Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities
Abstract
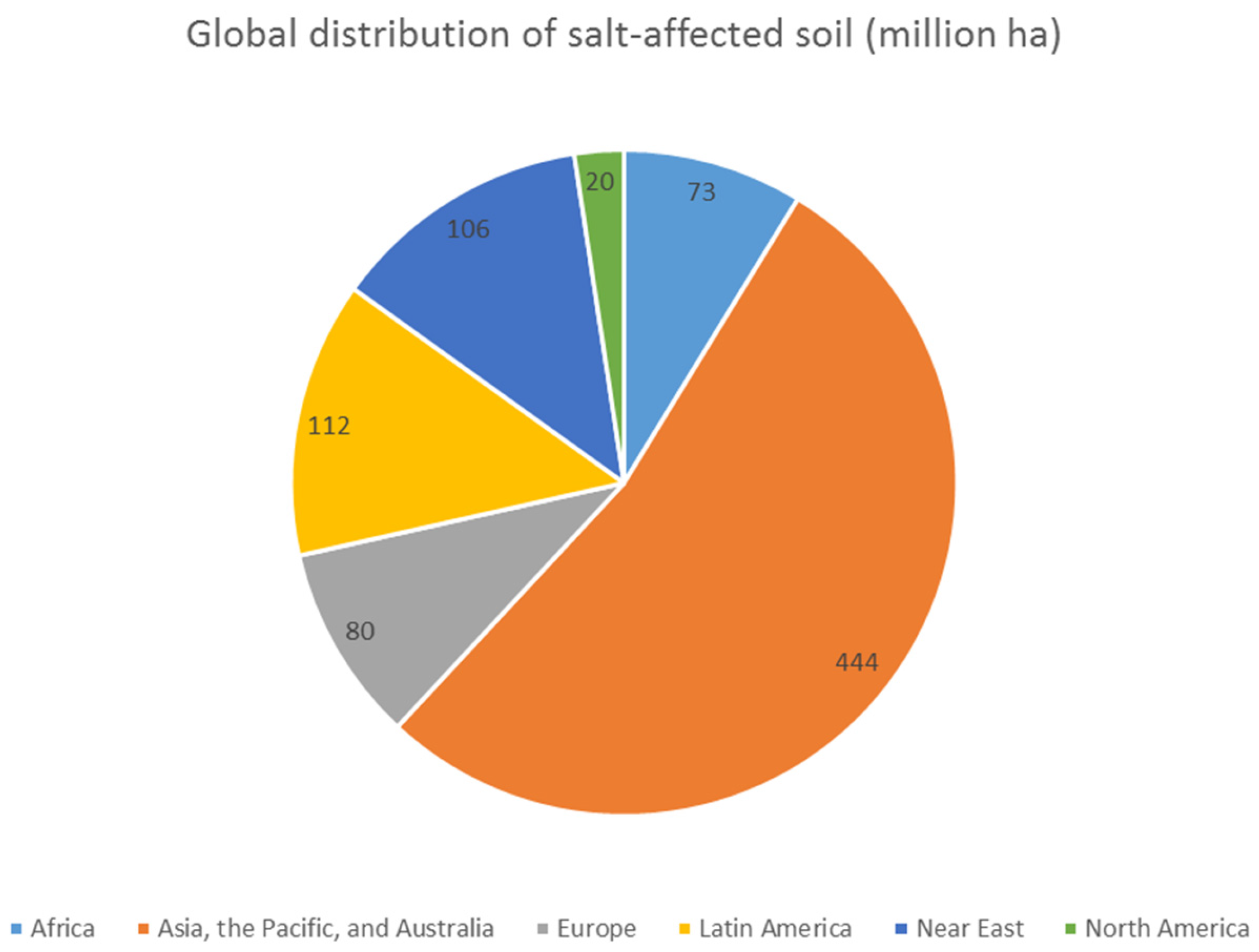
:1. Introduction
2. Rice Response to Salinity Stress
2.1. Biosynthesis and Accumulation of Organic Osmolytes
2.2. Ion Homeostasis and Compartmentation
2.3. Antioxidants—ROS Detoxification
2.4. Programmed Cell Death (PCD)
3. Approaches to Improve Salinity Stress Tolerance in Rice—Progress and Challenges
3.1. Conventional Breeding
3.2. Marker Assisted Selection
3.3. Genetic Engineering
4. Opportunity
4.1. Wild Rice—A Potential Candidate for Desalinization Crop
4.2. Wild Rice—An Invaluable Genetic Resource
4.3. The Next Frontier—The Use of Genome Editing for Integration Free Improvement of Crop Traits
4.3.1. Genome Editing Technologies
4.3.2. Strategies to Minimize Transgene Integration
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoang, T.M.L.; Williams, B.; Khanna, H.; Dale, J.; Mundree, S.G. Physiological basis of salt stress tolerance in rice expressing the antiapoptotic gene SfIAP. Funct. Plant Biol. 2014, 41, 1168–1177. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Salt-Affected Soils and Their Management. 1988. Available online: http://www.fao.org/docrep/x5871e/x5871e00.htm (accessed on 20 August 2016).
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Umali, D.L. Irrigation-Induced Salinity a Growing Problem for Development and the Environment; The Word Bank: Washington, DC, USA, 1993. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Declaration of the World Summit on Food Security. Rome, Italy, 16–18 November 2009. Available online: http://www.Fao.Org/wsfs/world-summit/wsfs-challenges/en/ (accessed on 20 August 2016).
- Food and Agriculture Organization. World Water Day 2012 Celebration. Un Conference Centre, Bangkok, 22 March 2012. Available online: http://www.Fao.Org/asiapacific/rap/home/meetings/list/detail/en/?Meetings_id=637&year=2012 (accessed on 20 August 2016).
- The United Nations Population Fund. Linking Population, Poverty and Development. Available online: http://www.unfpa.org/pds/trends.htm (accessed on 8 February 2016).
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A. The future of science: Food and water for life. Plant Cell 2009, 21, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Conti, L.; Tonelli, C.; Galbiati, M. Challenges and perspectives to improve crop drought and salinity tolerance. New Biotechnol. 2013, 30, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Plant adaptations to salt and water stress: Differences and commonalities. In Advances in Botanical Research; Ismail, T., Ed.; Elseiver: Wyman Street, Waltham, MA, USA, 2011; Volume 57, pp. 1–32. [Google Scholar]
- Linares, O.F. African rice (Oryza glaberrima): History and future potential. Proc. Natl. Acad. Sci. USA 2002, 99, 16360–16365. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.A.; Lu, B.-R.; Tomooka, N. The evolving story of rice evolution. Plant Sci. 2008, 174, 394–408. [Google Scholar] [CrossRef]
- Virmani, S.S.; llyas-Ahmed, M. Rice breeding for sustainable production. In Breeding Major Food Staples; Kang, M., Priyadarshan, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Hossain, M.; Fischer, K.S. Rice research for food security and sustainable agricultural development in Asia: Achievements and future challenges. GeoJournal 1995, 35, 286–298. [Google Scholar] [CrossRef]
- Khush, G. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Bibliography on Salt Tolerance. Fibres, Grains and Special Crops; Brown, G.E., Jr., Ed.; Salinity Laboratory United States Department of Agriculture Research Service: Riverside, CA, USA. Available online: http://www.Ars.Usda.Gov/services/docs.Htm?Docid=8908 (accessed on 20 August 2016).
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Dayton, L. Agribiotechnology: Blue-sky rice. Nature 2014, 514, S52–S54. [Google Scholar] [CrossRef] [PubMed]
- Maas, E.V.; Nieman, R.H. Physiology of plant tolerance to salinity. In Crop Tolerance to Suboptimal Land Conditions; ASS, CSA, SSSA: Madison, WI, USA, 1978; Volume 32, pp. 277–299. [Google Scholar]
- Heenan, D.P.; Lewin, L.G.; McCaffery, D.W. Salinity tolerance in rice varieties at different growth stages. Aust. J. Exp. Agric. 1988, 28, 343–349. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C.; Lesch, S.M. Timing of salinity stress affects rice growth and yield components. Agric. Water Manag. 2001, 48, 191–206. [Google Scholar] [CrossRef]
- Yeo, A.; Flowers, T. Salinity resistance in rice (Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils. Aust. J. Plant Physiol. 1986, 13, 161–173. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- James, R.A.; Rivelli, A.R.; Munns, R.; Caemmerer, S.V. Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Funct. Plant Biol. 2002, 29, 1393–1403. [Google Scholar] [CrossRef]
- Kader, M.A.; Lindberg, S. Cytosolic calcium and ph signaling in plants under salinity stress. Plant Signal. Behav. 2010, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Engler, J.; Iyer, S.; Gerats, T.; Van Montagu, M.; Caplan, A.B. Effects of osmoprotectants upon nacl stress in rice. Plant Physiol. 1997, 115, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Shriram, V.; Nikam, T.D.; Jawali, N.; Shitole, M.G. Sodium chloride-induced changes in mineral nutrients and proline accumulation in indica rice cultivars differing in salt tolerance. J. Plant Nutr. 2008, 31, 1999–2017. [Google Scholar] [CrossRef]
- Thu Hoai, N.T.; Shim, I.S.; Kobayashi, K.; Kenji, U. Accumulation of some nitrogen compounds in response to salt stress and their relationships with salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2003, 41, 159–164. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A.D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Rathinasabapathi, B. Metabolic engineering for stress tolerance: Installing osmoprotectant synthesis pathways. Ann. Bot. 2000, 86, 709–716. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Harinasut, P.; Tsutsui, K.; Takabe, T.; Nomura, M.; Takabe, T.; Kishitani, S. Exogenous glycinebetaine accumulation and increased salt-tolerance in rice seedlings. Bioscie. Biotechnol. Biochem. 1996, 60, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ros-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Cuin, T.A.; Miller, A.J.; Laurie, S.A.; Leigh, R.A. Potassium activities in cell compartments of salt-grown barley leaves. J. Exp. Bot. 2003, 54, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.R.F.; Silva, E.N.; Ferreira-Silva, S.L.; Voigt, E.L.; Viégas, R.A.; Silveira, J.A.G. High K+ supply avoids Na+ toxicity and improves photosynthesis by allowing favorable K+:Na+ ratios through the inhibition of Na+ uptake and transport to the shoots of jatropha curcas plants. J. Plant Nutr. Soil Sci. 2013, 176, 157–164. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Guo, R.; Shi, D.; Liu, B.; Lin, X.; Yang, C. Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Tobita, S. Effects of salinity on sodium content and photosynthetic responses of rice seedlings differing in salt tolerance. J. Plant Physiol. 2000, 157, 54–58. [Google Scholar] [CrossRef]
- Lee, K.-S.; Choi, W.-Y.; Ko, J.-C.; Kim, T.-S.; Gregorio, G.B. Salinity tolerance of japonica and indica rice (Oryza sativa L.) at the seedling stage. Planta 2003, 216, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Adak, M.K.; Ghosh, P.D.; Gupta, S.; Sen Gupta, D.N.; Mandal, C. Differential responses of two rice varieties to salt stress. Plant Biotechnol. Rep. 2011, 5, 89–103. [Google Scholar] [CrossRef]
- Kader, M.A.; Lindberg, S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. Determined by the fluorescent dye SBFI. J. Exp. Bot. 2005, 56, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R.O.N. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, H.; Sivakumar, P.; Chakrabarty, R.; Thomas, G. Scavenging of reactive oxygen species in nacl-stressed rice (Oryza sativa L.)—Differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003, 165, 1411–1418. [Google Scholar] [CrossRef]
- Borsani, O.; Zhu, J.; Verslues, P.E.; Sunkar, R.; Zhu, J.-K. Endogenous sirnas derived from a pair of natural cis-antisense transcripts regulate salt tolerance in arabidopsis. Cell 2005, 123, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fu, X.; Koo, Y.D.; Zhu, J.K.; Jenney, F.E.J.; Adams, M.W.; Zhu, Y.; Shi, H.; Yun, D.J.; Hasegawa, P.M.; et al. An enhancer mutant of arabidopsis salt overly sensitive 3 mediates both ion homeostasis and the oxidative stress response. Mol. Cell. Biol. 2007, 27, 5214–5224. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Jain, S.; Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2013, 22, 27–34. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Transgenic approaches for abiotic stress tolerance in plants: Retrospect and prospects. Plant Cell Rep. 2008, 27, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Hara-Nishimura, I.; Inoue, K.; Nishimura, M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991, 294, 89–93. [Google Scholar] [CrossRef]
- Greenberg, J.T. Programmed cell death: A way of life for plants. Proc. Natl. Acad. Sci. USA 1996, 93, 12094–12097. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Dickman, M. Plant programmed cell death: Can’t live with it; can’t live without it. Mol. Plant Pathol. 2008, 9, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Fomicheva, A.S.; Tuzhikov, A.I.; Beloshistov, R.E.; Trusova, S.V.; Galiullina, R.A.; Mochalova, L.V.; Chichkova, N.V.; Vartapetian, A.B. Programmed cell death in plants. Biochemistry 2012, 77, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Fu, B.-Y.; Xu, H.-X.; Zhu, L.-H.; Zhai, H.-Q.; Li, Z.-K. Cell death in response to osmotic and salt stresses in two rice (Oryza sativa L.) ecotypes. Plant Sci. 2007, 172, 897–902. [Google Scholar] [CrossRef]
- Li, J.-Y.; Jiang, A.-L.; Zhang, W. Salt stress-induced programmed cell death in rice root tip cells. J. Integr. Plant Biol. 2007, 49, 481–486. [Google Scholar] [CrossRef]
- Breseghello, F.; Coelho, A.S.G. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.M.; Francies, R.M.; Rasool, S.N.; Reddy, V.R.P. Breeding for tolerance stress triggered by salinity in rice. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 167–176. [Google Scholar]
- Ismail, A.; Heuer, S.; Thomson, M.; Wissuwa, M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol. Biol. 2007, 65, 547–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kim, D.S.; Lee, S.J.; Song, H.S.; Lim, Y.P.; Lee, Y.I. Selection and characterizations of radiation-induced salinitytolerant lines in rice. Breed. Sci. 2003, 53, 313–318. [Google Scholar] [CrossRef]
- Oo, K.S.; Lang, N.T. Developing salt tolerance by mutagenesis. Omonrice 2005, 13, 126–134. [Google Scholar]
- Mba, C.; Afza, R.; Jain, S.M.; Gregorio, G.B.; Zapata-Arias, F.J. Induced mutations for enhancing salinity tolerance in rice. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 413–454. [Google Scholar]
- Zapata, F.J.; Aldemita, R.R. Induction of salt tolerance in high yielding rice varieties through mutagenesis and anther culture. In Current Options for Cereal Improvement; Maluszyns-Ki, M., Ed.; Kluwer Acad: Dordrecht, The Netherlands, 1986; pp. 193–202. [Google Scholar]
- Miah, M.A.A.; Pathan, M.S.; Quayum, H.A. Production of salt tolerant rice breeding line via doubled haploid. Euphytica 1996, 91, 285–288. [Google Scholar] [CrossRef]
- Sathish, P.; Gamborg, O.L.; Nabors, M.W. Establishment of stable nacl-resistant rice plant lines from anther culture: Distribution pattern of K+/Na+ in callus and plant cells. Theor. Appl. Genet. 1997, 95, 1203–1209. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Nafisah, A.; Zhu, L.; Xu, J.; Li, Z. Selection efficiencies for improving drought/salt tolerances and yield using introgression breeding in rice (Oryza sativa L.). Crop J. 2013, 1, 134–142. [Google Scholar] [CrossRef]
- Yen, C.-C.; Lin, J.-H. Screening, inheritance and linkage marker analyses of salt tolerance in mutated scented japonica rice (Oryza sativa L.). Plant Prod. Sci. 2011, 14, 260–269. [Google Scholar] [CrossRef]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Norlyn, J.D.; Rush, D.W.; Kingsbury, R.W.; Kelley, D.B.; Cunningham, G.A.; Wrona, A.F. Saline culture of crops: A genetic approach. Science 1980, 210, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.C. Principles and strategies in breeding for higher salt tolerance. Plant Soil 1985, 89, 227–241. [Google Scholar] [CrossRef]
- Tal, M. Genetics of salt tolerance in higher plants: Theoretical and practical considerations. Plant Soil 1985, 89, 199–226. [Google Scholar] [CrossRef]
- Rajanaidu, N.; Zakri, A.H. Breeding for morpho-physiological traits in crop plants. In Plant Breeding and Genetic Engineering; Zakri, A.H., Ed.; SABRAO: Bangkok, Thailand, 1988; pp. 116–139. [Google Scholar]
- Yeo, A.R. Physiological criteria in screening and breeding. In Soil Mineral Stresses: Approaches to Crop Improvement; Yeo, A.R., Flowers, T.J., Eds.; Springer: Berlin, Germany, 1994; pp. 37–57. [Google Scholar]
- Li, Z.-K.; Xu, J.-L. Breeding for drought and salt tolerant rice (Oryza sativa L.): Progress and perspective. In Advances in Molecular Breeding toward Drought and Salt Tolerance Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 531–564. [Google Scholar]
- Lafitte, H.R.; Ismail, A.M.; Bennett, J. Abiotic stress tolerance in rice for asia: Progress and future. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004.
- Jena, K.K.; MacKill, D.J. Molecular markers and their use in marker-assisted selection in rice. Crop Sci. 2008, 48, 1266–1276. [Google Scholar] [CrossRef]
- Gorantla, M.; Babu, P.R.; Reddy, V.B.L.; Feltus, F.A.; Paterson, A.H.; Reddy, A.R. Functional genomics of drought stress response in rice: Transcript mapping of annotated unigenes of an indica rice (Oryza sativa L. cv. Nagina 22). Curr. Sci. 2005, 89, 496–514. [Google Scholar]
- Waziri, A.; Kumar, P.; Purty, R.S. Saltol QTL and their role in salinity tolerance in rice. Austin J. Biotechnol. Bioeng. 2016, 3, 1067–1072. [Google Scholar]
- Ren, Z.; Gao, J.; Li, L.; Cai, X.; Huang, W.; Chao, D.; Zhu, M.; Wang, Z.; Luan, S.; Lin, H. A rice quantitative trail locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005, 37, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Jena, K.K.; Seshu, D.V. Salt tolerance in wild rices. Int. Rice Res. Inst. Newsl. 1987, 12, 15. [Google Scholar]
- Flowers, T.J.; Flowers, S.A.; Hajibagheri, M.A.; Yeo, A.R. Salt tolerance in the halophytic wild rice, Porteresia coarctata takeoka. New Phytol. 1990, 114, 675–684. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Variability in the resistance of sodium chloride salinity within rice (Oryza sativa L.) varieties. New Phytol. 1981, 88, 363–373. [Google Scholar] [CrossRef]
- Quijano-Guerta, C.; Kirk, G.J.D. Tolerance of rice germplasm to salinity and other soil chemical stresses in tidal wetlands. Field Crops Res. 2002, 76, 111–121. [Google Scholar] [CrossRef]
- De Leon, T.B.; Linscombe, S.; Gregorio, G.B.; Subudhi, P.K. Genetic variation in southern USA rice genotypes for seedling salinity tolerance. Front. Plant Sci. 2015, 6, 374. [Google Scholar] [CrossRef] [PubMed]
- Sakina, A.; Ahmed, I.; Shahzad, A.; Iqbal, M.; Asif, M. Genetic variation for salinity tolerance in pakistani rice (Oryza sativa L.) germplasm. J. Agron. Crop Sci. 2016, 202, 25–36. [Google Scholar] [CrossRef]
- Bonilla, P.S.; Dvorak, J.; MacKill, D.; Deal, K.; Gregorio, G.B. RFLP and SSLP mapping of salinity tolerance genes in chromosome 1 of rice (Oryza sativa L.) using recombinant inbred lines. Philipp. Agric. Sci. 2002, 85, 64–74. [Google Scholar]
- Takeshisa, H.; Shimoda, Y.; Fukuta, Y.; Ueda, T.; Yano, M.; Yamaya, T.; Kameya, T.; Sato, T. Identification of quantitative trail loci for plant growth of rice in paddy field flooded with salt water. Field Crop Res. 2004, 89, 85–95. [Google Scholar] [CrossRef]
- Gregorio, G.B.; Senadhira, D.; Mendoza, R.D.; Manigbas, N.L.; Roxas, J.P.; Guerta, C.Q. Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res. 2002, 76, 91–101. [Google Scholar] [CrossRef]
- Suriya-Arunroj, D.; Supapoj, N.; Toojinda, T.; Vanavichit, A. Relative leaf water content as an efficient method for evaluating rice cultivars for tolerance to salt stress. Sci. Asia 2004, 30, 411–415. [Google Scholar] [CrossRef]
- Salvi, S.; Tuberosa, R. To clone or not to clone plant QTLs: Present and future challenges. Trends Plant Sci. 2005, 10, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sahi, C.; Singh, A.; Kumar, K.; Blumwald, E.; Grover, A. Salt stress response in rice: Genetics, molecular biology, and comparative genomics. Funct. Integr. Genom. 2006, 6, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Ponnaiah, M.; Krajewski, P.; Frova, C.; Gianfranceschi, L.; Pè, M.E.; Sari-Gorla, M. Addressing drought tolerance in maize by transcriptional profiling and mapping. Mol. Genet. Genom. 2009, 281, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.; Rai, V.; Bal, S.; Sinha, S.; Kumar, V.; Chauhan, M.S.; Gautam, R.K.; Singh, R.B.; Sharma, P.C.; Singh, A.K.; et al. Combining QTL mapping and transcriptome profiling of bulked rils for identification of functional polymorphism for salt tolerance genes in rice (Oryza sativa L.). Mol. Genet. Genom. 2010, 284, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.H.M.; Pandit, A.; Singh, R.K.; Sameena, S.; Chauhan, M.S.; Singh, A.K.; Sharma, P.C.; Gaikwad, K.; Sharma, T.R.; Mohapatra, T.; et al. Mapping of QTLs controlling Na+, K+ and Ci− ion concentrations in salt tolerant indica rice variety CSR27. J. Plant Biochem. Biotechnol. 2009, 18, 139–150. [Google Scholar] [CrossRef]
- Goff, S.A.; Ricke, D.; Lan, T.-H.; Presting, G.; Wang, R.; Dunn, M.; Glazebrook, J.; Sessions, A.; Oeller, P.; Varma, H.; et al. A draft sequence of the rice genome (Oryza sativa L. ssp. Japonica). Science 2002, 296, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.J.; Ocampo, D.M.; Egdane, J.; Katimbang, M.; Singh, R.K.; Gregorio, G.; Ismail, A.M. QTL mapping and marker assisted backcrossing for improving salinity tolerance in rice. In Proceedings of the Plant and Animal Genomes XV Conference, San Diago, CA, USA, 13–17 January 2007.
- Thomson, M.J.; de Ocampo, M.; Egdane, J.; Rahman, M.A.; Sajise, A.G.; Adorada, D.L.; Ismail, A.M. Characterizing the saltol quantitative trait locus for salinity tolerance in rice. Rice 2010, 3, 148–160. [Google Scholar] [CrossRef]
- Singh, R.K.; Gopalakrishnan, S.; Singh, V.P.; Prabhu, K.V.; Mohapatra, T.; Singh, N.K.; Sharma, T.R.; Nagarajan, M.; Vinod, K.K.; Singh, D.; et al. Marker assisted selection: A paradigm shift in basmati breeding. Indian J. Genet. 2011, 71, 120–128. [Google Scholar]
- Huyen, N.T.L.; Cuc, M.L.; Ismail, A.M.; Ham, H.L. Introgression the salinity tolerance QTLs saltol into AS996, the elite rice variety of vietnam. Am. J. Plant Sci. 2012, 3, 981–987. [Google Scholar] [CrossRef]
- Linh, H.L.; Linh, H.T.; Xuan, D.T.; Ham, H.L.; Ismail, A.M.; Khanh, D.T. Molecular breeding to improve salt tolerance of rice (Oryza sativa L.) in the red river delta of vietnam. Int. J. Plant Genom. 2012, 2012, 949038. [Google Scholar]
- Vu, H.T.T.; Le, D.D.; Ismail, A.M.; Le, H.H. Marker-assisted backcrossing (MABC) for improved salinity tolerance in rice (Oryza sativa L.) to cope with climate change in vietnam. Aust. J. Crop Sci. 2012, 6, 1649–1654. [Google Scholar]
- Hoque, A.B.M.Z.; Haque, M.A.; Sarker, M.R.A.; Rahman, M.A. Marker-assisted introgression of saltol locus into genetic background of BRRI Dhan-49. Int. J. Biosci. 2015, 6, 71–80. [Google Scholar]
- Martinez, V.A.; Hill, W.G.; Knott, S.A. On the use of double haploids for detecting QTL in outbred populations. Heredity 2002, 88, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, H. Salt and paraquat stress tolerance results from co-expression of the Suaeda salsa glutathione S-transferase and catalase in transgenic rice. Plant Cell Tissue Organ Cult. 2006, 86, 349–358. [Google Scholar] [CrossRef]
- Singla-pareek, S.; Yadav, S.K.; Pareek, A.; Reddy, M.K.; Sopory, S.K. Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res. 2008, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Hoshida, H.; Tanaka, Y.; Hibino, T.; Hayashi, Y.; Tanaka, A.; Takabe, T.; Takabe, T. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol. Biol. 2000, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Nagamiya, K.; Motohashi, T.; Nakao, K.; Prodhan, S.H.; Hattori, E.; Hirose, S.; Ozawa, K.; Ohkawa, Y.; Takabe, T.; Takabe, T. Enhancement of salt tolerance in transgenic rice expressing an Escherichia coli catalase gene, katE. Plant Biotechnol. Rep. 2007, 1, 49–55. [Google Scholar] [CrossRef]
- Moriwaki, T.; Yamamoto, Y.; Aida, T.; Funahashi, T.; Shishido, T.; Asada, M.; Prodhan, S.H.; Komamine, A.; Motohashi, T. Overexpression of the Escherichia coli catalase gene, katE, enhances tolerance to salinity stress in the transgenic indica rice cultivar, BR5. Plant Biotechnol. Rep. 2008, 2, 41–46. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hibino, T.; Hayashi, Y.; Tanaka, A.; Kishitani, S.; Takabe, T.; Yokota, S. Salt tolerance of transgenic rice overexpressing yeast mitochondrial Mn-SOD in chloroplasts. Plant Sci. 1999, 148, 131–138. [Google Scholar] [CrossRef]
- Prashanth, S.R.; Sadhasivam, V.; Parida, A. Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in indica rice var Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res. 2008, 17, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fan, Z.; Guo, L.; Li, Y.; Chen, Z.-L.; Qu, L.-J. Over-expression of the bacterial nhaA gene in rice enhances salt and drought tolerance. Plant Sci. 2005, 168, 297–302. [Google Scholar] [CrossRef]
- Ohta, M.; Hayashi, Y.; Nakashima, A.; Hamada, A.; Tanaka, A.; Nakamura, T.; Hayakawa, T. Introduction of a Na+/H+ antiporter gene from Atriplex gmelini confers salt tolerance to rice. FEBS Lett. 2002, 532, 279–282. [Google Scholar] [CrossRef]
- Fukuda, A.; Nakamura, A.; Tagiri, A.; Tanaka, H.; Miyao, A.; Hirochika, H.; Tanaka, Y. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004, 45, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Razzaque, S.; Elias, S.M.; Amin, U.S.M.; Haque, T.; Islam, S.M.T.; Lisa, L.A.; Naznin, F.; Rasul, N.M.; Seraj, Z.I. Effect of the vacuolar Na+/H+ antiporter transgene in a rice landrace and a commercial rice cultivar after its insertion by crossing. Acta Physiol. Plant. 2014, 37, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; An, R.; Tang, J.-H.; Cui, X.-H.; Hao, F.-S.; Chen, J.; Wang, X.-C. Over-expression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol. Breed. 2007, 19, 215–225. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, S.; Zhang, H.; Zhao, Y. Expression of yeast SOD2 in transgenic rice results in increased salt tolerance. Plant Sci. 2006, 170, 216–224. [Google Scholar] [CrossRef]
- Verma, D.; Singla-Pareek, S.L.; Rajagopal, D.; Reddy, M.K.; Sopory, S.K. Functional validation of a novel isoform of Na+/H+ antiporter from Pennisetum glaucum for enhancing salinity tolerance in rice. J. Biosci. 2007, 32, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Kitamoto, H.K.; Nakamura, A.; Fukuda, A.; Tanaka, Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiol. 2007, 144, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Wu, R. Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci. 2001, 160, 869–875. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, A.N. Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol. Biol. 1998, 38, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Kathuria, H.; Ferjani, A.; Sakamoto, A.; Mohanty, P.; Murata, N.; Tyagi, A. Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor. Appl. Genet. 2002, 106, 51–57. [Google Scholar] [PubMed]
- Su, J.; Hirji, R.; Zhang, L.; He, C.; Selvaraj, G.; Wu, R. Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stress-protectant glycine betaine. J. Exp. Bot. 2006, 57, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Pandian, S.K.; Ramesh, M. Transgenic indica rice cv. ADT 43 expressing a Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. Plant Cell Tissue Organ Cult. 2011, 107, 383–395. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; Kavi Kishor, P.B.; Jawali, N.; Shitole, M.G. Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol. Rep. 2009, 4, 37–48. [Google Scholar] [CrossRef]
- Roy, M.; Wu, R. Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. 2002, 163, 987–992. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.-K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [PubMed]
- In-Cheol, J.; Se-Jun, O.; Ju-Seok, S.; Won-Bin, C.; Sang Ik, S.; Chung Ho, K.; Youn Shic, K.; Hak-Soo, S.; Choi, Y.D.; Nahm, B.H.; et al. Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol. 2003, 131, 516–524. [Google Scholar]
- Hoang, T.M.L.; Moghaddam, L.; Williams, B.; Khanna, H.; Dale, J.; Mundree, S.G. Development of salinity tolerance in rice by constitutive-overexpression of genes involved in the regulation of programmed cell death. Front. Plant Sci. 2015, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Duan, X.; Wang, B.; Hong, B.; Ho, T.D.; Wu, R. Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol. 1996, 110, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Rohila, J.S.; Jain, R.K.; Wu, R. Genetic improvement of basmati rice for salt and drought tolerance by regulated expression of a barley HVA1 cdna. Plant Sci. 2002, 163, 525–532. [Google Scholar] [CrossRef]
- Saijo, Y.; Hata, S.; Kyozuka, J.; Shimamoto, K.; Izui, K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000, 23, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 2003, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qian, Q.; Zhu, D. Expression of a calcineurin gene improves salt stress tolerance in transgenic rice. Plant Mol. Biol. 2005, 58, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Z. Tobacco OPBP1 enhances salt tolerance and disease resistance of transgenic rice. Int. J. Mol. Sci. 2008, 9, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Se-Jun, O.; Sang Ik, S.; Youn Shic, K.; Hyun-Jun, J.; Kim, S.Y.; Minjeong, K.; Kim, Y.-K.; Nahm, B.H.; Kim, J.-K. Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol. 2005, 138, 341–351. [Google Scholar]
- Stam, M.; Mol, J.N.M.; Kooter, J.M. Review article: The silence of genes in transgenic plants. Ann. Bot. 1997, 79, 3–12. [Google Scholar] [CrossRef]
- Waterhouse, P.M.; Graham, M.W.; Wang, M.B. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense rna. Proc. Natl. Acad. Sci. USA 1998, 95, 13959–13964. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.B.; Waterhouse, P.M. High-efficiency silencing of a beta-glucuronidase gene in rice is correlated with repetitive transgene structure but is independent of DNA methylation. Plant Mol. Biol. 2000, 43, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Mette, M.F.; Aufsatz, W.; van der Winden, J.; Matzke, M.A.; Matzke, A.J.M. Transcriptional silencing and promoter methylation triggered by double-stranded rna. EMBO J. 2000, 19, 5194–5201. [Google Scholar] [CrossRef] [PubMed]
- Dietz-Pfeilstetter, A. Stability of transgene expression as a challenge for genetic engineering. Plant Sci. 2010, 179, 164–167. [Google Scholar] [CrossRef]
- Vaucheret, H. Post-transcriptional small rna pathways in plants: Mechanisms and regulations. Genes Dev. 2006, 20, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Rajeevkumar, S.; Anunanthini, P.; Sathishkumar, R. Epigenetic silencing in transgenic plants. Front. Plant Sci. 2015, 6, 693. [Google Scholar] [CrossRef] [PubMed]
- Kumpatla, S.P.; Hall, T.C. Recurrent onset of epigenetic silencing in rice harboring a multi-copy transgene. Plant J. 1998, 14, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Naito, S.; Shimamoto, K. Post-transcriptional gene silencing in cultured rice cells. Plant Cell Physiol. 2000, 41, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Miki, D.; Shimamoto, K. De novo DNA methylation induced by sirna targeted to endogenous transcribed sequences is gene-specific and OsMet1-independent in rice. Plant J. 2008, 56, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Keren, R.; Miyamoto, S. Reclamation of saline, sodic and boron-affected soils. In Agricultural Salinity Assessment and Management; Tanji, K.K., Wallender, W.W., Eds.; American Society of Civil Engineers: New York, NY, USA, 2011. [Google Scholar]
- Bhumbla, D.; Abrol, I. Saline and sodic soils. In Soils and Rice; International Rice Research Institute: Manila, Philippines, 1978; pp. 719–738. [Google Scholar]
- Tian, L.; Tan, L.; Liu, F.; Cai, H.; Sun, C. Identification of quantitative trait loci associated with salt tolerance at seedling stage from Oryza rufipogon. J. Genet. Genom. 2011, 38, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Wurm, P.A.S.; Campbell, L.C.; Batten, G.D.; Bellairs, S.M. Australian Native Rice: A New Sustainable Wild Food Enterprise; Rural Industries Research and Development Corporation (RIRDC): Barton, Australia, 2012. [Google Scholar]
- Cheng, Z.-Q.; Huang, X.-Q.; Zhang, Y.-Z.; Qian, J.; Yang, M.-Z.; Wu, C.-J.; Liu, J.-F. Diversity in the content of some nutritional components in husked seeds of three wild rice species and rice varieties in yunnan province of china. J. Integr. Plant Biol. 2005, 47, 1260–1270. [Google Scholar] [CrossRef]
- Garcia-Oliveira, A.L.; Tan, L.; Fu, Y.; Chuanqing, S. Genetic identification of quantitative trait loci for contents of mineral nutrients in rice grain. J. Integr. Plant Biol. 2009, 51, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.F.; van der Linden, G. Future-proof crops: Challenges and strategies for climate resilience improvement. Curr. Opin. Plant Biol. 2016, 30, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Q.; Gao, D.; Wang, J.; Lang, Y.; Liu, T.; Li, B.; Bai, Z.; Luis Goicoechea, J.; Liang, C.; et al. Whole-genome sequencing of Oryza brachyantha reveals mechanisms underlying Oryza genome evolution. Nat. Commun. 2013, 4, 1595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-J.; Zhu, T.; Xia, E.-H.; Shi, C.; Liu, Y.-L.; Zhang, Y.; Liu, Y.; Jiang, W.-K.; Zhao, Y.-J.; Mao, S.-Y. Rapid diversification of five Oryza AA genomes associated with rice adaptation. Proc. Natl. Acad. Sci. USA 2014, 111, E4954–E4962. [Google Scholar] [CrossRef] [PubMed]
- Brozynska, M.; Furtado, A.; Henry, R.J. Genomics of crop wild relatives: Expanding the gene pool for crop improvement. Plant Biotechnol. J. 2015, 14, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.E.; Nock, C.J.; Ishikawa, R.; Rice, N.; Henry, R.J. Chloroplast genome sequence confirms distinctness of Australian and Asian wild rice. Ecol. Evol. 2012, 2, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Brozynska, M.; Omar, E.S.; Furtado, A.; Crayn, D.; Simon, B.; Ishikawa, R.; Henry, R.J. Chloroplast genome of novel rice germplasm identified in northern Australia. Trop. Plant Biol. 2014, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ballini, E.; Berruyer, R.; Morel, J.B.; Lebrun, M.H.; Nottéghem, J.L.; Tharreau, D. Modern elite rice varieties of the ‘green revolution’ have retained a large introgression from wild rice around the Pi33 rice blast resistance locus. New Phytol. 2007, 175, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Lee, D.S.; Song, Z.P.; Suh, H.S.; LU, B.R. Gene flow from cultivated rice (Oryza sativa) to its weedy and wild relatives. Ann. Bot. 2004, 93, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Gao, L. Population structure and conservation genetics of wild rice Oryza rufipogon (Poaceae): A region-wide perspective from microsatellite variation. Mol. Ecol. 2004, 13, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Pusadee, T.; Jamjod, S.; Rerkasem, B.; Schaal, B.A. Life-history traits and geographical divergence in wild rice (Oryza rufipogon) gene pool in indochina peninsula region. Ann. Appl. Biol. 2016, 168, 52–65. [Google Scholar] [CrossRef]
- Song, Z.P.; Lu, B.-R.; Zhu, Y.G.; Chen, J.K. Gene flow from cultivated rice to the wild species Oryza rufipogon under experimental field conditions. New Phytol. 2003, 157, 657–665. [Google Scholar] [CrossRef]
- Wurm, P.A.S. A surplus of seeds: High rates of post-dispersal seed predation in a flooded grassland in monsoonal Australia. Aust. J. Ecol. 1998, 23, 385–392. [Google Scholar] [CrossRef]
- Sengupta, S.; Majumder, A.L. Porteresia coarctata (Roxb.) tateoka, a wild rice: A potential model for studying salt-stress biology in rice. Plant Cell Environ. 2010, 33, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.C.; Waters, D.L.E.; Wood, R.H.; Rose, T.J. Morphological characterisation of Australian ex situ wild rice accessions and potential for identifying novel sources of tolerance to phosphorus deficiency. Genet. Resour. Crop Evol. 2016, 63, 327–337. [Google Scholar] [CrossRef]
- Atwell, B.J.; Wang, H.; Scafaro, A.P. Could abiotic stress tolerance in wild relatives of rice be used to improve Oryza sativa? Plant Sic. 2013, 215–216, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Woodroffe, C.; Chappell, J.; Thom, B. Geomorphological Dynamics and Evolution of the South Alligator Tidal River and Plains, Northern Territory; Australian National University North Australia Research Unit: Darwin, Australia, 1986. [Google Scholar]
- Hope, G.S.; Hughes, P.J.; Russell-Smith, J. Geomorphological Fieldwork and the Evolution of the Landscape of Kakadu National Park; Jones, R., Ed.; Archeological Research in Kakadu National Park Australian national Parks and Wildlife Service: Canberra, Australia, 1985; pp. 229–240. [Google Scholar]
- Hart, B.; Ottaway, E.; Noller, B. Magela Creek system, Northern Australia. II. Material budget for the floodplain. Mar. Freshw. Res. 1987, 38, 861–876. [Google Scholar] [CrossRef]
- White, L.A. Dark cracking clays of the estuarine flood plains of the Northern Territory. In The Properties and Utilisation of Cracking Clay Soils; McGarity, J.W., Hoult, E.H., So, H.B., Eds.; The Properties adn Utiliastion of Cracking Clay Soils. Rev. Rural Sci. 5, 29–35.
- Wasson, R.J.E. Modern Seditmentation and Late Quarternary Evolution of the Magela Creek Plain; Supervising Scientist for the Alligator Rivers Region: Darwin, Australia, 1992; p. 322. [Google Scholar]
- Mollah, W.S. Humpty Doo: Rice in the Northern Territory; Australian National University North Australia Research Unit: Darwin, Australia, 1982. [Google Scholar]
- Cockfield, G.; Mushtaq, S.; White, N. Relocation of intensive agriculture to Northern Australia: The case of the rice industry. In Government of Queensland; Technical Report; University of Southern Queensland: Toowoomba, Australia, 2012. [Google Scholar]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.; Eicholz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.-G.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OSERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-F.; Li, H.; Qin, R.-Y.; Li, J.; Qiu, C.-H.; Yang, Y.-C.; Ma, H.; Li, L.; Wei, P.-C.; Yang, J.-B. Generation of inheritable and “transgene clean” targeted genome-modified rice in later generations using the CRISPR/Cas9 system. Sci. Rep. 2015, 5, 11491. [Google Scholar] [CrossRef] [PubMed]
| Gene | Protein | Gene Sources | DS | Comments | Reference |
|---|---|---|---|---|---|
| Machinery: Antioxidants and ROS detoxification | |||||
| CAT1 and GST | Catalase and Glutathione S-transferase | Suaeda salsa | G, V | Enhanced salt tolerance. Increased GST, CAT and SOD activities; Decreased H2O2 and Electrolytes leakage. | [115] |
| GlyII | Glyoxalase II | Oryza sativa | V | Enhanced salt tolerance. Increase Glyoxalase (1, 2, 3 and 4) activities. Increased shoot/root dry weight; accumulate less Na+, more K+. | [116] |
| GS2 | Chloroplastic Glutamine synthetase | Oryza sativa | V | Enhanced salt tolerance. Higher quantum yield of PSII, less Na+ accumulation. | [117] |
| katE | Catalase | Escherichia coli | V | Enhanced salt tolerance. Increase catalase activities. Less damage by NaCl at both developmental stages. | [118,119] |
| Mn-SOD | Mitochondrial manganese superoxide dismutase | Saccharomyces cerevisiae | V | Enhanced salt tolerance. Increase SOD activities. Higher quantum yield of PSII. | [120] |
| Sod1 dismutase | Cytosolic copper zinc superoxide | Avicennia marina | V | Enhanced salt tolerance. Higher fresh weight and dry weight in transgenic plants than non-transgenics. | [121] |
| Machinery: Ion homeostasis and compartmentation | |||||
| nhaA | Na+/H+ antiporter | Escherichia coli | G, V, R | Improved salt tolerance. Increase germination, shoot height. Transport more Na+ to vacuoles. Better yield. | [122] |
| AgNHX1 | Vacuolar-type Na+/H+ antiporter | Atriplex gmelini | V | Improved salt tolerance. | [123] |
| OsNHX1 | Vacuolar (Na+, K+)/H+ antiporter | Oryza sativa | V, R | Improved salt tolerance. Transgenics had better growth, decrease osmotic potential under NaCl stress. | [124,125,126] |
| SOS2 | Plasma membrane Na+/H+ antiporter | Schizosaccharo-myces pombe | V | Enhanced salt tolerance. Transgenic plants had higher photosynthesis, low concentration of ROS, less Na+ accumulation, low Na+/K+ ratio, better yield component compare to non-transgenic. | [127] |
| PgNHX1 | Vacuolar Na+/H+ antiporter | Pennisetum glaucum | V, R | Enhanced salt tolerance. Delay senescence, shoot and root lengths are better than non-transgenic under NaCl stress. | [128] |
| OsKAT1 | Shaker K+ channel | Oryza sativa | cell | Cell culture line increase growth and cell K+ content during NaCl stress. | [129] |
| Machinery: Osmotic adjustment | |||||
| ADC | Arginine decarboxylase | Avena sativa | V | Enhanced salt tolerance. Increase in biomass under salinity–stress conditions. | [130] |
| codA | Choline oxidase | Arthrobacter globiformis | V | Enhanced salt tolerance. Transgenic plants accumulate glycinebetaine. Better yield under NaCl compare to non-transgenics. | [131,132] |
| COX | choline oxidase | Arthrobacter pascens | V | Enhanced salt tolerance. Transgenic plants accumulate glycinebetaine. increase dry weight under NaCl compare to non-transgenics. | [133] |
| P5SC | Δ1-pyrroline-5 carboxylate synthetase | Vigna aconitifolia | V | Enhanced salt tolerance. Transgenic plants maintain shoot height and fresh and dry weight under NaCl stress. Accumulate more proline under NaCl stress. | [134] |
| P5CSF129A | Δ1-pyrroline-5 carboxylate synthetase | Vigna aconitifolia | V | Enhanced salt tolerance. Transgenic plants maintain shoot height and fresh and dry weight under NaCl stress. Accumulate more proline, less lipid peroxidation. | [135] |
| SAMDC | S-adenosylmethionine decarboxylase | Tritordeum | V | Enhanced salt tolerance. Transgenic plants had better shoot length and better fresh weight under NaCl stress. | [136] |
| TPS and TPP | Trehalose-6-phosphate synthase and Trehalose-6-phosphate phosphatase | Escherichia coli | V | Enhanced salt tolerance. Transgenic plants accumulate more trehalose. Better dry weight, better shoot height, maintain maximum quantum yield of PSII. Accumulate less Na+, maintain Na+/K+ ratio. | [137,138] |
| Machinery: Programmed cell death | |||||
| AtBAG4 | AtBAG4 | Arabidopsis | V R | Enhanced salt tolerance. Transgenic plants maintain cell membrane integrity, photosynthesis, low concentration of ROS, inhibit cell death, less Na+ accumulation, maintain low Na+/K+ ratio, better yield component compare to non-transgenic. | [1,139] |
| p35 | p35 | Baculovirus | |||
| Hsp70 | Hsp70 | Citrus tristeza virus | |||
| SfIAP | IAP | Spodoptera frugiperda | |||
| Machinery: Signal transduction | |||||
| HVA1 | LEA | Hordeum vulgare | V | Enhanced salt tolerance. Transgenic plants maintain better growth, plant height, root fresh weight under NaCl stress. | [140,141] |
| OsCDPK7 | CDPK | Oryza sativa | V | Enhanced salt tolerance. Transgenic plants survived better under NaCl stress. | [142] |
| OsMAPK5 | MAPK | Oryza sativa | V | Enhanced salt tolerance | [143] |
| CNAtr | Calcineurin | Mouse | V | Enhanced salt tolerance. Less Na+ accumulated in roots. Root growth was less inhibited than shoot growth under NaCl stress. | [144] |
| Machinery: Transcription factor | |||||
| OPBP1 AP2/ERF | transcription factor | Tobacco | V | Enhanced salt tolerance. Transgenic plants survived salt shock while non-transgenic die off. | [145] |
| CBF3/DREBIA (CBF3) and ABF3 | transcription factor | Arabidopsis | V | Enhanced salt tolerance. Transgenic improved growth and maximum quantum yield of PSII. | [146] |
| Challenges | ||
| Limited parental resources for conventional breeding | Complexity of salinity tolerance in rice.
|
| Opportunities | ||
| Wild rice—an excellent candidate for desalinization crop | Wild rice—an invaluable genetic resource for conventional breeding and other approaches | The next frontier—the use of genome editing for integration free improvement of crop traits |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, T.M.L.; Tran, T.N.; Nguyen, T.K.T.; Williams, B.; Wurm, P.; Bellairs, S.; Mundree, S. Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy 2016, 6, 54. https://doi.org/10.3390/agronomy6040054
Hoang TML, Tran TN, Nguyen TKT, Williams B, Wurm P, Bellairs S, Mundree S. Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy. 2016; 6(4):54. https://doi.org/10.3390/agronomy6040054
Chicago/Turabian StyleHoang, Thi My Linh, Thach Ngoc Tran, Thuy Kieu Tien Nguyen, Brett Williams, Penelope Wurm, Sean Bellairs, and Sagadevan Mundree. 2016. "Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities" Agronomy 6, no. 4: 54. https://doi.org/10.3390/agronomy6040054
APA StyleHoang, T. M. L., Tran, T. N., Nguyen, T. K. T., Williams, B., Wurm, P., Bellairs, S., & Mundree, S. (2016). Improvement of Salinity Stress Tolerance in Rice: Challenges and Opportunities. Agronomy, 6(4), 54. https://doi.org/10.3390/agronomy6040054






