Leaf Eh and pH: A Novel Indicator of Plant Stress. Spatial, Temporal and Genotypic Variability in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil, Nutrients, Plants, and Water Management
2.2. Experimental Settings
2.2.1. Diurnal Change Experiments
- an O. sativa sub. japonica upland rice variety: IDSA6 = IRAT216
- an O. sativa sub. indica upland rice variety: IRBLTA-2Pi
- a crossing between O. glaberrima × O. sativa, upland rice variety: Nerica4
2.2.2. Intra-Leaf Variability Experiments
- in “upland” conditions, without fertilizer in the first season.
- in “upland” (aerobic) and in “lowland” (permanently submerged) conditions with NPK fertilizer in the second season.
- in “upland” and in “lowland” conditions with Hoagland solution in the third season.
2.2.3. Inter-Leaf Variability Experiments
2.3. Eh and pH Measurements
2.3.1. Soil Eh and pH
2.3.2. Leaf Eh and pH
2.4. Statistical Analysis
3. Results
3.1. Temporal Variations of Eh, pH and Eh@pH7 in Rice Leaves
3.1.1. Diurnal Variations
- temperature, with a median R2 of 0.48 and 0.39 respectively, as seen in Table S1.2, and
- solar radiation, with median R2 of 0.58 and 0.47 respectively, as seen in Table S1.3.
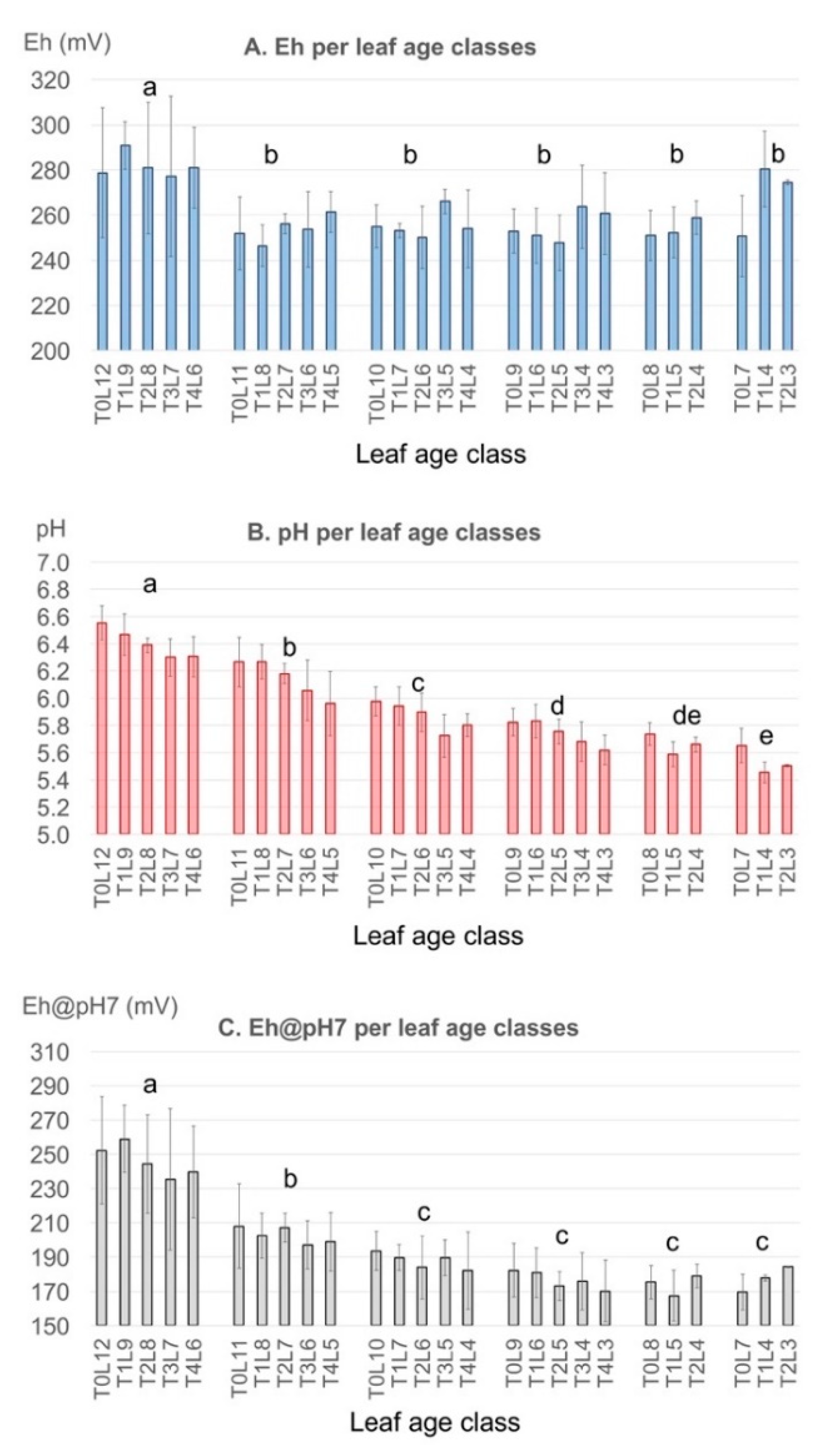
3.1.2. Plant Age
3.1.3. Cropping Season
3.2. Varietal Differences in Eh, pH and Eh@pH7
3.3. Spatial Variations of Leaf Eh, pH and Eh@pH7 in Rice Leaves
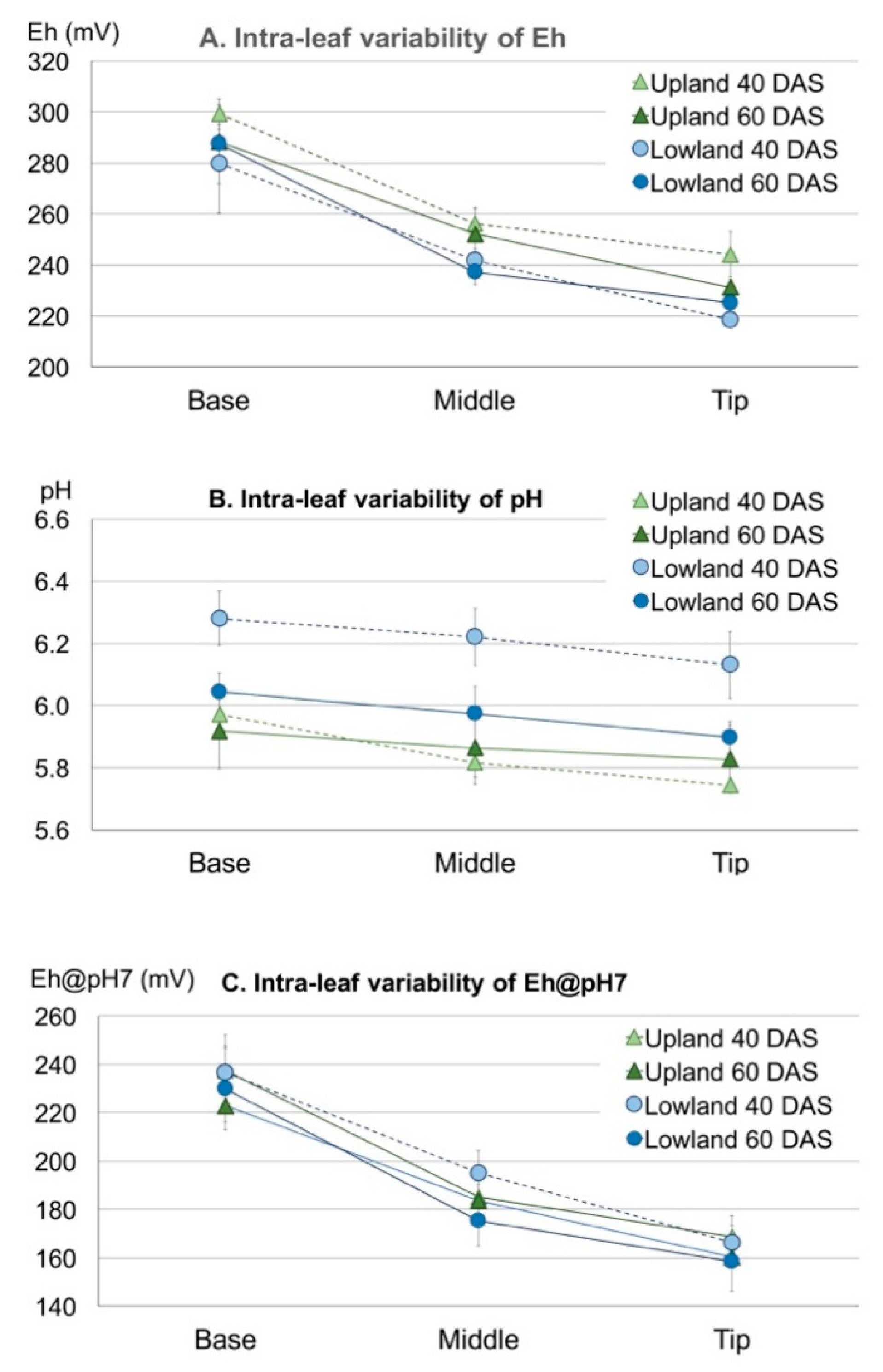
3.3.1. Intra-Leaf Variability
3.3.2. Inter-Leaf Variability: Eh-pH “Gradients” in Rice Plants
4. Discussion
4.1. Spatial and Temporal Variability
4.2. Validation of The Measurement Method
- As photosynthesis and respiration strongly affect redox level in plants, the measurements should be done in a time window during which solar radiation and temperature variations, hence when Eh variations, are minor: between 11:00 a.m. and 16:00 p.m. in our experimental conditions.
- Because of the high spatial and temporal variability of leaf Eh-pH, the measurement should be always conducted on the same part of the same leaf: in the case of upright monocotyledon plants such as rice, we propose to use the middle part of the last fully expanded leaf, as it has the lowest Eh of the plant and the second highest pH, and presents a low variability with time. The last, youngest leaf, which is in rapid development, is not recommended for measurement as its Eh is very variable. Old, senescent leaves are not recommended either, as their Eh also evolves rapidly with leaf age and thus leads to very variable measurements.
- As plant age impacts leaf Eh and pH, this parameter should also be taken into account when comparing plants.
4.3. Leaf Eh-pH and Eh@pH7 as an Indicator of Plant Stresses
4.4. Spatial and Temporal Variations of Leaf Eh-pH and Disease Patterns and Dynamics
4.4.1. Fungi
- Roumen [42] reports that at the 6th leaf stage, blast lesion density was lower in one week-old leaves than in younger, 1–2 days-old leaves, i.e., blast development was higher in the high Eh conditions of immature leaves than in photosynthetically active leaves, at lower Eh.
- The decline in blast lesion density with leaf age was faster under higher radiation and temperature conditions, i.e., lower Eh and under more active photosynthesis [42].
- Susceptibility of rice to blast decreases from the vegetative stage to the reproductive stage [43], which could be related to decreasing leaf pH (going below 6) and Eh@pH7.
4.4.2. Viruses
4.4.3. Bacteria
4.5. Eh-pH Homeostasis as a Mechanism of Resistance/Tolerance to Major Diseases?
4.6. Leaf Eh-pH and Eh@pH7 as an Indicator of Plant Health
- the processes of co-evolution of plants and their pathogens [83].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rengel, Z. Handbook of Plant Growth. pH as the Master Variable; Marcel Dekker: New York, NY, USA, 2002; ISBN 0-8247-0761-3. [Google Scholar]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Intracellular redox compartmentation and ROS-related communication in regulation and signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Foyer, C.H. Redox regulation of plant development. Antioxid. Redox Signal. 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R.; Tudzynski, P. Reactive oxygen species in development and infection processes. Semin. Cell Dev. Biol. 2016, 57, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, C.; Chand, R.; Navathe, S.; Sharma, S. Histo-chemical and biochemical analysis reveals association of er1 mediated powdery mildew resistance and redox balance in pea. Plant Physiol. Biochem. 2016, 106, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M. Contrasting regulation of NO and ROS in potato defense-associated metabolism in response to pathogens of different lifestyles. PLoS ONE 2016, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Loake, G.J. Redox-based protein modifications: The missing link in plant immune signalling. Curr. Opin. Plant Biol. 2011, 14, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.H.; Datnoff, L.E. Mineral nutrition and suppression of plant disease. Encycl. Agric. Food Syst. 2014, 4, 231–244. [Google Scholar] [CrossRef]
- Tanaka, A.; Christensen, M.J.; Takemoto, D.; Park, P.; Scott, B. Reactive oxygen species play a role in regulating a fungus–perennial ryegrass mutualistic interaction. Society 2006, 18, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.K.; Andrio, E.; Marino, D.; Pauly, N.; Dunand, C. Reactive oxygen species during plant-microorganism early interactions. J. Integr. Plant Biol. 2010, 52, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.A.; García-Cristobal, J.; Bonilla, A.; Ramos, B.; Gutierrez-Mañero, J. Beneficial rhizobacteria from rice rhizosphere confers high protection against biotic and abiotic stress inducing systemic resistance in rice seedlings. Plant Physiol. Biochem. 2014, 82, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Pasala, R.; Minhas, P.S.; Suprasanna, P. Plant bioregulators for sustainable agriculture: Integrating redox signaling as a possible unifying mechanism. Adv. Agron. 2016, 137, 237–278. [Google Scholar]
- Kerchev, P.; De Smet, B.; Waszczak, C.; Messens, J.; Van Breusegem, F. Redox strategies for crop improvement. Antioxid. Redox Signal. 2015, 23, 1186–1205. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: Advantages and limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A.; Miller, R.L. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Husson, O.; Husson, B.; Brunet, A.; Babre, D.; Alary, K.; Sarthou, J.P.; Charpentier, H.; Durand, M.; Benada, J.; Henry, M. Practical improvements in soil redox potential (Eh) measurement for characterisation of soil properties. Application for comparison of conventional and conservation agriculture cropping systems. Anal. Chim. Acta 2016, 906, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Husson, O.; Brunet, A.; Babre, D.; Charpentier, H.; Durand, M.; Sarthou, J.-P.J.; Temp, M. Conservation agriculture systems alter the electrical characteristics (Eh, pH and EC) of four soil types in France. Soil Tillage Res. 2018, 176, 57–68. [Google Scholar] [CrossRef]
- Benada, J. The measurement of redox potential in plants and some applications on the growth and development of cereals. Flora Abt. A 1968, 159, 104–127. [Google Scholar] [CrossRef]
- Benada, J. Measurement of redox potential and ph in plants and their function in the mechanism of plant resistance and in plant physiology. Int. J. Adv. Res. Electr. Electron. Instrum. Eng. 2016, 1111–1116. [Google Scholar] [CrossRef]
- Howell, D.C. Statistical Methods for Psychology; Cengage Learning: Stamford, CT, USA, 2009; ISBN 9780495597841. [Google Scholar]
- Addinsoft XLSTAT. Available online: https://www.xlstat.com/fr/solutions/premium (accessed on 28 September 2018).
- Yoshida, S. Fundamentals of Rice Crop Science; The Internstional Rice Research Interest: Los Banos, Philippines, 1981. [Google Scholar]
- Kusumi, K.; Hirotsuka, S.; Kumamaru, T.; Iba, K. Increased leaf photosynthesis caused by elevated stomatal methylation and chromatin patterning conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J. Exp. Bot. 2012, 63, 5636–5644. [Google Scholar] [CrossRef] [PubMed]
- Hatfi, J.L.; Walthall, C.L. Meeting global food needs: Realizing the potential via genetics × environment × management interactions. Agron. J. 2015, 107, 1215–1226. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Tong, J.; He, X.; Xu, Z.; Xu, L.; Wei, P.; Huang, Y.; Brestic, M.; Ma, H.; Shao, H. A novel soybean intrinsic protein gene, GmTIP2;3, involved in responding to osmotic stress. Front. Plant Sci. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Sharma, R.; Handa, N.; Kaur, H.; Rattan, A.; Yadav, P.; Gautam, V.; Kaur, R.; Bhardwaj, R. Redox homeostasis in plants under abiotic stress: Role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front. Environ. Sci. 2015, 3, 1–12. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Gadjev, I.; Stone, J.M.; Gechev, T.S. Programmed cell death in plants: New insights into redox regulation and the role of hydrogen peroxide. Int. Rev. Cell Mol. Biol. 2008, 270, 87–144. [Google Scholar] [PubMed]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Psychiatr. Danub. 2016, 7 (Suppl. S1), 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; My, T.; Nguyen, N.; Kahlen, K.; Stützel, H. Quantification of the effects of architectural traits on dry mass production and light interception of tomato canopy under different temperature regimes using a dynamic functional–structural plant model. J. Exp. Bot. 2014, 65, 6399–6410. [Google Scholar] [CrossRef] [PubMed]
- Poole, L.B.; Karplus, P.A.; Claiborne, A. Protein sulfenic acids in redox signaling. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Lee, F.N.; Counce, P.A.; Gibbons, J.H.; Barr, H.; Pyricularia, S. Mediation of partial resistance to rice blast through anaerobic induction of ethylene. Phytopathology 2004, 94, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Srikant, K.; Hegde, Y.R. Physiological and nutritional studies on Pyricularia grisea, the causal agent of blast of rice. Karnataka J. Agric. Sci. 2004, 17, 851–853. [Google Scholar]
- Roumen, E.C. Effect of leaf age on components of partial resistance in rice to leaf blast. Euphytica 1992, 63, 271–279. [Google Scholar] [CrossRef]
- Challagulla, V.; Bhattarai, S.; Midmore, D.J. In-vitro vs in-vivo inoculation: Screening for resistance of Australian rice genotypes against blast fungus. Rice Sci. 2015, 22, 132–137. [Google Scholar] [CrossRef]
- Ndjiondjop, M.-N.; Albar, L.; Fargette, D.; Fauquet, C.; Ghesquière, A. The genetic basis of high resistance to rice yellow mottle virus (RYMV) in cultivars of two cultivated rice species. Plant Dis. 1999, 83, 931–935. [Google Scholar] [CrossRef]
- Joseph, A.; Olufolaji, D.; Nwilene, F.; Onasanya, A.; Omole, M.; Onasanya, R.; Sere, Y. Effect of leaf age on rice yellow mottle virus severity and chlorophyll content with mechanical inoculation and vector transmission method. Trends Appl. Sci. Res. 2011, 6, 691–698. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, S.R.; Yenjerappa, S.T.; Naik, M.K.; Mallesh, S.B.; Kalibavi, C.M. Studies on cultural and physiological characters of Xanthomonas oryzae pv. oryzae causing bacterial blight of rice. Karnataka J. Agric. Sci. 2013, 26, 214–216. [Google Scholar]
- Fuangthong, M.; Mongkolsuk, S. Isolation and characterization of a multiple peroxide resistant mutant from Xanthomonas campestris pv. phaseoli. FEMS Microbiol. Lett. 1997, 152, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Jha, G.; Sonti, R.V. Attack and defense in Xanthomonas-rice interactions. Proc. Indian Natn Sci. Acad. 2009, 75, 49–68. [Google Scholar]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Mew, T. Effect of plant age and leaf maturity on the quantitative resitance of rice cultivars to Xanthomonas campestris pv. oryzae. Plant Dis. 1991, 75, 901–904. [Google Scholar] [CrossRef]
- Benada, J. Redox Potential and pH in Plants and Their Function in the Mechanism of Resistance to Diseases and in Plant Physiology; Agrotest fyto s.r.o. Zemědělský výzkumný ústav Kroměříž: Kroměříž, Czech Republic, 2008; ISBN 978-80-87555-06-4. [Google Scholar]
- Séré, Y.; Fargette, D.; Abo, M.E.; Wydra, K.; Bimerew, M.; Onasanya, A.; Akator, S.K. Managing the major diseases of rice in Africa. In Realizing Africa’s Rice Promise; Wopereis, M., Johnson, D., Ahmadi, N., Tollens, E., Jalloh, A., Eds.; CABI Publishing: Wallingford, UK, 2013; pp. 213–228. [Google Scholar]
- Ponnamperuma, F.N. The chemistry of submerged soils. Adv. Agron. 1972, 24, 29–96. [Google Scholar]
- Vanacker, H.; Carver, T.L.W.; Foyer, C.H. Pathogen-induced changes in the antioxidant status of the apoplast in barley leaves. Plant Physiol. 1998, 117, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Thornton, C.R.; Kershaw, M.J.; Debao, L.; Talbot, N.J. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol. Microbiol. 2003, 47, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ji, Y.; Bhuiyan, N.H.; Pilot, G.; Selvaraj, G.; Zou, J.; Wei, Y. Amino acid homeostasis modulates salicylic acid-associated redox status and defense responses in Arabidopsis. Plant Cell Online 2010, 22, 3845–3863. [Google Scholar] [CrossRef] [PubMed]
- Galhano, R.; Talbot, N.J. The biology of blast: Understanding how Magnaporthe oryzae invades rice plants. Fungal Biol. Rev. 2011, 25, 61–67. [Google Scholar] [CrossRef]
- Williams, B.; Kabbage, M.; Kim, H.J.; Britt, R.; Dickman, M.B. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 2011, 7, e1002107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lysøe, E.; Harris, L.J.; Walkowiak, S.; Subramaniam, R.; Divon, H.H.; Riiser, E.S.; Llorens, C.; Gabaldón, T.; Kistler, H.C.; Jonkers, W.; et al. The genome of the generalist plant pathogen Fusarium avenaceum is enriched with genes involved in redox, signaling and secondary metabolism. PLoS ONE 2014, 9, e112703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samalova, M.; Meyer, A.J.; Gurr, S.J.; Fricker, M.D. Robust anti-oxidant defences in the rice blast fungus Magnaporthe oryzae confer tolerance to the host oxidative burst. New Phytol. 2014, 201, 556–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, M.J.; Wang, Z.-Y.; Jones, M.A.; Smirnoff, N.; Talbot, N.J. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA 2007, 104, 11772–11777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.A.; Gibson, R.P.; Quispe, C.F.; Littlechild, J.A.; Talbot, N.J. An NADPH-dependent genetic switch regulates plant infection by the rice blast fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 21902–21907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landraud, P.; Chuzeville, S.; Billon-Grande, G.; Poussereau, N.; Bruel, C. Adaptation to pH and role of PacC in the rice blast fungus Magnaporthe oryzae. PLoS ONE 2013, 8, e69236. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Guo, W.; Chen, Y.; Dong, S.; Zhang, X.; Zhang, H.; Song, W.; Wang, W.; Wang, Q.; Lv, R.; et al. The basic leucine zipper transcription factor Moatf1 mediates oxidative stress responses and is necessary for full virulence of the rice blast fungus Magnaporthe oryzae. Mol. Plant Microbe Interact. 2010, 23, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Brugidou, C.; Opalka, N.; Yeager, M.; Beachy, R.N.; Fauquet, C. Stability of rice yellow mottle virus and cellular compartmentalization during the infection process in Oryza sativa (L.). Virology 2002, 297, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gillet, F.X.; Cattoni, D.I.; Petiot-Bécard, S.; Delalande, F.; Poignavent, V.; Brizard, J.P.; Bessin, Y.; Dorsselaer, A.V.; Declerck, N.; Sanglier-Cianférani, S.; et al. The RYMV-encoded viral suppressor of RNA silencing P1 is a zinc-binding protein with redox-dependent flexibility. J. Mol. Biol. 2013, 425, 2423–2435. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh, T.; Thumanu, K.; Wongkaew, S.; Boonkerd, N.; Teaumroong, N.; Phansak, P.; Buensanteai, N. Salicylic acid-induced accumulation of biochemical components associated with resistance against Xanthomonas oryzae pv. oryzae in rice. J. Plant Interact. 2017, 12, 108–120. [Google Scholar] [CrossRef]
- Rasool, B.; Mcgowan, J.; Pastok, D.; Marcus, S.E.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; Hancock, R.D.; Foyer, C.H. Redox control of aphid resistance through altered cell wall composition and nutritional quality. Plant Physiol. 2017, 175, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.H.; Mundt, C.C. Effect of plant age and leaf position on susceptibility to wheat stripe rust. Phytopathology 2017, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Develey-Rivière, M.P.; Galiana, E. Resistance to pathogens and host developmental stage: A multifaceted relationship within the plant kingdom. New Phytol. 2007, 175, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Chant, S.R.; Gbaja, I.S. Effect of nutrition on the interaction of viruses and Fusarium Oxysporum in tomato seedlings. Phytoparasitica 1985, 13, 47–57. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Fagard, M.; Launay, A.; Clément, G.; Courtial, J.; Dellagi, A.; Farjad, M.; Krapp, A.; Soulié, M.C.; Masclaux-Daubresse, C. Nitrogen metabolism meets phytopathology. J. Exp. Bot. 2014, 65, 5643–5656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotta, A.; Rahikainen, M.; Konert, G.; Finazzi, G.; Kangasjärvi, S. Signalling crosstalk in light stress and immune reactions in plants. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130235. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Ippolito, F.; Scala, F. A black future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J. Plant Pathol. 2015, 97, 223–234. [Google Scholar] [CrossRef]
- Joseph, S.; Chia, C.; Munroe, P.; Donne, S.; Thomas, T.; Nielsen, S.; Marjo, C.; Rutlidge, H.; Li, L.; Taylor, P.; et al. Shifting paradigms: Development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag. 2013, 4, 323–343. [Google Scholar] [CrossRef]
- Graber, E.R.; Frenkel, O.; Jaiswal, A.K.; Elad, Y. How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag. 2014, 5, 169–183. [Google Scholar] [CrossRef]
- Tollenaere, C.; Lacombe, S.; Wonni, I.; Barro, M.; Ndougonna, C.; Gnacko, F.; Sérémé, D.; Jacobs, J.; Hebrard, E.; Cunnac, S.; et al. Virus-bacteria rice co-infection in Africa: Field estimation, reciprocal effects, molecular mechanisms, and evolutionary implications. Front. Plant Sci. 2017, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.C.C.; da Silva, G.B.; Silva-Lobo, V.L.; Côrtes, M.V.C.B.; Moraes, A.J.G.; Prabhu, A.S. Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biol. Control 2011, 58, 160–166. [Google Scholar] [CrossRef]
- Bart, R.; Ronald, P.; Hake, S. Fertility versus disease resistance, a hard choice. Genes Dev. 2006, 20, 1215–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, L.; Yang, Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid–inducible mitogen-activated protein kinase. Plant Cell 2003, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Rausher, M.D. Co-evolution and plant resistance to natural enemies. Nature 2001, 411, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Meynard, J.M.; Dedieu, B.; Bos, A.P.B. Re-design and co-design of farming systems. An overview of methods and practices. In Farming Systems Research into the 21st Century: The New Dynamic; Darnhofer, I., Gibon, D., Dedieu, B., Eds.; Springer: New York, NY, USA, 2012; pp. 407–432. [Google Scholar]




| Trial Name | Season | Fertilization | Soil Type | Varieties | Water Management | Sowing Date | Measurement 1 | Measurement 2 |
|---|---|---|---|---|---|---|---|---|
| Diurnal change | Season I | - | Clayey | Nerica 4, IRBLTA-2Pi, IDSA6 | Upland | 11 December 2015 | 28 January 2016, 48 DAS | 22 February 2016, 73 DAS |
| Diurnal change | Season II | NPK | Clayey | Nerica 4, IRBLTA-2Pi, IDSA6 | Upland | 25 April 2016 | 20 June 2016, 56 DAS | 21 June 2016, 57 DAS |
| Diurnal change | Season III | NPK | Clayey | Nerica 4, IRBLTA-2Pi, Azucena | Upland | 12 August 2016 | 20–21 September 2016, 39–40 DAS | 10–11 October 2016, 59–60 DAS * |
| Diurnal change | Season IV | Hoagland | Clayey | Nerica 4, IRBLTA-2Pi, Azucena | Upland | 2 December 2016 | 10–11 January 2017, 39–40 DAS | 30–31 January 2017, 59–60 DAS * |
| Intra leaf variability | Season I | - | Clayey | Nerica 4 | Upland | 11 February 2016 | 7 March 2016, 25 DAS | |
| Intra leaf variability | Season II | NPK | Clayey | Nerica 4 | Upland and lowland | 17 August 2016 | 17–20 October 2016, 61–64 DAS | 2 November 2016, 80 DAS |
| Intra leaf variability | Season III | Hoagland | Sandy | Nerica 4 | Upland and lowland | 13 January 2017 | 22 February 2017, 40 DAS | 14 March 2017, 60 DAS |
| Intra leaf variability | Season IV | Hoagland | Clayey | IR 64 | Lowland | 22 February 2017 | 19 April 2017, 55 DAS | |
| Inter leaf variability | Season I | - | Clayey | Nerica 4 | Upland | 11 February 2016 | 22–23 March 2016, 39–40 DAS | 12–13 April 2016, 61–62 DAS |
| Inter leaf variability | Season II | NPK | Clayey | Nerica 4 | Upland | 17 August 2016 | 27–28 September 2016, 40–41 DAS | 17–20 October 2016, 61–64 DAS |
| Inter leaf variability | Season III | Hoagland | Clayey | Nerica 4 | Upland, 2 soil gradients | 19–22 November 2016 | 19–22 December 2016, 30 DAS | 16–21 January 2017, 58 DAS |
| Inter leaf variability | Season IV | Hoagland | Clayey | Nerica 4 and IR 64 | Upland and lowland | 13 February 2017 | 28–31 March 2017, 43–46 DAS | |
| Inter leaf variability | Season V | Field + NPK | Sandy | Nerica 4 | Upland | 20 February 2017 | 5–7 April 2017, 44–46 DAS |
| Season III | Variety | n | Days after Sowing | F | p | Season IV | Variety | n | Days after Sowing | F | p | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 DAS | 60 DAS | 39 DAS | 59 DAS | ||||||||||
| Eh (mV) | Nerica4 | 10 | 235.7 b | 265.4 a | 47.97 | <0.0001 | Eh (mV) | Nerica4 | 10 | 240.3 b | 256.1 a | 22.19 | 0.000 |
| IRBLTA-2Pi | 10 | 241.8 b | 276.8 a | 48.57 | <0.0001 | IRBLTA-2Pi | 10 | 250.7 b | 267.2 a | 38.84 | <0.0001 | ||
| Azucena | 10 | 253.9 b | 279.4 a | 42.13 | <0.0001 | Azucena | 10 | 246.1 b | 268.1 a | 31.57 | <0.0001 | ||
| pH | Nerica4 | 10 | 6.16 a | 5.85 b | 56.06 | <0.0001 | pH | Nerica4 | 10 | 6.50 a | 5.76 b | 592.81 | <0.0001 |
| IRBLTA-2Pi | 10 | 6.20 a | 6.17 a | 1.14 | 0.302 | IRBLTA-2Pi | 10 | 6.79 a | 5.89 b | 381.94 | <0.0001 | ||
| Azucena | 9 | 6.09 a | 5.84 b | 23.61 | 0.000 | Azucena | 10 | 6.44 a | 5.78 b | 168.91 | <0.0001 | ||
| Eh@pH7 (mV) | Nerica4 | 10 | 185.0 b | 196.4 a | 4.48 | 0.049 | Eh@pH7 (mV) | Nerica4 | 10 | 210.2 a | 182.1 b | 43.38 | <0.0001 |
| IRBLTA-2Pi | 10 | 195.0 b | 226.8 a | 24.39 | 0.000 | IRBLTA-2Pi | 10 | 239.0 a | 200.5 b | 167.23 | <0.0001 | ||
| Azucena | 9 | 199.2 a | 209.9 a | 4.13 | 0.059 | Azucena | 10 | 210.6 a | 194.7 b | 13.92 | 0.002 | ||
| Temperature (°C) | Nerica4 | 10 | 30.03 a | 29.25 b | 7.01 | 0.016 | Temperature (°C) | Nerica4 | 10 | 27.40 b | 29.43 a | 16.72 | 0.001 |
| IRBLTA-2Pi | 10 | 29.97 a | 29.20 b | 5.49 | 0.031 | IRBLTA-2Pi | 10 | 27.39 b | 29.41 b | 16.00 | 0.001 | ||
| Azucena | 10 | 29.92 a | 29.15 b | 5.97 | 0.025 | Azucena | 10 | 27.52 a | 29.44 a | 14.35 | 0.001 | ||
| Solar radiation (Wm−2) | Nerica4 | 10 | 49.61 a | 28.44 b | 5.28 | 0.031 | Solar radiation (Wm−2) | Nerica4 | 10 | 38.13 a | 30.73 a | 1.08 | 0.312 |
| IRBLTA-2Pi | 10 | 47.31 a | 27.66 b | 8.32 | 0.010 | IRBLTA-2Pi | 10 | 32.45 a | 34.68 a | 0.07 | 0.788 | ||
| Azucena | 10 | 49.91 a | 27.30 b | 10.09 | 0.005 | Azucena | 10 | 30.85 a | 36.79 a | 0.57 | 0.459 | ||
| Variety | n | Season | F | p | ||
|---|---|---|---|---|---|---|
| Season II | Season III | |||||
| Eh (mV) | Nerica4 | 10 | 249.6 b | 265.2 a | 27.39 | <0.0001 |
| IRBLTA-2Pi | 10 | 264.1 a | 276.8 b | 3.88 | 0.065 | |
| pH | Nerica4 | 10 | 5.95 a | 5.85 b | 5.63 | 0.030 |
| IRBLTA-2Pi | 10 | 6.24 a | 6.17 a | 2.60 | 0.125 | |
| Eh@pH7 (mV) | Nerica4 | 10 | 186.4 b | 196.4 a | 5.84 | 0.027 |
| IRBLTA-2Pi | 10 | 218.6 a | 226.8 a | 1.23 | 0.283 | |
| Temperature (°C) | Nerica4 | 10 | 29.71 a | 29.25 a | 2.27 | 0.150 |
| IRBLTA-2Pi | 10 | 29.72 a | 29.20 a | 2.56 | 0.128 | |
| Solar radiation (Wm−2) | Nerica4 | 10 | 68.91 a | 28.44 b | 19.74 | 0.000 |
| IRBLTA-2Pi | 10 | 77.35 a | 27.66 b | 25.66 | <0.0001 | |
| DAS | n | Varieties | F | p | Temperature (°C) | Solar Radition (Wm−2) | |||
|---|---|---|---|---|---|---|---|---|---|
| IRBLTA-2Pi | Azucena | Nerica4 | |||||||
| Season III | |||||||||
| Eh (mV) | 39–40 DAS | 20 | 242.6 b | 250.8 a | 237.6 b | 9.54 | 0.000 | 29.20 | 27.80 |
| 60 DAS | 10 | 276.8 a | 279.4 a | 265.2 b | 4.48 | 0.021 | 29.22 | 51.04 | |
| pH | 39–40 DAS | 20 | 6.24 a | 6.06 b | 6.18 a | 10.05 | 0.000 | 29.20 | 27.80 |
| 60 DAS | 10 | 6.17 a | 5.84 b | 5.85 b | 45.31 | <0.0001 | 29.22 | 51.04 | |
| Eh@pH7 (mV) | 39–40 DAS | 20 | 198.1 a | 194.2 ab | 188.2 b | 3.04 | 0.056 | 29.20 | 27.80 |
| 60 DAS | 10 | 226.8 a | 209.9 b | 196.4 c | 12.86 | 0.000 | 29.22 | 51.04 | |
| Season IV | |||||||||
| Eh (mV) | 39–40 DAS | 20 | 252.8 a | 252.3 a | 243.6 b | 5.61 | 0.006 | 27.33 | 29.04 |
| 59 DAS | 10 | 267.2 a | 268.1 a | 256.5 b | 8.06 | 0.002 | 29.43 | 36.53 | |
| pH | 39–40 DAS | 20 | 6.79 a | 6.50 b | 6.43 B | 46.11 | <0.0001 | 27.33 | 29.04 |
| 59 DAS | 10 | 5.89 a | 5.78 b | 5.76 b | 11.98 | 0.000 | 29.43 | 36.53 | |
| Eh@pH7 (mV) | 39–40 DAS | 20 | 241.0 a | 222.4 b | 210.1 c | 31.22 | <0.0001 | 27.33 | 29.04 |
| 59 DAS | 10 | 200.5 a | 194.7 a | 182.1 b | 12.01 | 0.000 | 29.43 | 36.53 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, O.; Audebert, A.; Benada, J.; Soglonou, B.; Tano, F.; Dieng, I.; Bousset, L.; Sarthou, J.-P.; Joseph, S.; Menozzi, P.; et al. Leaf Eh and pH: A Novel Indicator of Plant Stress. Spatial, Temporal and Genotypic Variability in Rice (Oryza sativa L.). Agronomy 2018, 8, 209. https://doi.org/10.3390/agronomy8100209
Husson O, Audebert A, Benada J, Soglonou B, Tano F, Dieng I, Bousset L, Sarthou J-P, Joseph S, Menozzi P, et al. Leaf Eh and pH: A Novel Indicator of Plant Stress. Spatial, Temporal and Genotypic Variability in Rice (Oryza sativa L.). Agronomy. 2018; 8(10):209. https://doi.org/10.3390/agronomy8100209
Chicago/Turabian StyleHusson, Olivier, Alain Audebert, Jaroslav Benada, Brigitte Soglonou, Firmin Tano, Ibnou Dieng, Lydia Bousset, Jean-Pierre Sarthou, Stephen Joseph, Philippe Menozzi, and et al. 2018. "Leaf Eh and pH: A Novel Indicator of Plant Stress. Spatial, Temporal and Genotypic Variability in Rice (Oryza sativa L.)" Agronomy 8, no. 10: 209. https://doi.org/10.3390/agronomy8100209
APA StyleHusson, O., Audebert, A., Benada, J., Soglonou, B., Tano, F., Dieng, I., Bousset, L., Sarthou, J.-P., Joseph, S., Menozzi, P., Boulakia, S., & Futakuchi, K. (2018). Leaf Eh and pH: A Novel Indicator of Plant Stress. Spatial, Temporal and Genotypic Variability in Rice (Oryza sativa L.). Agronomy, 8(10), 209. https://doi.org/10.3390/agronomy8100209





