Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps
Abstract
:1. Introduction
2. Mycorrhizal Roots Have Two Uptake Pathways for Nutrients

Contribution of the Arbuscular Mycorrhizal Symbiosis to Plant Nutrition
3. Nitrogen Uptake by Arbuscular Mycorrhizal Fungi
3.1. Uptake of Inorganic N Sources
| Pathway/Enzyme | Fungal Species | Tissue | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spore | ERM | AM | IRM | ||||||
| Inorganic N uptake | |||||||||
| NH4+ uptake | Fumo, Rhir, Acla, Gima | + | + | nd | nd | [50,58,60,61,62,64,70,71] | |||
| NH4+ transporter | Rhir | + | + | + | + | [53,72,73] | |||
| NO3− uptake | Glfa, Rhir, Fumo | + | + | nd | nd | [62,63,64,65,70,71] | |||
| NO3− transporter | Rhir | + | + | + | + | [71,72,74,75,76] | |||
| Nitrate permease | Rhir | + | + | + | + | [76] | |||
| Organic N uptake | |||||||||
| Acla, Rhir, Gima | nd | +/− | nd | nd | [60] | ||||
| Fumo | nd | + | + | nd | [45,58] | ||||
| nd | − | nd | nd | [77] | |||||
| Amino acid transporter | + | + | + | + | [72] | ||||
| Amino acid permease | Fumo | − | + | + | nd | [78] | |||
| Peptide transporter | Rhir | nd | + | + | + | [79] | |||
| Alanine | Rhir | − | nd | nd | nd | [71] | |||
| Arginine | Rhir | − | + | nd | nd | [50,71] | |||
| Cysteine | Rhir | − | + | nd | nd | [80] | |||
| Glycine | Rhir, Fumo, un | + | + | nd | nd | [62,71] | |||
| Glutamate | Fumo, Rhir | + | + | nd | nd | [62,71] | |||
| Glutamine | Fumo, Rhir | + | + | nd | nd | [71,81] | |||
| Methionine | Rhir | − | + | nd | nd | [80] | |||
| Ornithine | Rhir | + | nd | nd | nd | [71] | |||
| Urea 4 | Gima | + | + | nd | nd | [71,82] | |||
| Nitrate reduction | |||||||||
| Nitrate reductase1 | Glfa, Rhir, Fumo, Glma, Glsp | + | nd | + | nd | [72,83,84,85,86] | |||
| Nitrate reductase (NADH) | Un, Fumo | + | + | + | nd | [52,87,88,89] | |||
| Nitrate reductase (NADPH) | Rhir | nd | + | + | + | [52,90] | |||
| Nitrite reductase | Rhir | nd | + | + | + | [72,84] | |||
| GDH pathway | |||||||||
| GDH1 | Clet | nd | nd | + | + | [91] | |||
| GDH (NADH) | Fumo, Rhir | + | + | + | + | [71,72,92] | |||
| GDH (NADPH) | Glfa | nd | nd | +/− | nd | [83,93] | |||
| GS/GOGAT pathway | |||||||||
| Glutamine synthetase | Glfa, Fumo, Rhir | + | + | + | + | [71,72,74,75,81,83,84,93,94] | |||
| Glutamate synthase (NADH) | Rhir | + | + | + | + | [64,72,74,75,84,94] | |||
| Glutamate synthase (NADPH) | Glfa, Rhir | nd | nd | + | + | [72,83] | |||
| Amino acid biosynthesis | |||||||||
| Transaminases | Glfa | nd | nd | + | nd | [83,95] | |||
| Asparagine synthase | Rhir | + | + | + | + | [72] | |||
| Arginine biosynthesis | |||||||||
| Carbamoyl-P synthase | Rhir | + | + | + | + | [72,74,75] | |||
| Argininosuccinate synthase | Rhir | + | + | + | + | [72,74,75] | |||
| Argininosuccinate lyase | Rhir | + | + | + | + | [72,74,75] | |||
| Arginine breakdown | |||||||||
| Arginase | Rhir | + | + | + | + | [72,74,75] | |||
| Urease | Rhcl, Rhir | + | + | + | + | [72,74,75] | |||
| Urease accessory protein | Rhir | + | + | + | + | [71,72,75,94] | |||
| Ornithine aminotransferase | Rhir | + | + | + | + | [71,72,74,75,94] | |||
| Polyamine biosynthesis | |||||||||
| Ornithine decarboxylase | Rhir | + | + | + | + | [72,74,75] | |||
| N uptake from interface | Plant species | ||||||||
| Plant NH4+ transporter | Medicago truncatula | na | na | + | na | [39,41] | |||
| Lotus japonicus | na | na | + | na | [38] | ||||
| Glycine max | na | na | + | na | [36] | ||||
| Sorghum bicolor | na | na | + | na | [37] | ||||
3.2. Uptake of Organic N by Hyphae
4. Nitrogen Assimilation and Transport by Arbuscular Mycorrhizal Fungi
4.1. Nitrate Reduction in Arbuscular Mycorrhizal Fungi
4.2. Nitrogen Assimilation into Amino Acids in Arbuscular Mycorrhizal Fungi
4.3. Nitrogen Transport from the Extraradical Mycelium to the Intraradical Mycelium
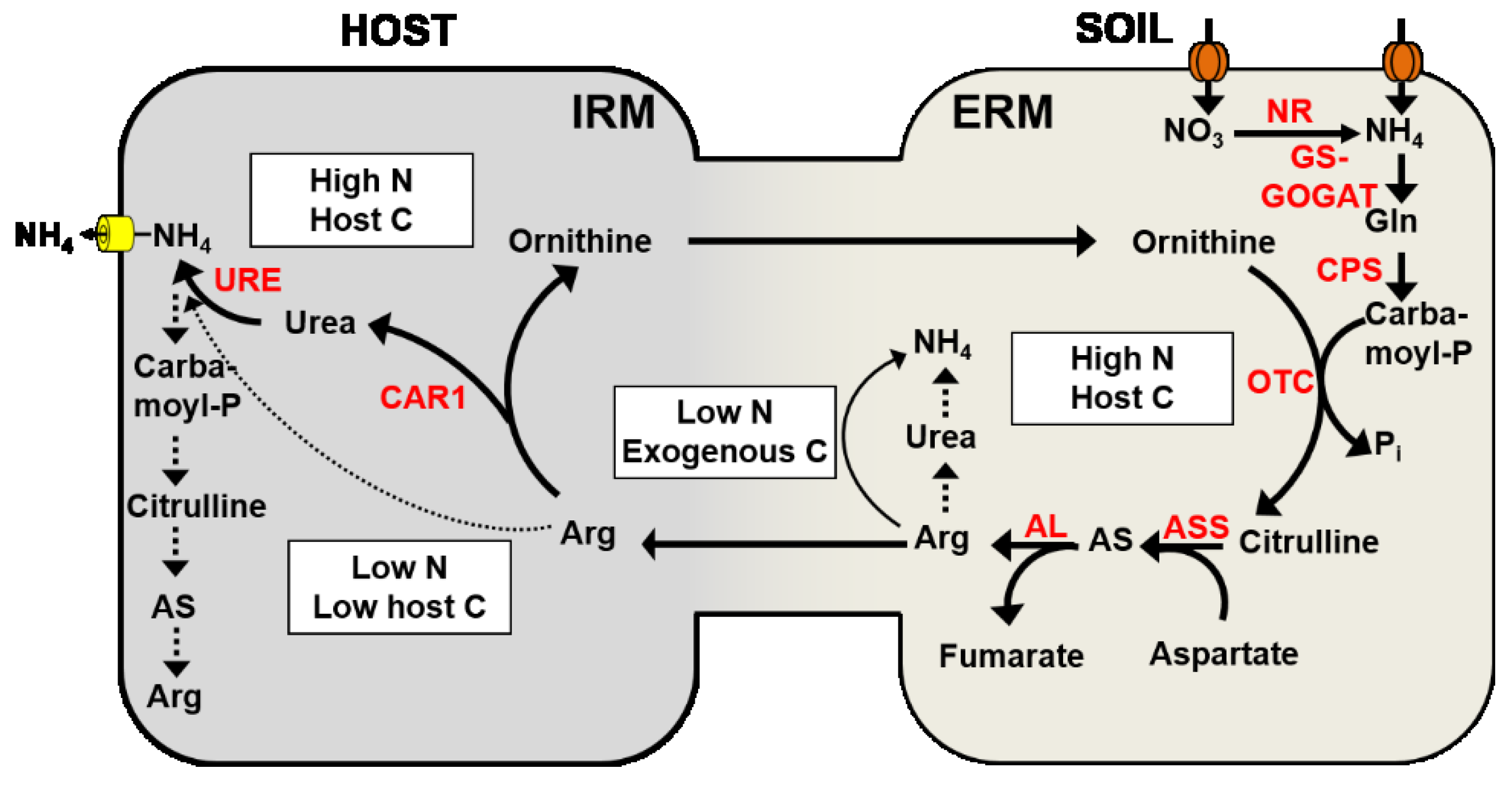
4.4. Nitrogen Transport across the Mycorrhizal Interface
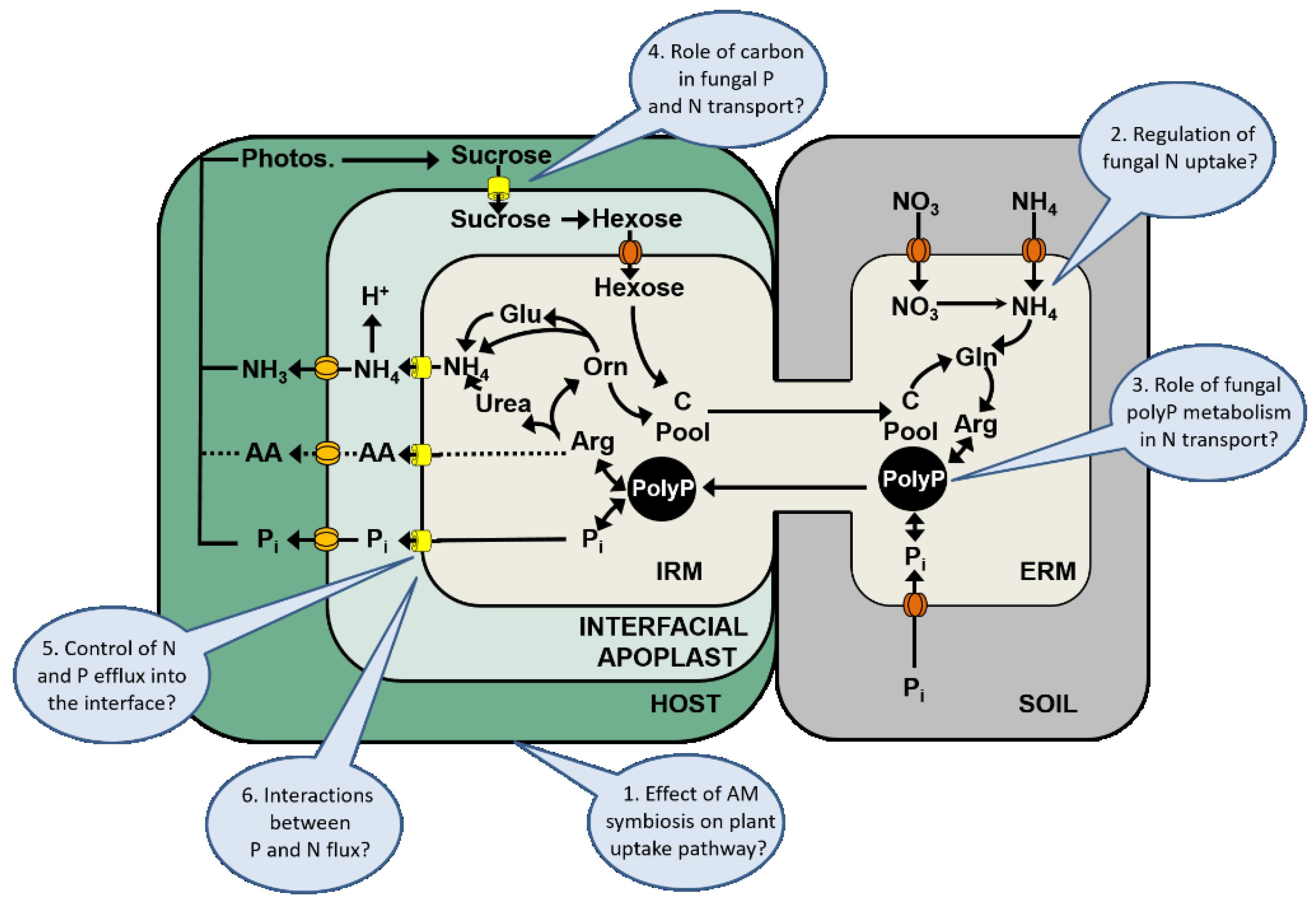
5. Conclusions
5.1. Effect of the Arbuscular Mycorrhizal Symbiosis on the Plant Uptake Pathway
5.2. Regulation of Fungal N Uptake
5.3. Role of Fungal PolyP Metabolism in N Transport
5.4. Role of Carbon in Fungal P and N Transport
5.5. Control of Fungal N and P Efflux into the Interface
5.6. Interactions between P and N Flux
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizae in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.H.; Graham, J.H. Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant Soil 2002, 244, 263–271. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Cameron, D.D. Arbuscular mycorrhizal fungi as (agro)ecosystem engineers. Plant Soil 2010, 333, 1–5. [Google Scholar] [CrossRef]
- Wright, D.P.; Read, D.J.; Scholes, J.D. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ. 1998, 21, 881–891. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. How useful is the mutualism-parasitism continuum of arbuscular mycorrhizal functioning? Plant Soil 2013, 363, 7–18. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H. The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil 2013, 363, 411–419. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Mensah, J.A.; Fellbaum, C.R. Common mycorrhizal networks and their effect on the bargaining power of the fungal partner in the arbuscular mycorrhizal symbiosis. Comp. Integr. Biol. 2015, in press. [Google Scholar]
- Fellbaum, C.R.; Mensah, J.A.; Cloos, A.J.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Fungal nutrient allocation in common mycelia networks is regulated by the carbon source strength of individual host plants. New Phytol. 2014, 203, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Walder, F.; Niemann, H.; Natarajan, M.; Lehmann, M.F.; Boller, T.; Wiemken, A. Mycorrhizal networks: Common goods of plants shared under unequal terms of trade. Plant Physiol. 2012, 159, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Babikova, Z.; Gilbert, L.; Bruce, T.J.A.; Birkett, M.; Caulfield, J.C.; Woodcock, C.; Pickett, J.A.; Johnson, D. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett. 2013, 16, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Teste, F.P.; Veneklaas, E.J.; Dixon, K.W.; Lambers, H. Is nitrogen transfer among plants enhanced by contrasting nutrient-acquisition strategies? Plant Cell Environ. 2015, 38, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gorzelak, M.A.; Asay, A.K.; Pickles, B.J.; Simard, S.W. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AOB Plants 2015. [Google Scholar] [CrossRef] [PubMed]
- Barto, E.K.; Hilker, M.; Muller, F.; Mohney, B.K.; Weidenhamer, J.D.; Rillig, M.C. The fungal fast lane: Common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS ONE 2011, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Weremijewicz, J.; Janos, D.P. Common mycorrhizal networks amplify size inequality in Andropogon gerardii monocultures. New Phytol. 2013, 198, 203–213. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, M.; Qiu, G.Y.; Zhou, J. Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J. Plant Ecol. 2009, 2, 107–118. [Google Scholar] [CrossRef]
- Babikova, Z.; Johnson, D.; Bruce, T.J.A.; Pickett, J.; Gilbert, L. Underground allies: How and why do mycelial networks help plants defend themselves? Bioassays 2013, 36, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.L.; Hartley, A.E.; Vogelsang, K.M.; Bever, J.D.; Schultz, P.A. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol. 2005, 167, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: The contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 2004, 162, 511–524. [Google Scholar] [CrossRef]
- Bücking, H.; Kuhn, A.J.; Schröder, W.H.; Heyser, W. The fungal sheath of ectomycorrhizal pine roots: An apoplastic barrier for the entry of calcium, magnesium, and potassium into the root cortex? J. Exp. Bot. 2002, 53, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, P.; Heyser, W. Apoplastic transport through the fungal sheath of Pinus sylvestris/Suillus bovinus ectomycorrhizae. Bot. Acta 1992, 105, 427–434. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Li, H.Y.; Smith, S.E.; Holloway, R.E.; Zhu, Y.G.; Smith, F.A. Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol. 2006, 172, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J.; Liu, H.; Harrison, M.J. The spatial expression patterns of a phosphate transporter (MtPt1) from Medicago truncatula indicate a role in phosphate transport at the root/soil interface. Plant J. 2001, 25, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, U.; Guo, W.B.; Fischer, K.; Isayenkov, S.; Ludwig-Müller, J.; Hause, B.; Yan, X.L.; Küster, H.; Franken, P. Overlapping expression patterns and differential transcript levels of phosphate transporter genes in arbuscular mycorrhizal, Pi-fertilised and phytohormone-treated Medicago truncatula roots. Planta 2009, 229, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Dewbre, G.R.; Liu, J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 2002, 14, 2413–2429. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, U.; Kroken, U.; Roux, C.; Briggs, S.P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 13324–13329. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Chague, V.; Melamed-Bessudo, C.; Kapulnik, Y.; Jain, A.; Raghothama, K.G.; Levy, A.A.; Silber, A. Functional characterization of LePt4: A phosphate transporter in tomato with mycorrhiza-enhanced expression. J. Exp. Bot. 2007, 58, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, S.H. Relative quantitative rt-pcr to study the expression of plant nutrient transporters in arbuscular mycorrhizas. Plant Sci. 2001, 160, 899–904. [Google Scholar] [CrossRef]
- Kobae, Y.; Tamura, Y.; Takai, S.; Banba, M.; Hata, S. Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean. Plant Cell Physiol. 2010, 51, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Koegel, S.; Lahmidi, N.A.; Arnould, C.; Chatagnier, O.; Walder, F.; Ineichen, K.; Boller, T.; Wipf, D.; Wiemken, A.; Courty, P.E. The family of ammonium transporters (AMT) in Sorghum bicolor: Two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol. 2013, 198, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Guether, M.; Neuhauser, B.; Balestrini, R.; Dynowski, M.; Ludewig, U.; Bonfante, P. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009, 150, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.K.; Javot, H.; Deewatthanawong, P.; Torres-Jerez, I.; Tang, Y.; Blancaflor, E.B.; Udvardi, M.K.; Harrison, M.J. Medicago truncatula and Glomus intraradices gene expression in cortical cells harboring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Penmetsa, R.V.; Terzaghi, N.; Cook, D.R.; Harrison, M.J. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2007, 104, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B.; et al. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter 4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 2015. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tienda, J.; Valderas, A.; Camañes, G.; García-Agustín, P.; Ferrol, N. Kinetics of NH4+ uptake by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mycorrhiza 2012, 22, 485–491. [Google Scholar] [CrossRef] [PubMed]
- George, E.; Marschner, H.; Jakobsen, I. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit. Rev. Biotechnol. 1995, 15, 257–270. [Google Scholar] [CrossRef]
- Hawkins, H.J.; George, E. Effect of plant nitrogen status on the contribution of arbuscular mycorrhizal hyphae to plant nitrogen uptake. Physiol. Plant. 1999, 105, 694–700. [Google Scholar] [CrossRef]
- Saia, S.; Benitéz, E.; Garcia-Garrido, J.M.; Settanni, L.; Amato, G.; Giambalvo, D. The effect of arbuscular mycorrhizal fungi on total plant nitrogen uptake and nitrogen recovery from soil organic material. J. Agric. Sci. 2014, 152, 370–378. [Google Scholar] [CrossRef]
- Mensah, J.A.; Koch, A.M.; Antunes, P.M.; Hart, M.M.; Kiers, E.T.; Bücking, H. High functional diversity within arbuscular mycorrhizal fungal species is associated with differences in phosphate and nitrogen uptake and fungal phosphate metabolism. Mycorrhiza 2015, 25, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.; Cruz, C.; Ferrol, N. Nitrogen and carbon/nitrogen dynamics in arbuscular mycorrhiza: The great unknown. Mycorrhiza 2015, 25, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Azcon-Aguilar, C.; Alba, C.; Montilla, M.; Barea, J.M. Isotopic (15N) evidence of the use of less available N forms by VA mycorrhizas. Symbiosis 1993, 15, 39–48. [Google Scholar]
- Toussaint, J.P.; St-Arnaud, M.; Charest, C. Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and RI t-DNA roots of Daucus carota l. In an vitro compartmented system. Can. J. Microbiol. 2004, 50, 251–260. [Google Scholar] [PubMed]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yano, K. Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ. 2005, 28, 1247–1254. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Schmelzer, E.; Bothe, H. Expression of nitrate transporter genes in tomato colonized by an arbuscular mycorrhizal fungus. Physiol. Plant. 2002, 115, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tienda, J.; Testillano, P.S.; Balestrini, R.; Fiorilli, V.; Azcón-Aguilar, C.; Ferrol, N. GintAmt2, a new member of the ammonium transporter family in the arbuscular mycorrhizal fungus Glomus intraradices. Fungal Genet. Biol. 2011, 48, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, L.; Huguet, S.; Wipf, D.; Pauly, N.; Truong, H.N. Combined phosphate and nitrogen limitation generates a nutrient stress transcriptome favorable for arbuscular mycorrhizal symbiosis in Medicago truncatula. New Phytol. 2013, 199, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Penmetsa, R.V.; Breuillin, F.; Bhattarai, K.K.; Noar, R.D.; Gomez, S.K.; Zhang, Q.; Cook, D.R.; Harrison, M.J. Medicago truncatula MtPt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. Plant J. 2011, 68, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Ames, R.N.; Reid, C.P.P.; Porter, L.K.; Cambardella, C. Hyphal uptake and transport of nitrogen from two 15N-labelled sources by Glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol. 1983, 95, 381–396. [Google Scholar] [CrossRef]
- Barea, J.M.; Azcón-Aguilar, C.; Azcón, R. Vesicular-arbuscular mycorrhiza improve both symbiotic N2 fixation and N uptake from soil as assessed with a 15N technique under field conditions. New Phytol. 1987, 106, 717–725. [Google Scholar] [CrossRef]
- Frey, B.; Schüepp, H. Acquisition of nitrogen by external hyphae of arbuscular mycorrhizal fungi associated with Zea mays L. New Phytol. 1993, 124, 221–230. [Google Scholar] [CrossRef]
- Johansen, A.; Jakobsen, I.; Jensen, E.S. Hyphal transport by a vesicular-arbuscular mycorrhizal fungus of N applied to the soil as ammonium or nitrate. Biol. Fertil. Soils 1993, 16, 66–70. [Google Scholar] [CrossRef]
- Hawkins, H.J.; Johansen, A.; George, E. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil 2000, 226, 275–285. [Google Scholar] [CrossRef]
- Tobar, R.; Azcón, R.; Barea, J.M. Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol. 1994, 126, 119–122. [Google Scholar] [CrossRef]
- Johansen, A.; Finlay, R.D.; Olsson, P.A. Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 1996, 133, 705–712. [Google Scholar] [CrossRef]
- Bago, B.; Vierheilig, H.; Piché‚, Y.; Azcón-Aguilar, C. Nitrate depletion and pH changes induced by the extraradical mycelium of the arbuscular mycorrhizal fungus Glomus intraradices grown in monoxenic cultures. New Phytol. 1996, 133, 273–280. [Google Scholar] [CrossRef]
- Bago, B.; Azcón-Aguilar, C. Changes in the rhizospheric pH induced by arbuscular mycorrhiza formation in onion (Allium cepa L.). Z. Pflanzenernähr. Bodenkd. 1997, 160, 333–339. [Google Scholar] [CrossRef]
- Howitt, S.M.; Udvardi, M.K. Structure, function and regulation of ammonium transporters in plants. Biochim. Biophys. Acta 2000, 1465, 152–170. [Google Scholar] [CrossRef]
- D’Apuzzo, E.; Rogato, A.; Simon-Rosin, U.; El Alaoui, H.; Barbulova, A.; Betti, M.; Dimou, M.; Katinakis, P.; Marquez, A.; Marini, A.M.; et al. Characterization of three functional high-affinity ammonium transporters in Lotus japonicus with differential transcriptional regulation and spatial expression. Plant Physiol. 2004, 134, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Schüßler, A.; Walker, C. The Glomeromycota. A species list with new families and new genera; Libraries at the Royal Botanic Garden Edinburgh, The Royal Botanic Garden Kew, Botanische Staatssammlung Munich, and Oregon State University: Gloucester, UK, 2010. [Google Scholar]
- Hawkins, H.J.; George, E. Reduced 15N-nitrogen transport through arbuscular mycorrhizal hyphae to Triticum aestivum L. supplied with ammonium vs. nitrate nutrition. Ann. Bot. 2001, 87, 303–311. [Google Scholar] [CrossRef]
- Gachomo, E.; Allen, J.W.; Pfeffer, P.E.; Govindarajulu, M.; Douds, D.D.; Jin, H.R.; Nagahashi, G.; Lammers, P.J.; Shachar-Hill, Y.; Bücking, H. Germinating spores of Glomus intraradices can use internal and exogenous nitrogen sources for de novo biosynthesis of amino acids. New Phytol. 2009, 184, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, E.; Kohler, A.; Dozolme-Seddas, P.; Balestrini, R.; Benabdellah, K.; Colard, A.; Croll, D.; da Silva, C.; Gomez, S.K.; Koul, R.; et al. The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 2012, 193, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pedrosa, A.; González-Guerrero, M.; Valderas, A.; Azcón-Aguilar, C.; Ferrol, N. GintAmt1 encodes a functional high-affinity ammonium transporter that is expressed in the extraradical mycelium of Glomus intraradices. Fungal Genet. Biol. 2006, 43, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Kasiborski, B.; Koul, R.; Lammers, P.J.; Bücking, H.; Shachar-Hill, Y. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: Gene characterization and the coordination of expression with nitrogen flux. Plant Physiol. 2010, 153, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frey, N.F.D.; Gianinazzi-Pearson, V.; et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 2014, 111, 563–563. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Robinson, D.; Fitter, A.H. An arbuscular mycorrhizal inoculum enhances root proliferation in, but not nitrogen capture from, nutrient-rich patches in soil. New Phytol. 2000, 145, 575–584. [Google Scholar] [CrossRef]
- Cappellazzo, G.; Lanfranco, L.; Fitz, M.; Wipf, D.; Bonfante, P. Characterization of an amino acid permease from the endomycorrhizal fungus Glomus mosseae. Plant Physiol. 2008, 147, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Belmondo, S.; Fiorilli, V.; Pérez-Tienda, J.; Ferrol, N.; Marmeisse, R.; Lanfranco, L. A dipeptide transporter from the arbuscular mycorrhizal fungus Rhizophagus irregularis is upregulated in the intraradical phase. Front. Plant Sci. 2014, 5, 436. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Shachar-Hill, Y. Sulfur transfer through an arbuscular mycorrhiza. Plant Physiol. 2009, 149, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, M.; Trujillo, C.G.; Serrano, E.; Fischer, R.; Requena, N. Different nitrogen sources modulate activity but not expression of glutamine synthetase in arbuscular mycorrhizal fungi. Fungal Genet. Biol. 2004, 41, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Takahashi, K.; Koide, R.T.; Kimura, M. Determination of the nitrogen source for arbuscular mycorrhizal fungi by 15N application to soil and plants. Mycorrhiza 2001, 10, 267–273. [Google Scholar] [CrossRef]
- Cliquet, J.B.; Stewart, G.R. Ammonia assimilation in Zea mays L. infected with a vesicular-arbuscular mycorrhizal fungus Glomus fasciculatum. Plant Physiol. 1993, 101, 865–871. [Google Scholar] [PubMed]
- Subramanian, K.S.; Charest, C. Arbuscular mycorrhizae and nitrogen assimilation in maize after drought and recovery. Physiol. Plant. 1998, 102, 285–296. [Google Scholar] [CrossRef]
- Kaldorf, M.; Zimmer, W.; Bothe, H. Genetic evidence for the occurence of assimilatory nitrate reductase in arbuscular mycorrhizal and other fungi. Mycorrhiza 1994, 5, 23–28. [Google Scholar] [CrossRef]
- Ho, I.; Trappe, J.M. Nitrate reducing capacity of two vesicular-arbuscular mycorrhizal fungi. Mycologia 1975, 67, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.J.; Smith, S.E.; Nicholas, D.J.D.; Wallace, W. Activity of nitrate reductase in Trifolium subterraneum: Effects of mycorrhizal infection and phosphate nutrition. New Phytol. 1983, 94, 63–79. [Google Scholar] [CrossRef]
- Vázquez, M.; Barea, J.; Azcón, R. Impact of soil nitrogen concentration on Glomus spp.-Sinorhizobium interactions as affecting growth, nitrate reductase activity and protein content of Medicago sativa. Biol. Fertil. Soils 2001, 34, 57–63. [Google Scholar]
- Hawkins, H.J.; Cramer, M.D.; George, E. Root respiratory quotient and nitrate uptake in hydroponically grown non-mycorrhizal and mycorrhizal wheat. Mycorrhiza 1999, 9, 57–60. [Google Scholar] [CrossRef]
- Kaldorf, M.; Schmelzer, E.; Bothe, H. Expression of maize and fungal nitrate reductase genes in arbuscular mycorrhiza. MPMI 1998, 11, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Nemec, S. Histochemical characterization of Glomus etunicatus infection of Citrus limon fibrous roots. Can. J. Bot. 1981, 59, 609–617. [Google Scholar]
- MacDonald, R.M.; Lewis, M. The occurence of some acid phosphatases and dehydrogenases in the vesicular-arbuscular mycorrhizal fungus Glomus mosseae. New Phytol. 1978, 80, 135–141. [Google Scholar] [CrossRef]
- Smith, S.E.; St John, B.J.; Smith, F.A.; Nicholas, D.J.D. Activity of glutamine synthetase and glutamate dehydrogenase in Trifolium subterraneum L. and Allium cepa L.: Effects of mycorrhizal infection and phosphate nutrition. New Phytol. 1985, 99, 211–227. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.R.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Faure, S.; Cliquet, J.B.; Thephany, G.; Boucaud, J. Nitrogen assimilation in Lolium perenne colonized by the arbuscular mycorrhizal fungus Glomus fasciculatum. New Phytol. 1998, 138, 411–417. [Google Scholar] [CrossRef]
- Valentine, A.J.; Osborne, B.A.; Mitchell, D.T. Form of inorganic nitrogen influences mycorrhizal colonisation and photosynthesis of cucumber. Sci. Hortic. 2002, 92, 229–239. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, N.; Gao, K.; Chen, F.; Yuan, L.; Mi, G. Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS ONE 2013, 8, e61031. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.D.; Lewis, O.A.M. The influence of NO3− and NH4+ nutrition on the carbon and nitrogen partitioning characteristics of wheat (Triticum aestivum) and maize (Zea mays) plants. Plant Soil 1993, 154, 289–300. [Google Scholar] [CrossRef]
- Cramer, M.D.; Lewis, O.A.M.; Lips, S.H. Inorganic carbon fixation and metabolism in maize roots as affected by nitrate and ammonium nutrition. Physiol. Plant. 1993, 89, 632–639. [Google Scholar] [CrossRef]
- Cooper, T.G.; Sumrada, R.A. What is the function of nitrogen catabolite repression in Saccharomyces cerevisiae? J. Bacteriol. 1983, 155, 623–627. [Google Scholar] [PubMed]
- Javelle, A.; Morel, M.; Rodriguez-Pastrana, B.R.; Botton, B.; Andr‚, B.; Marini, A.M.; Brun, A.; Chalot, M. Molecular characterization, function and regulation of ammonium transporters (Amt) and ammonium-metabolizing enzymes (GS, NADP-GDH) in the ectomycorrhizal fungus Hebeloma cylindrosporum. Mol. Microbiol. 2003, 47, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Navarro, F.J.; Machín, F.; Martín, Y.; Siverio, J.M. Down-regulation of eukaryotic nitrate transporter by nitrogen-dependent ubiquitinylation. J. Biol. Chem. 2006, 281, 13268–13274. [Google Scholar] [CrossRef] [PubMed]
- Schulten, H.-R.; Schnitzer, M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1998, 26, 1–15. [Google Scholar] [CrossRef]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Fitter, A.H. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13754–13759. [Google Scholar] [CrossRef] [PubMed]
- Thirkell, J.D.; Cameron, D.D.; Hodge, A. Resolving the “nitrogen paradox” of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ. 2015. [Google Scholar] [CrossRef] [PubMed]
- Atul-Nayyar, A.; Hamel, C.; Hanson, K.; Germida, J. The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 2009, 19, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.J.; Firestone, M.K.; Nuccio, E.; Hodge, A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 2012, 80, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Chang. Biol. 2015, 21, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, E.E.; Hodge, A.; Pett-Ridge, J.; Herman, D.J.; Weber, P.K.; Firestone, M.K. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ. Microbiol. 2013, 15, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Cliquet, J.B.; Murray, P.J.; Boucaud, J. Effect of the arbuscular mycorrhizal fungus Glomus fasciculatum on the uptake of amino nitrogen by Lolium perenne. New Phytol. 1997, 137, 345–349. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Digman, M.A.; Gratton, E.; Treseder, K.K. Organic nitrogen uptake by arbuscular mycorrhizal fungi in a boreal forest. Soil Biol. Biochem. 2012, 55. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, M.D.; Garcia, M.O.; Treseder, K.K. Amino acid uptake in arbuscular mycorrhizal plants. PLoS ONE 2012, 7, e47643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guescini, M.; Zeppa, S.; Pierleoni, R.; Sisti, D.; Stocchi, L.; Stocchi, V. The expression profile of the Tuber borchii nitrite reductase suggests its positive contribution to host plant nitrogen nutrition. Curr. Genet. 2007, 51, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bailly, J.; Debaud, J.C.; Verner, M.C.; Plassard, C.; Chalot, M.; Marmeisse, R.; Fraissinet-Tachet, L. How does a symbiotic fungus modulate expression of its host-plant nitrite reductase? New Phytol. 2007, 175, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Temple, S.J.; Vance, C.P.; Gantt, S.J. Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 1998, 3, 51–56. [Google Scholar] [CrossRef]
- Botton, B.; Chalot, M. Nitrogen assimilation: Enzymology in ectomycorrhizas. In Mycorrhiza; Varma, A., Hock, B., Eds.; Springer-Verlag: Berlin, Germany, 1995; pp. 325–363. [Google Scholar]
- Rolin, D.; Pfeffer, P.E.; Douds, D.D.; Farrell, H.M.; Shachar-Hill, Y. Arbuscular mycorrhizal symbiosis and phosphorus nutrition: Effects on amino acid production and turnover in leek. Symbiosis 2001, 30, 1–14. [Google Scholar]
- Forde, B.G. Nitrate transporters in plants: Structure, function and regulation. Biochim. Biophys. Acta 2000, 1465, 219–235. [Google Scholar] [CrossRef]
- Marzluf, G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 1997, 61, 17–32. [Google Scholar] [PubMed]
- Cruz, C.; Egsgaard, H.; Trujillo, C.; Ambus, P.; Requena, N.; Martins-Loucao, M.A.; Jakobsen, I. Enzymatic evidence for the key role of arginine in nitrogen translocation by arbuscular mycorrhizal fungi. Plant Physiol. 2007, 144, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Moran, K.J.; Sanders, F.; Nockolds, C.; Tinker, P.B. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas. III. Polyphosphate granules and phosphorus translocation. New Phytol. 1980, 84, 649–659. [Google Scholar] [CrossRef]
- Cramer, C.L.; Davis, H.H. Polyphosphate-cation interaction in the amino acid-containing vacuole of Neurospora crassa. J. Biol. Chem. 1984, 259, 5152–5157. [Google Scholar] [PubMed]
- Cramer, C.L.; Vaughn, L.E.; Davis, R.H. Basic amino acids and inorganic polyphosphates in Neurospora crassa: Independent regulation of vacuolar pools. J. Bacteriol. 1980, 142, 945–952. [Google Scholar] [PubMed]
- Westenberg, B.; Boller, T.; Wiemken, A. Lack of arginine- and polyphosphate-storage pools in a vacuole-deficient mutant (end1) of Saccharomyces cerevisiae. FEBS Lett. 1989, 254, 133–136. [Google Scholar] [CrossRef]
- Bücking, H.; Heyser, W. Elemental composition and function of polyphosphates in ectomycorrhizal fungi - an X-ray microanalytical study. Mycol. Res. 1999, 103, 31–39. [Google Scholar] [CrossRef]
- George, E.; Häussler, K.U.; Vetterlein, D.; Gorgus, E.; Marschner, H. Water and nutrient translocation by hyphae of Glomus mosseae. Can. J. Bot. 1992, 70, 2130–2137. [Google Scholar] [CrossRef]
- Bücking, H.; Heyser, W. Uptake and transfer of nutrients in ectomycorrhizal associations: Interactions between photosynthesis and phosphate nutrition. Mycorrhiza 2003, 13, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Shachar-Hill, Y. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 2005, 165, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Guether, M.; Volpe, V.; Balestrini, R.; Requena, N.; Wipf, D.; Bonfante, P. LjLht1.2—A mycorrhiza-inducible plant amino acid transporter from Lotus japonicus. Biol. Fertil. Soils 2011, 47, 925–936. [Google Scholar] [CrossRef]
- Beever, R.E.; Burns, D.J.W. Phosphorus uptake, storage and utilization by fungi. Adv. Bot. Res. 1980, 8, 127–219. [Google Scholar]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Plassard, C. Aquaporins: For more than water at the plant–fungus interface? New Phytol. 2011, 190, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Bago, A.; Sutka, M.; Paz, J.A.; Cano, C.; Amodeo, G.; Ruiz-Lozano, J.M. Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Mol. Plant Microb. Interact. 2009, 22, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.A.; Huang, W.; Liu, F.C.; Tang, N.W.; Liu, Y.; Lin, H.; Zhao, B. Functional analysis of the novel mycorrhiza-specific phosphate transporter AsPt1 and Pht1 family from Astragalus sinicus during the arbuscular mycorrhizal symbiosis. New Phytol. 2013, 198, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Wang, C.; Shou, H.; Whelan, J. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett. 2012, 586, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Carbonnel, S.; Gutjahr, C. Control of arbuscular mycorrhiza development by nutrient signals. Front. Plant Sci. 2014, 5, 462. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bücking, H.; Kafle, A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy 2015, 5, 587-612. https://doi.org/10.3390/agronomy5040587
Bücking H, Kafle A. Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy. 2015; 5(4):587-612. https://doi.org/10.3390/agronomy5040587
Chicago/Turabian StyleBücking, Heike, and Arjun Kafle. 2015. "Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps" Agronomy 5, no. 4: 587-612. https://doi.org/10.3390/agronomy5040587
APA StyleBücking, H., & Kafle, A. (2015). Role of Arbuscular Mycorrhizal Fungi in the Nitrogen Uptake of Plants: Current Knowledge and Research Gaps. Agronomy, 5(4), 587-612. https://doi.org/10.3390/agronomy5040587






