has led to the development of various formulations, which are more or less suitable [93,94]. In these formulations, immobilization processes are used and allow the fixation or trapping of cells. Encapsulation within a polymer, such as agarose or alginate, both biocompatible and biodegradable matrices, ensures the protection and release of bacteria. To develop efficient bioremediation method for the atrazine herbicide present in soils, alginate beads containing bentonite (clay), powdered activated carbon and/or skimmed milk have been evaluated for the suitability for the release of R. erythropolis into the soil and for the efficiency of atrazine degradation. This bead formulation provides adequate numbers of R. erythropolis cells to degrade atrazine in either water or soil. The addition of bentonite accelerates cell release, and skimmed milk increases the shelf life of the biologically active agent [93]. For extended storage at 4 °C, cell viability is greatly prolonged by growing cell-encapsulated beads in nutrient broth media. Air-dried bead formulations more suited for agricultural and bioremediation applications possess a capacity for atrazine degradation similar to that of the currently used wet bead formulations [94]. R. erythropolis has also been used as a biocatalyst for cholesterol degradation in aqueous media. In this work, two immobilization methods were compared: the percentage of metabolite degraded was higher for inkjet immobilized bacteria than for atomized immobilized bacteria [95]. An efficient and convenient alginate-based formulation also containing an organic co-solvent has been developed with the Rhodococcus sp. A5270 strain, able to catalyze the enantioselective biotransformation of nitriles [96].
The use of additives to the encapsulation matrix may enhance the fitness of these bacteria in soils. In the same way, the condition of the multiplication of the bacteria prior to the encapsulation may promote the ability for biodegradation. Nevertheless, it is necessary first to assess the consequences of the conditions of the biomass production of the added compounds in the formulation for spreading in the natural environment, so as to avoid metabolic interactions harmful to the survival of the biocontrol agents and to the efficiency of decontamination. Indeed, most bacteria can use diverse carbon sources as substrates. Generally, for reasons of efficiency (i.e., reduction in the diversity of enzyme synthesized), there is a hierarchy in the use of nutrients by the bacterial cell. In the presence of several substrates, selection steps, called metabolic preference and catabolic repression, are observed [97]. This type of metabolic interference has substantial consequences for the metabolic function of cells. To illustrate this, we precultured the biocontrol agent, strain R138, in various minimal media containing succinate (a substrate of basal metabolism, commonly found in the rhizosphere) or γ-caprolactone (a biostimulating molecule, substrate for the γ-lactone pathway) and in a Luria-Bertani rich medium. These liquid cultures were then applied to wounded potato tubers inoculated with a virulent soft-rot pathogen. Only the formulation containing the γ-caprolactone provided rapid protection (from the second day); also, it provided more effective biocontrol over time (at least seven days). This shows that the metabolism of this type of biocontrol agent must be conditioned to obtain the best results for quorum quenching applications. Thus, an example of field treatment would be underground seed-tubers with adequate alginate beads containing the activated (preconditioned) biocontrol agent. During a second step, when environmental conditions are favorable for the growth and virulence of the pathogen, it may be necessary to spray crops with a liquid treatment containing biostimulating molecules.
5. Conclusions
Microbiological control methods are complementary to other control methods, based on prophylactic measures, chemical treatments or genetic approaches [98]. Biocontrol formulations are an expanding market and now make up 1% of total pesticide sales. Fravel [99] and Montesinos [100] have drawn up lists of biocontrol products and strains authorized by the United States Environmental Protection Agency (USEPA) and the European Protection Agency (EPA). Most of these strains belong to the bacterial genera, Bacillus and Pseudomonas, and the fungal genera, Aspergillus and Trichoderma. The beneficial effects are the consequences of diverse mechanisms, including those involving microbial siderophores, the biosynthesis of antibiotics, surfactants and phytohormones, nutrient and spatial competition, mycoparasitism, predation, cell lysis by phages and plant-induced systemic resistance. However, there is skepticism about the reliability of biocontrol, partly because published accounts of control include evidence of a lack of consistency [101,102]. Moreover, antibiotic and cyanide production by biocontrol agents can affect not only the pathogen, but, in some cases, plant development [103,104]. Consequently, investigations are underway to screen for novel protectors and new antagonistic properties that target novel, and often poorly understood, pathogen traits.
In this review, we have presented and discussed advances in a new approach to biocontrol based on the interference of microbial communication involved in sensing cellular density and the microenvironment. Because of the diversity of its pathogens and its ranking as the fourth most important food crop in the world, the potato was chosen as the plant model for developing this novel biocontrol strategy. This choice is relevant to diseases affecting organs underground, because these organs are generally out of the reach of germicidal treatments [52]. One of the major microbial diseases in Europe is caused by soft-rot bacteria; these bacteria destroy potato crops and tubers during storage or transport to the supply areas [55], and therefore, soft-rot quorum sensing has emerged as a relevant target for biocontrol by a quorum quenching mechanism involving the bacterium, R. erythropolis. R. erythropolis expresses NAHSL-degrading activity, displays biocontrol capabilities for protecting potato tubers and is, therefore, an attractive candidate for developing a quorum quenching-based biocontrol strategy against soft-rot bacteria. This may be of great value to farming, because the application of a biostimulating γ-lactone in the environment of the crop selects and enhances R. erythropolis populations with the two beneficial activities: quenching the pathogenicity of soft-rot bacteria and eliminating the amending molecule, which is, therefore, fully biodegradable. This biostimulated quorum quenching strategy could be extended to numerous pathogens of horticultural and vegetable crops, including several ranked among the top ten bacterial pathogens [105], such as A. tumefaciens, Ralstonia solanacearum, Pseudomonas syringae and Erwinia amylovora, all of which use various N-hexanoyl/octanoyl-L-HSL as signaling molecules [2,106,107,108].
According to current evaluations, quorum quenching strategies appear to respect the microbial balance better than other biocontrol strategies, like, for example, predation or antibiosis. These other methods are suspected to be associated with rapid recolonization by an uncontrolled microflora [52,90]. However, the application of a biocontrol inoculum, with or without a biostimulator, inevitably leads to changes in microbial balance. Treatment with γ-caprolactone or γ-heptalactone alters the microbial diversity in the rhizosphere by increasing the relative density of flora capable of degrading NAHSL. Fortunately, this effect is temporary and its duration is correlated with the duration of γ-lactone assimilation [18,85]. Other changes in the microflora structure have also been recorded [85,89], but they do not appear to be greater than those observed during the phase change associated with plant growth, experimental culture conditions (field and year), climatic variations or cultural practices [56,109,110,111,112].
Another important criticism that can be leveled at this approach is that the target signals (NAHSL) are not fully specific to soft-rot bacteria and could be synthesized and used by other bacteria associated with plants [1,2]. Some neutral and/or beneficial plant bacteria also produce NAHSL, raising the issue of the possible disruption of the functions of these bacteria that are beneficial to the plant crop. Indeed, NAHSL can lead to diverse positive reactions in plants, such as plant growth, the induction of plant defenses and adventitious root formation. These reactions depend on the type of NAHSL produced. For example, inoculation with long-chain (C14) NAHSL-producing Sinorhizobium meliloti strains enhances the resistance of Arabidopsis plants to pathogenic bacteria, whereas inoculation with short-chain (C8)-producing Rhizobium etli had no such effect. Furthermore, plants seem to influence the amount of NAHSL in the rhizosphere: the presence of plants decreases the concentration of NAHSL in bacterial culture without having a major impact on bacterial growth. This diminution may be due to a quorum quenching activity of root exudates [113]. Finally, plants can also produce NAHSL analogs, which select and promote favorable rhizospheric microflora or, conversely, perturb quorum sensing by pathogenic populations [114]. Therefore, spreading a biocontrol quencher in the plant’s environment could have a synergistic or antagonistic action, such that the desired effect could be enhanced or attenuated. Today, only some leguminous plants [115,116,117] and barley [118], but not S. tuberosum, were shown to interfere with quorum sensing by degrading NAHSL or producing NAHSL analogs, suggesting that the impact of the plant-host will not be decisive, at least for potato crops. On the opposite side, the bacterial production of NAHSL could have a more important role on the plant health, inducing or reinforcing the systemic resistance of certain Solanaceae species (like tomato) to other bacterial or fungal pathogens than those fought by the quorum quenching strategy [119,120]. Extensive study and trials are therefore needed to address these questions, such that the biocontrol potential of R. erythropolis can be fully exploited.
Acknowledgments
This work was supported by grants from Conseil Régional de Haute-Normandie, Ministère délégué à l’Enseignement Supérieur et à la Recherche, and the Grand Réseau de Recherche Végétal Agronomie Sols et Innovation (GRR VASI), the Fonds Européens de Développement Régional (FEDER, European Union) and by SIPRE-Comité Nord Stations de Recherche et de Création Variétale de pomme de terre. We are grateful to Jan Nešvera (Laboratory of Molecular Genetics of Bacteria, Institute of Microbiology, Academy of Sciences of the Czech Republic) for the gift of the plasmid, pEPR1, allowing the construction of the qsdA::gfp transcriptional fusion in strain R138. We also thank Alex Edelman for linguistic support. Works are related to the European Cooperation in Science and Technology COST631 action-Understanding and Modelling Plant-Soil Interactions in the Rhizosphere Environment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Von Bodman, S.B.; Bauer, W.D.; Coplin, D.L. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2003, 41, 455–482. [Google Scholar] [CrossRef]
- Cha, C.; Gao, P.; Chen, Y.C.; Shaw, P.; Farrand, S.K. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 1998, 11, 1119–1129. [Google Scholar] [CrossRef]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Fray, R.G.; Throup, J.P.; Daykin, M.; Wallace, A.; Williams, P.; Stewart, G.S.; Grierson, D. Plants genetically modified to produce N-acylhomoserine lactones communicate with bacteria. Nat. Biotechnol. 1999, 17, 1017–1120. [Google Scholar] [CrossRef]
- Maë, A.; Montesano, M.; Koiv, V.; Palva, E.T. Transgenic plants producing the bacterial pheromone N-acylhomoserine lactone exhibit enhanced resistance to the bacterial phyto-pathogen Erwinia carotovora. Mol. Plant-Microbe Interact. 2001, 14, 1035–1042. [Google Scholar] [CrossRef]
- Barnard, A.M.; Salmond, G.P. Quorum sensing in Erwinia species. Anal. Bioanal. Chem. 2007, 387, 415–423. [Google Scholar] [CrossRef]
- Charkowsky, A.O. The Soft Rot Erwinia. In Plant-Associated Bacteria; Gnanamanickam, S., Ed.; Springer: Dordrecht, the Netherlands, 2006; pp. 423–505. [Google Scholar]
- Charkowski, A.; Blanco, C.; Condemine, G.; Expert, D.; Franza, T.; Hayes, C.; Hugouvieux-Cotte-Pattat, N.; López Solanilla, E.; Low, D.; Moleleki, L.; Pirhonen, M.; Pitman, A.; Perna, N.; Reverchon, S.; Rodríguez Palenzuela, P.; San Francisco, M.; Toth, I.; Tsuyumu, S.; van der Waals, J.; van der Wolf, J.; Van Gijsegem, F.; Yang, C.H.; Yedidia, I. The role of secretion systems and small molecules in soft-rot enterobacteriaceae pathogenicity. Annu. Rev. Phytopathol. 2012, 50, 425–449. [Google Scholar] [CrossRef]
- Crépin, A.; Barbey, C.; Cirou, A.; Tannières, M.; Orange, N.; Feuilloley, M.; Dessaux, Y.; Burini, J.F.; Faure, D.; Latour, X. Biological control of pathogen communication in the rhizosphere: A novel approach applied to potato soft rot due to Pectobacterium atrosepticum. Plant Soil 2012, 358, 27–37. [Google Scholar] [CrossRef]
- Czajkowski, R.; Jafra, S. Quenching of acyl-homoserine lactone-dependent quorum-sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 2009, 56, 1–16. [Google Scholar]
- Czajkowski, R.; Pérombelon, M.C.M.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Põllumaa, L.; Alamäe, T.; Mäe, A. Quorum sensing and expression of virulence in pectobacteria. Sensors 2012, 12, 3327–3349. [Google Scholar] [CrossRef]
- Smadja, B.; Latour, X.; Faure, D.; Chevalier, S.; Dessaux, Y.; Orange, N. Involvement of N-acylhomoserine lactones throughout the plant infection by Erwinia carotovora subsp. atroseptica (Pectobacterium atrosepticum). Mol. Plant-Microbe Interact. 2004, 17, 1269–1278. [Google Scholar] [CrossRef]
- Jafra, S.; Jalink, H.; van der Schoor, R.; van der Wolf, J.M. Pectobacterium carotovorum subsp. carotovorum strains show diversity in production of response to N-acyl homoserine lactones. J. Phytopathol. 2006, 154, 729–739. [Google Scholar] [CrossRef]
- Molina, L.; Constantinescu, F.; Michel, L.; Reimmann, C.; Duffy, B.; Défago, G. Degradation of pathogen quorum-sensing molecules by soil bacteria: A preventive and curative biological control mechanism. FEMS Microbiol. Ecol. 2003, 45, 71–81. [Google Scholar] [CrossRef]
- Uroz, S.; D’Angelo-Picard, C.; Carlier, A.; Elasri, M.; Sicot, C.; Petit, A.; Oger, P.; Faure, D.; Dessaux, Y. Novel bacteria degrading N-acylhomoserine lactones and their use as quenchers of quorum-sensing-regulated functions of plant-pathogenic bacteria. Microbiology 2003, 149, 1981–1989. [Google Scholar] [CrossRef]
- Crépin, A.; Beury-Cirou, A.; Barbey, C.; Farmer, C.; Helias, V.; Burini, J.F.; Faure, D.; Latour, X. N-acyl homoserine lactones in diverse Pectobacterium and Dickeya plant pathogens: Diversity, abundance, and involvement in virulence. Sensors 2012, 12, 3484–3497. [Google Scholar]
- Cirou, A.; Diallo, S.; Kurt, C.; Latour, X.; Faure, D. Growth promotion of quorum-quenching bacteria in the rhizosphere of Solanum tuberosum. Environ. Microbiol. 2007, 9, 1511–1522. [Google Scholar] [CrossRef]
- D'Angelo-Picard, C.; Faure, D.; Penot, I.; Dessaux, Y. Diversity of N-acyl homoserine lactone-producing and -degrading bacteria in soil and tobacco rhizosphere. Environ. Microbiol. 2005, 7, 1796–808. [Google Scholar]
- Jafra, S.; Przysowa, J.; Czajkowski, R.; Michta, A.; Garbeva, P.; van der Wolf, J.M. Detection and characterization of bacteria from the potato rhizosphere degrading N-acyl-homoserine lactone. Can. J. Microbiol. 2006, 52, 1006–1015. [Google Scholar] [CrossRef]
- Ma, A.; Lv, D.; Zhuang, X.; Zhuang, G. Quorum quenching in culturable phyllosphere bacteria from tobacco. Int. J. Mol. Sci. 2013, 14, 14607–14619. [Google Scholar] [CrossRef]
- Bell, K.S.; Philp, J.C.; Aw, D.W.; Christofi, N. The genus Rhodococcus. J. Appl. Microbiol. 1998, 85, 195–210. [Google Scholar]
- de Carvalho, C.C.; da Fonseca, M.M. The remarkable Rhodococcus erythropolis. Appl. Microbiol. Biotechnol. 2005, 67, 715–726. [Google Scholar] [CrossRef]
- Ruberto, L.A.M.; Vasquez, S.; Lobalbo, A.; Mac Cormack, W.P. Psychrotolerant hydrocarbon-degrading Rhodococcus strains isolated from polluted Antarctic soils. Antarct. Sci. 2005, 17, 47–56. [Google Scholar] [CrossRef]
- Chang, W.N.; Liu, C.W.; Liu, H.S. Hydrophobic cell surface and bioflocculation behavior of Rhodococcus erythropolis RID B-4865-2009. Process. Biochem. 2009, 44, 955–962. [Google Scholar] [CrossRef]
- Schreiberová, O.; Hedbávná, P.; Cejková, A.; Jirků, V.; Masák, J. Effect of surfactants on the biofilm of Rhodococcus erythropolis, a potent degrader of aromatic pollutants. N. Biotechnol. 2012, 30, 62–68. [Google Scholar] [CrossRef]
- Larkin, M.J.; Kulakov, L.A.; Allen, C.C.R. Biodegradation and Rhodococcus—Masters of catabolic versatility. Curr. Opin. Microbiol. 2005, 16, 282–290. [Google Scholar]
- Larkin, M.J.; Kulakov, L.A.; Allen, C.C. Biodegradation by members of the genus Rhodococcus: Biochemistry, physiology, and genetic adaptation. Adv. Appl. Microbiol. 2006, 59, 1–29. [Google Scholar]
- Martinkova, L.; Uhnakova, B.; Patek, M.; Nesvera, J.; Kren, V. Biodegradation potential of the genus Rhodococcus. Environ. Int. 2009, 35, 162–177. [Google Scholar]
- van der Geize, R.; Dijkhuizen, L. Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr. Opin. Microbiol. 2004, 7, 255–261. [Google Scholar] [CrossRef]
- Warhurst, A.M.; Fewson, C.A. Biotransformations catalyzed by the genus Rhodococcus. Crit. Rev. Biotechnol. 1994, 14, 29–73. [Google Scholar] [CrossRef]
- Ciapina, E.M.; Melo, W.C.; Santa Anna, L.M.; Santos, A.S.; Freire, D.M.; Pereira, N., Jr. Biosurfactant production by Rhodococcus erythropolis grown on glycerol as sole carbon source. Appl. Biochem. Biotechnol. 2006, 129–132, 880–886. [Google Scholar]
- Pacheco, G.J.; Ciapina, E.M.; Gomes Ede, B.; Junior, N.P. Biosurfactant production by Rhodococcus erythropolis and its application to oil removal. Braz. J. Microbiol. 2010, 41, 685–693. [Google Scholar] [CrossRef]
- Pirog, T.; Sofilkanych, A.; Shevchuk, T.; Shulyakova, M. Biosurfactants of Rhodococcus erythropolis IMV Ас-5017: Synthesis intensification and practical application. Appl. Biochem. Biotechnol. 2013, 170, 880–894. [Google Scholar]
- Qi, Y.; Zhao, L.; Olusheyi, O.Z.; Tan, X. Isolation and preliminary characterization of a 3-chlorobenzoate degrading bacteria. J. Environ. Sci. 2007, 19, 332–337. (in Chinese). [Google Scholar] [CrossRef]
- Whyte, L.G.; Hawari, J.; Zhou, E.; Bourbonniere, L.; Inniss, W.E.; Greer, C.W. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl. Environ. Microbiol. 1998, 64, 2578–2584. [Google Scholar]
- Whyte, L.G.; Slagman, S.J.; Pietrantonio, F.; Bourbonniere, L.; Koval, S.F.; Lawrence, J.R.; Inniss, W.E.; Greer, C.W. Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15. Appl. Environ. Microbiol. 1999, 65, 2961–2968. [Google Scholar]
- Yakimov, M.M.; Giuliano, L.; Bruni, V.; Scarfì, S.; Golyshin, P.N. Characterization of antarctic hydrocarbon-degrading bacteria capable of producing bioemulsifiers. New Microbiol. 1999, 22, 249–256. [Google Scholar]
- de Carvalho, C.C.; Fatal, V.; Alves, S.S.; da Fonseca, M.M. Adaptation of Rhodococcus erythropolis cells to high concentrations of toluene. Appl. Microbiol. Biotechnol. 2007, 76, 1423–1430. [Google Scholar] [CrossRef]
- de Carvalho, C.C. Adaptation of Rhodococcus erythropolis cells for growth and bioremediation under extreme conditions. Res. Microbiol. 2012, 163, 125–136. [Google Scholar] [CrossRef]
- De Schrijver, A.; De Mot, R. Degradation of pesticides by actinomycetes. Crit. Rev. Microbiol. 1999, 25, 85–119. [Google Scholar] [CrossRef]
- Huang, L.; Ma, T.; Li, D.; Liang, F.L.; Liu, R.L.; Li, G.Q. Optimization of nutrient component for diesel oil degradation by Rhodococcus erythropolis. Mar. Pollut. Bull. 2008, 56, 1714–1718. [Google Scholar]
- Yu, B.; Xu, P.; Shi, Q.; Ma, C. Deep desulfurization of diesel oil and crude oils by a newly isolated Rhodococcus erythropolis strain. Appl. Environ. Microbiol. 2006, 72, 54–58. [Google Scholar] [CrossRef]
- Fanget, N.V.; Foley, S. Starvation/stationary-phase survival of Rhodococcus erythropolis SQ1: A physiological and genetic analysis. Arch. Microbiol. 2011, 193, 1–13. [Google Scholar] [CrossRef]
- Christofi, N.; Ivshina, I.B. Microbial surfactants and their use in field studies of soil remediation. J. Appl. Microbiol. 2002, 93, 915–929. [Google Scholar] [CrossRef]
- de Carvalho, C.C.; da Fonseca, M.M. Degradation of hydrocarbons and alcohols at different temperatures and salinities by Rhodococcus erythropolis DCL14. FEMS Microbiol. Ecol. 2005, 51, 389–399. [Google Scholar] [CrossRef]
- Leigh, M.B.; Prouzová, P.; Macková, M.; Macek, T.; Nagle, D.P.; Fletcher, J.S. Polychlorinated biphenyl (PCB)-degrading bacteria associated with trees in a PCB-contaminated site. Appl. Environ. Microbiol. 2006, 72, 2331–2342. [Google Scholar] [CrossRef]
- Leilei, Z.; Mingxin, H.; Suiyi, Z. Enzymatic remediation of the polluted crude oil by Rhodococcus. Afr. J. Microbiol. Res. 2012, 6, 1540–1547. [Google Scholar]
- Lofgren, J.; Haddad, S.; Kendall, K. Emerging Technologies in Hazardous Waste Management; Tedder, W., Pohland, F.G., Eds.; ACS Symposium Series; ACS Publication: Columbus, OH, USA, 1995; Volume 607, pp. 252–263. [Google Scholar]
- Rappert, S.; Li, R.; Kokova, M.; Antholz, M.; Nagorny, S.; Francke, W.; Müller, R. Degradation of 2,5-dimethylpyrazine by Rhodococcus erythropolis strain DP-45 isolated from a waste gas treatment plant of a fishmeal processing company. Biodegradation 2007, 18, 585–596. [Google Scholar] [CrossRef]
- Solyanikova, I.; Golovleva, L. Biochemical features of the degradation of pollutants by Rhodococcus as a basis for contaminated wastewater and soil cleanup. Mikrobiologiia 2011, 80, 579–594. [Google Scholar]
- Diallo, S.; Crepin, A.; Barbey, C.; Orange, N.; Burini, J.F.; Latour, X. Mechanisms and recent advances in biological control mediated through the potato rhizosphere. FEMS Microbiol. Ecol. 2011, 75, 351–364. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). International Year of the Potato 2008, New Light on A Hidden Treasure; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; p. 144.
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Hélias, V.; Pirhonen, M.; Tsror (Lahkim), L.; Elphinstone, J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Crépin, A.; Barbey, C.; Beury-Cirou, A.; Helias, V.; Taupin, L.; Reverchon, S.; Nasser, W.; Faure, D.; Dufour, A.; Orange, N.; Feuilloley, M.; Heurlier, K.; Burini, J.F.; Latour, X. Quorum sensing signaling molecules produced by reference and emerging soft-rot bacteria (Dickeya and Pectobacterium spp.). PLoS One 2012, 7, e35176. [Google Scholar] [CrossRef]
- Smadja, B.; Latour, X.; Trigui, S.; Burini, J-F.; Chevalier, S.; Orange, N. Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can. J. Microbiol. 2004, 50, 19–27. [Google Scholar] [CrossRef]
- Liu, H.; Coulthurst, S.J.; Pritchard, L.; Hedley, P.E.; Ravensdale, M.; Humphris, S.; Burr, T.; Takle, G.; Brurberg, M.B.; Birch, P.R.; Salmond, G.P.; Toth, I.K. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 2008, 4, e1000093. [Google Scholar] [CrossRef]
- Monson, R.; Burr, T.; Liu, H.; Hedley, P.; Toth, I.; Salmond, G.P. Identification of genes in the VirR regulon of Pectobacterium atrosepticum and characterization of their roles in quorum sensing-dependent virulence. Environ. Microbiol. 2012, 15, 687–701. [Google Scholar]
- Latour, X.; Diallo, S.; Chevalier, S.; Morin, D.; Smadja, B.; Burini, J.F.; Haras, D.; Orange, N. Thermoregulation of N-acyl homoserine lactones-based quorum sensing in the soft rot bacterium Pectobacterium atrosepticum. Appl. Environ. Microbiol. 2007, 73, 4078–4081. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.J.; Shin, M.H.; Kim, J.A.; Kim, H.K.; Lee, J.K. N-acylhomoserine lactonase producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol. Lett. 2006, 261, 102–108. [Google Scholar] [CrossRef]
- Uroz, S.; Chhabra, S.R.; Camara, M.; Williams, P.; Oger, P.; Dessaux, Y. N-acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 2005, 151, 3313–3322. [Google Scholar] [CrossRef]
- Uroz, S.; Oger, P.M.; Chapelle, E.; Adeline, M.T.; Faure, D.; Dessaux, Y. A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl. Environ. Microbiol. 2008, 74, 1357–1366. [Google Scholar] [CrossRef]
- Barbey, C.; Crépin, A.; Bergeau, D.; Ouchiha, A.; Mijouin, L.; Taupin, L.; Orange, N.; Feuilloley, M.; Dufour, A.; Burini, J.F.; Latour, X. In planta biocontrol of Pectobacterium atrosepticum by Rhodococcus erythropolis involves silencing of pathogen communication by the rhodococcal gamma-lactone catabolic pathway. PLoS One 2013, 8, e66642. [Google Scholar] [CrossRef]
- Barbey, C.; Crépin, A.; Cirou, A.; Budin-Verneuil, A.; Orange, N.; Feuilloley, M.; Faure, D.; Dessaux, Y.; Burini, J.F.; Latour, X. Catabolic pathway of gamma-caprolactone in the biocontrol agent Rhodococcus erythropolis. J. Proteome Res. 2012, 11, 206–216. [Google Scholar] [CrossRef]
- Afriat, L.; Roodveldt, C.; Manco, G.; Tawfik, D.S. The latent promiscuity of newly identified microbial lactonases is linked to a recently diverged phosphotriesterase. Biochemistry 2006, 45, 13677–13686. [Google Scholar] [CrossRef]
- Curragh, H.; Flynn, O.; Larkin, M.J.; Stafford, T.M.; Hamilton, J.T.; Harper, D.B. Haloalkane degradation and assimilation by Rhodococcus rhodochrous NCIMB 13064. Microbiology 1994, 140, 1433–1442. [Google Scholar] [CrossRef]
- Singh, B.K. Organophosphorus-degrading bacteria: Ecology and industrial applications. Nat. Rev. Microbiol. 2009, 7, 156–164. [Google Scholar] [CrossRef]
- Sekine, M.; Tanikawa, S.; Omata, S.; Saito, M.; Fujisawa, T.; Tsukatani, N.; Tajima, T.; Sekigawa, T.; Kosugi, H.; Matsuo, Y.; et al. Sequence analysis of three plasmids harboured in Rhodococcus erythropolis strain PR4. Environ. Microbiol. 2006, 8, 334–346. [Google Scholar] [CrossRef]
- Ramos, J.L.; Martinez-Bueno, M.; Molina-Henares, A.J.; Teran, W.; Watanabe, K.; Zhang, X.; Gallegos, M.T.; Brennan, R.; Tobes, R. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005, 69, 326–356. [Google Scholar]
- Cuthbertson, L.; Nodwell, J.R. The TetR family of regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef]
- Si, D.; Urano, N.; Shimizu, S.; Kataoka, M. LplR, a repressor belonging to the TetR family, regulates expression of the L-pantoyl lactone dehydrogenase gene in Rhodococcus erythropolis. Appl. Environ. Microbiol. 2012, 78, 7923–7930. [Google Scholar] [CrossRef]
- Cha, C.J.; Cain, R.B.; Bruce, N.C. The modified beta-ketoadipate pathway in Rhodococcus rhodochrous N75: Enzymology of 3-methylmuconolactone metabolism. J. Bacteriol. 1998, 180, 6668–6673. [Google Scholar]
- Alvarez, H.M. Relationship between β-oxidation pathway and the hydrocarbon-degrading profile in actinomycetes bacteria. Intern. Biodeterior. Biodegrad. 2003, 52, 35–42. [Google Scholar] [CrossRef]
- Van der Vlugt-Bergmans, C.J.; van der Werf, M.J. Genetic and biochemical characterization of a novel monoterpene epsilon-lactone hydrolase from Rhodococcus erythropolis DCL14. Appl. Environ. Microbiol. 2001, 67, 733–741. [Google Scholar] [CrossRef]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.H.; Rogers, C.J.; Meijler, M.M.; Moss, J.A.; Clapham, B.; Brogan, A.P.; Dickerson, T.J.; Janda, K.D. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA. 2005, 102, 309–314. [Google Scholar] [CrossRef]
- Roche, D.M.; Byers, J.T.; Smith, D.S.; Glansdorp, F.G.; Spring, D.R.; Welch, M. Communications blackout? Do N-acylhomoserine-lactone-degrading enzymes have any role in quorum sensing? Microbiology 2004, 150, 2023–2028. [Google Scholar] [CrossRef]
- Reading, N.C.; Sperandio, V. Quorum sensing: the many language of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef]
- Takano, E. Gamma-butyrolactones: Streptomyces signalling molecules regulating antibiotic production and differentiation. Curr. Opin. Microbiol. 2006, 9, 287–294. [Google Scholar] [CrossRef]
- Nishida, H.; Ohnishi, Y.; Beppu, T.; Horinouchi, S. Evolution of γ-butyrolactone synthases and receptors in Streptomyces. Environ. Microbiol. 2007, 9, 1986–1994. [Google Scholar] [CrossRef]
- Carlier, A.; Uroz, S.; Smadja, B.; Fray, R.; Latour, X.; Dessaux, Y.; Faure, D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM-paralogous gene, aiiB, also encoding N-Acyl homoserine lactonase activity. Appl. Environ. Microbiol. 2003, 69, 4989–4993. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zhang, L.H. Quorum sensing and quorum-quenching enzymes. J. Microbiol. 2005, 43, 101–109. [Google Scholar]
- Barbey, C.; Kwasiborski, A.; Burini, J-F.; Faure, D.; Latour, X. Identification of a wide range of catabolic enzymes involved in the assimilation of various N-acyl homoserine lactones by Rhodococcus erythropolis. 2013; unpublished. [Google Scholar]
- Reimmann, C.; Ginet, N.; Michel, L.; Keel, C.; Michaux, P.; Krishnapillai, V.; Zala, M.; Heurlier, K.; Triandafillu, K.; Harms, H.; Défago, G.; Haas, D. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 2002, 148, 923–932. [Google Scholar]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Appl. Environ. Microbiol. 2008, 74, 1357–1366. [Google Scholar] [CrossRef]
- Cirou, A.; Raffoux, A.; Diallo, S.; Latour, X.; Dessaux, Y.; Faure, D. Gamma-caprolactone stimulates the growth of quorum-quenching Rhodococcus populations in a large-scale hydroponic system for culturing Solanum tuberosum. Res. Microbiol. 2011, 162, 945–950. [Google Scholar] [CrossRef]
- Maga, J.A. Lactones in foods. CRC Crit. Rev. Food Sci. Nutr. 1976, 8, 1–56. [Google Scholar]
- Murib, J.H.; Kahn, J.H. Process for Preparing Gamma-Caprolactone by Isomerization of Epsilon-Caprolactone. U.S. Patent 4,611,069, 9 September 1986. [Google Scholar]
- Nuñez, M.T.; Martin, V.S. Efficient oxidation of phenyl group to carboxylic acids with ruthenium tetraoxide. A simple synthesis of (R)-γ-caprolactone, the pheromone of Trogoderma granarium. J. Org. Chem. 1990, 55, 1928–1932. [Google Scholar] [CrossRef]
- Cirou, A.; Mondy, S.; An, S.; Charrier, A.; Sarrazin, A.; Thoison, O.; DuBow, M.; Faure, D. Efficient biostimulation of the native and introduced quorum-quenching Rhodococcus erythropolis is revealed by a combination of analytical chemistry, microbiology and pyrosequencing. Appl. Environ. Microbiol. 2012, 78, 481–492. [Google Scholar] [CrossRef]
- Latour, X.; Faure, D.; Diallo, S.; Cirou, A.; Smadja, B.; Dessaux, Y.; Orange, N. Control of bacterial diseases of potato caused by Pectobacterium spp. (Erwinia carotovora). Cah. Agric. 2008, 17, 355–359. [Google Scholar]
- Van Peer, R.; Schippers, B. Plant growth responses to bacterization with selected Pseudomonas spp. strains and rhizosphere microbial development in hydroponic cultures. Revue canadienne de microbiologie 1989, 35, 456–463. [Google Scholar] [CrossRef]
- Cirou, A.; Uroz, S.; Chapelle, E.; Latour, X.; Orange, N.; Faure, D.; Dessaux, Y. Quorum sensing as a target for novel biocontrol strategies. In Plant Pathology in the 21st Century; Gisi, U., Chet, I., Gullino, M.L., Eds.; Springer: Berlin, Germany, 2009; pp. 121–132. [Google Scholar]
- Vancov, T.; Jury, K.; Van Zwieten, L. Atrazine degradation by encapsulated Rhodococcus erythropolis NI86/21. J. Appl. Microbiol. 2005, 99, 767–775. [Google Scholar] [CrossRef]
- Vancov, T.; Jury, K.; Rice, N.; van Zwieten, L.; Morris, S. Enhancing cell survival of atrazine degrading Rhodococcus erythropolis NI86/21 cells encapsulated in alginate beads. J. Appl. Microbiol. 2007, 102, 212–220. [Google Scholar] [CrossRef]
- Mobed-Miremadi, M.; Darbha, S. Immobilization of R. erythropolis in alginate-based artificial cells for simulated plaque degradation in aqueous media. J. Microencapsul. 2013. [Google Scholar] [CrossRef]
- Guo, X.L.; Deng, G.; Xu, J.; Wang, M.X. Immobilization of Rhodococcus sp. AJ270 in alginate capsules and its application in enantioselective biotransformation of trans-2-methyl-3-phenyl-oxiranecarbonitrile and amide. Enzyme Microb. Tech. 2006, 39, 1–5. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Alabouvette, C.; Olivain, C.; Steinberg, C. Biological control of plant pathogens: The European situation. Eur. J. Plant Pathol. 2006, 114, 329–341. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef]
- Latour, X.; Delorme, S.; Mirleau, P.; Lemanceau, P. Identification of traits implicated in the rhizosphere competence of fluorescent pseudomonads: Description of a strategy based on population and model strain studies. Agron. Sustain. Dev. 2003, 23, 397–405. [Google Scholar]
- Mark, G.L.; Morrissey, J.P.; Higgins, P.; O’Gara, F. Molecular-based strategies to exploit Pseudomonas biocontrol strains for environmental biotechnology applications. FEMS Microbiol. Ecol. 2006, 56, 161–177. [Google Scholar]
- Brazelton, J.N.; Pfeufer, E.E.; Sweat, T.A.; McSpadden-Gardener, B.B.; Coenen, C. 2,4-Diacetylphloroglucinol alters plant root development. Mol. Plant-Microbe Interact. 2008, 21, 1349–1358. [Google Scholar] [CrossRef]
- Schippers, B.; Bakker, A.W.; Bakker, P.A.H.M. Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Ann. Rev. Phytopathol. 1987, 25, 339–358. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; Toth, I.; Salmond, G.; Foster, G.D. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Flavier, A.B.; Ganova-Raeva, L.M.; Schell, M.A.; Denny, T.P. Hierarchical autoinduction in Ralstonia solanacearum: Control of acyl-homoserine lactone production by a novel autoregulatory system responsive to 3-hydroxypalmitic acid methyl ester. J. Bacteriol. 1997, 179, 7089–7097. [Google Scholar]
- Quiñones, B.; Dulla, G.; Lindow, S.E. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol. Plant Microbe Interact. 2005, 18, 682–693. [Google Scholar] [CrossRef]
- Venturi, V.; Venuti, C.; Devescovi, G.; Lucchese, C.; Friscina, A.; Degrassi, G.; Aguilar, C.; Mazzucchi, U. The plant pathogen Erwinia amylovora produces acyl-homoserine lactone signal molecules in vitro and in planta. FEMS Microbiol. Lett. 2004, 241, 179–183. [Google Scholar] [CrossRef]
- Becker, R.; Behrendt, U.; Hommel, B.; Kropf, S.; Ulrich, A. Effect of transgenic fructan producing potatoes on the community structure of rhizosphere and phyllosphere bacteria. FEMS Microbiol. Ecol. 2008, 66, 411–425. [Google Scholar] [CrossRef]
- Heuer, H.; Kroppenstedt, R.M.; Lottmann, J.; Berg, G.; Smalla, K. Effect of T4 lysozyme release from transgenic potato roots on bacterial rhizosphere communities are negligible relative to natural factors. Appl. Environ. Microbiol. 2002, 68, 1325–1335. [Google Scholar] [CrossRef]
- Lottmann, J.; Heuer, H.; Smalla, K.; Berg, G. Influence of transgenic T4-lysozyme-producing potato plants on potentially beneficial plant-associated bacteria. FEMS Microbiol. Ecol. 1999, 29, 365–377. [Google Scholar] [CrossRef]
- Van Overbeek, L.; van Elsas, J.D. Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanum tuberosum L.). FEMS Microbiol. Ecol. 2008, 64, 283–296. [Google Scholar] [CrossRef]
- Zarkani, A.A.; Stein, E.; Röhrich, C.R.; Schikora, M.; Evguenieva-Hackenberg, E.; Degenkolb, T.; Vilcinskas, A.; Klug, G.; Kogel, K.H.; Schikora, A. Homoserine lactones influence the reaction of plants to rhizobia. Int. J. Mol. Sci. 2013, 14, 17122–17146. [Google Scholar] [CrossRef]
- Bais, H.P. Shoot the messages not the messengers. Plant Soil 2012, 358, 7–10. [Google Scholar] [CrossRef]
- Delalande, L.; Faure, D.; Raffoux, A.; Uroz, S.; D’Angelo-Picard, C.; Elasri, M.; Carlier, A.; Berruyer, R.; Petit, A.; Williams, P.; Dessaux, Y. N-hexanoyl-l-homoserine lactone, a mediator of bacterial quorum-sensing regulation, exhibits plant-dependent stability and may be inactivated by germinating Lotus corniculatus seedlings. FEMS Microbiol. Ecol. 2005, 52, 13–20. [Google Scholar] [CrossRef]
- Keshavan, N.D.; Chowdhary, P.K.; Haines, D.C.; Gonzalez, J.E.L. Canavanine made by Medicago sativa interferes with quorum sensing in Sinorhizobium meliloti. J. Bacteriol. 2005, 187, 8427–8436. [Google Scholar] [CrossRef]
- Teplitski, M.; Robinson, J.B.; Bauer, W.D. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant-Microbe Interact. 2000, 13, 637–648. [Google Scholar] [CrossRef]
- Götz, C.; Fekete, A.; Gebefuegi, I.; Forczek, S.T.; Fuksová, K.; Li, X.; Englmann, M.; Gryndler, M.; Hartmann, A.; Matucha, M.; Schmitt-Kopplin, P.; Schröder, P. Uptake, degradation and chiral discrimination of N-acyl-d/l-homoserine lactones by barley (Hordeum vulgare) and yam bean (Pachyrhizus erosus) plants. Anal. Bioanal. Chem. 2007, 389, 1447–1457. [Google Scholar] [CrossRef]
- Schikora, A.; Schenk, S.T.; Stein, E.; Molitor, A.; Zuccaro, A.; Kogel, K.-H. N-acyl-homoserine lactone confers resistance toward biotrophic and hemibiotrophic pathogens via altered activation of AtMPK6. Plant Physiol. 2011, 157, 1407–1418. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Van Breusegem, F.; Eberl, L.; Hartmann, A.; Langebartels, C. Induction of systemic resistance in tomato by N-acyl-l-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
