Abstract
Silicon (Si) is a beneficial element that alleviates the effects of stress factors including drought (D). Strawberry is a Si-accumulator species sensitive to D; however, the function of Si in this species is obscure. This study was conducted to examine the effect of Si and inoculation with an arbuscular mycorrhizal fungus (AMF) on physiological and biochemical responses of strawberry plants under D. Plants were grown for six weeks in perlite and irrigated with a nutrient solution. The effect of Si (3 mmol L−1), AMF (Rhizophagus clarus) and D (mild and severe D) was studied on growth, water relations, mycorrhization, antioxidative defense, osmolytes concentration, and micronutrients status. Si and AMF significantly enhanced plant biomass production by increasing photosynthesis rate, water content and use efficiency, antioxidant enzyme defense, and the nutritional status of particularly Zn. In contrast to the roots, osmotic adjustment did not contribute to the increase of leaf water content suggesting a different strategy of both Si and AMF for improving water status in the leaves and roots. Our results demonstrated a synergistic effect of AMF and Si on improving the growth of strawberry not only under D but also under control conditions.
1. Introduction
Although silicon (Si) is not considered an essential element for higher plants, numerous studies have demonstrated that Si is a beneficial element that alleviates abiotic and biotic stresses in plants [1,2,3]. Si is a quasi-essential element for the growth of rice, wheat, sorghum, potato, cucumber, zucchini, and soybean, under various biotic and abiotic stress conditions [4]. According to the Si tissue concentration, plants are classified into Si-accumulators and non-accumulators. The differences in Si accumulation among species can be attributed to the differential ability of roots to take up Si [2].
Drought (D) adversely influences several features of plant growth and development, and a prolonged D severely diminishes plant productivity [5]. Water loss through transpiration is reduced by stomatal closure as an immediate response of plants upon being exposed to D; however, it reduces also nutrient uptake and limits plant ability for dry matter production. In addition, reduced intercellular CO2 concentration leads to an excess excitation energy that causes enhanced leakage of electrons to molecular oxygen and increases the production of reactive oxygen species (ROS) [6,7]. These cytotoxic ROS destroy normal metabolism through oxidative damage to lipids, proteins, and nucleic acids [8]. Plants have developed complex physiological and biochemical adjustments to tolerate D, including the activation of antioxidative enzymes, maintenance of cell turgor, and water status through the accumulation of organic osmolytes such as soluble carbohydrates and free amino acids, particularly proline [9,10].
Si supplementation of plants alleviates D stress. Several mechanisms including the activation of photosynthetic enzymes [11], the activation of enzymatic antioxidant defense systems, increased water use efficiency [12,13], nutrient uptake [14], root growth and hydraulic conductance [15], and the accumulation of organic osmolytes [16] are involved in Si-mediated growth improvement under D [11,17].
The association of roots with arbuscular mycorrhizal fungi (AMF) is the most abundant symbiosis in the plant kingdom [18]. The colonization of roots by AMF enhances the plant growth by increasing nutrient uptake and plant tolerance to stress [19,20]. Several studies evaluated the effects of AMF-inoculation in horticultural plants such as citrus, apple, and strawberry [21,22,23]. AMF symbiosis increased the rate of photosynthesis, stomatal conductance, and leaf water potential in colonized plants under D [24]. Moreover, AMF had a significant direct contribution to the uptake of phosphorus (P), zinc (Zn), and copper (Cu) under water stress [25].
Strawberry (Fragaria x ananasa Duch.) plants are extremely sensitive to drought because of a shallow root system, large leaf area, and high-water content of fruits. When the strawberry plants are not sufficiently irrigated, both yield and fruit size are reduced [22]. As a Si-accumulating species [26,27], strawberry has both functional influx (Lsi1) and efflux (Lsi2) transporters for Si uptake, and under a constant soluble Si application can absorb 3% Si per dry weight [26]. However, to the best of our knowledge, there is no study on the effect of Si on strawberry under abiotic stresses including D. Another obscure aspect in this regard is Si effect on the association of roots with AMF in this species. Therefore, given the potential of both Si and AMF for mitigation of drought stress effects, the objectives of the present study are (1) to elucidate the influence of Si on photosynthesis, water status, and activity of antioxidative defense system in strawberry plants under D conditions and (2) to investigate the Si effect on the response of mycorrhizal plants when exposed to D stress. We hypothesized the existence of a synergistic effect of Si and AMF on the protection against D in strawberry plants.
2. Materials and Methods
2.1. Preparation of Plant and Fungus Materials
The first-generation strawberry (Fragaria × ananassa var. Paros) plantlets of genetically different individuals originating from a strawberry field were prepared as donor mother plants. Second-generation strawberry plantlets from 10 cm stolons of these genetically different mother plants were propagated in a growth chamber. Four independent biological replicates were used per treatment. The offset plants were grown in a standard peat–perlite (1:1) mixture for one week to allow root development.
Inoculum of Rhizophagus clarus (Walker & Schüßler; isolated in symbiosis with Poa annua L. in a grassland in Cuba) (MUCL 46238–GINCO–BEL; Synonymy: Glomus clarum Nicolson & Schenck; [28]) was provided by the Department of Soil Science, University of Tabriz, Iran. Originally, fungi were obtained from Pal Axel Lab, Lund University, Sweden. R. clarus was propagated with Trifolium repens L. plants in 3.5 L pots containing sterile sandy loam soil. Rorison’s nutrient solution, prepared with deionized water [29] with 50% strength of phosphorus, was added to the pots twice a week to bring the soil moisture to water holding capacity (WHC). The pots were incubated in a greenhouse with 28/20 °C day/night and 16/8 h light/dark periods. After four months, the tops of the plants were excised and the pot materials containing soil and mycorrhizal roots were thoroughly mixed and used as fungal inoculum.
2.2. Plant Treatments
The experiment was conducted using a completely randomized design with three factors including irrigation regimes (three levels), Si treatments (two levels), and AMF inoculation (two levels). Each treatment combination was represented by four independent pots as four replicates.
One-week-old strawberry seedlings were transferred to 3 L pots (one plant per pot) filled with washed perlite and containing 60 g autoclaved and non-autoclaved AMF inoculum in −AMF and +AMF treatments, respectively. The pots were irrigated daily with water or Hoagland nutrient solution at WHC of the perlite after weighing. The total volume of nutrient solution applied to the plants was 200 mL pot−1 week−1. To avoid the accumulation of salts in the substrate, electric conductivity in the perlite was measured in samples taken weekly from the bottom of the pots. Si as sodium silicate (Na2SiO3, Sigma–Aldrich, Munich, Germany) prepared as the solution (0.6 mM, pH = 6.1) was added to the pots weekly by irrigation leading to a concentration of 3 mmol L−1 perlite (~84 mg L−1 perlite) at the end of the experiment after 6 weeks. One week after starting the Si application, the different irrigation regimes (IR) included well-watered (WW, 90% WHC), mild drought (MD, 75% WHC), and severe drought (SD, 35% WHC) and were assigned randomly to the pots, and watering was omitted from D treatments until they reached the respective WHC. This was achieved 4 and 6 days after starting a different IR for the MD and SD treatments, respectively. Well-watered and D plants received the same amount of nutrient solution, and the respective WHC was achieved by adjusting the volume of water used for irrigation.
In order to determine the possible effect of Na as the accompanying ion in the Si salt applied to the plants, an experiment was conducted parallel to the main experiment with an additional control (without the addition of salt or Si) and 6 mmol L−1 NaCl containing an equivalent Na with 3 mmol L−1 Na2SiO3. The dry weight (g plant−1) of plants under control (0.48 ± 0.05) and 6 mmol L−1 salt (0.51 ± 0.04) was not significantly different (Tukey test, p < 0.001).
Plants were grown under controlled environmental conditions with a temperature regime of 25 °C/18 °C day/night, 14/10 h light/dark periods, a relative humidity of 30%, and at a photon flux density of about 400 μmol m−2 s−1.
2.3. Plant Harvest
Six-week-old plants (five weeks after starting Si treatments and four weeks after reaching the respective WHC) were harvested. Shoots and roots were separated, washed with distilled water, and blotted dry on filter paper. After determination of the fresh weight (FW), the dry weight (DW) was determined after drying at 60 °C for 48 h. Subsamples were taken for biochemical analyses before drying. Before harvest, the gas exchange parameters were determined in attached leaves.
For evaluation of the AMF colonization, the fine roots (1 g FW) were cleared in 10% (v/v) KOH and stained with 0.05% (v/v) trypan blue in lacto–glycerin. The colonization rate of the roots (%) was estimated by counting the proportion of root length containing fungal structures (arbuscules, vesicles and hyphae) using the gridline intersect method [30,31]. In brief, stained root segments were spread out evenly in a 10 cm diameter Petri dish. A grid of lines was marked on the bottom of the dish to form 0.5 cm2. Vertical and horizontal gridlines were observed with a binocular device, and the presence or absence of fungal structures was recorded at each point where the roots intersected a line. Three sets of observations were made recording all the root-gridline intersects. Each of the three replicate records was made on a fresh rearrangement of the same root segments [30,31].
2.4. Leaf Osmotic Potential and Relative Water Content
The leaf osmotic potential (ψs) was determined in the second leaves harvested 1 h after the light was turned on in the growth chamber. The leaves were homogenized in a prechilled mortar and pestle and centrifuged at 4000 g for 20 min at 4 °C. The osmotic pressure of the samples was measured by an osmometer (Micro–Osmometer, Herman Roebling Messtechnik, Germany), and the milliosmol data were recalculated to MPa. For the determination of the relative water content (RWC%), the leaf disks (5 mm diameter) were prepared, and after the determination of the fresh weight (FW), they were submerged for 20 h in distilled water; thereafter, they were blotted dry gently on a paper towel, and the turgid weight (TW) was determined. The dry weight (DW) of the samples was determined after drying in an oven at 70 °C for 24 h, and the RWC% was calculated according to the formula (FW − DW)/(TW − DW) × 100.
2.5. Measurements of Photosynthetic Gas Exchange
Before the harvest gas exchange parameters were determined with the attached leaves. The net CO2 fixation rate (μmol m−2 s−1), transpiration rate (mmol m−2 s−1), and stomatal conductance (mol m−2 s−1) were determined with a calibrated portable gas exchange system (LCA–4, ADC Bioscientific Ltd., Hoddesdon, UK). Water use efficiency (WUE) was calculated as the ratio of photosynthesis/transpiration (μmol mmol−1).
2.6. Biochemical Determinations
For the determination of carbohydrates, leaf and root samples (100 mg) were homogenized in a 100 mM potassium phosphate buffer (pH 7.5) at 4 °C. After centrifugation at 12,000 g for 15 min, the supernatant was used for the determination of total soluble sugars. An aliquot of the supernatant was mixed with an anthrone–sulfuric acid reagent and incubated for 10 min at 100 °C. After cooling, the absorbance was determined at 625 nm. The standard curve was created using glucose (Sigma–Aldrich, Munich, Germany) [32]. The total soluble protein was determined by the Bradford (1976) method using a commercial reagent (Roti®Quant, Roth GmbH, Karlsruhe, Germany) and bovine serum albumin (BSA) as standard. Total free α-amino acids were assayed using a ninhydrin colorimetric method. Glycine (Sigma–Aldrich, Munich, Germany) was used to produce a standard curve [33]. For the determination of proline, samples were homogenized with 3% (v/v) sulfosalicylic acid and the homogenate was centrifuged at 3000 g for 20 min. The supernatant was treated with acetic acid and acid ninhydrin and boiled for 1 h, and then the absorbance was determined at 520 nm. Proline (Sigma–Aldrich, Munich, Germany) was used to produce a standard curve [34].
2.7. Determination of Enzyme Activities and Concentration Of Oxidants
Fresh leaf samples (100 mg) were ground in liquid nitrogen using a mortar and pestle. Each enzyme assay was tested for linearity between the volume of crude extract and the measured activity. All measurements were undertaken through spectrophotometry (Specord 200, Analytical Jena AG, Jena, Germany) according to optimized protocols described elsewhere [35]. The activity of ascorbate peroxidase (APX, EC 1.11.1.11) was measured by determining the ascorbic acid oxidation; one unit of APX oxidizes ascorbic acid at a rate of 1 μmol min−1 at 25 °C. The catalase (CAT, EC 1.11.1.6) activity was assayed by monitoring the decrease in absorbance of H2O2 at 240 nm; unit activity was taken as the amount of enzyme which decomposes 1 μmol of H2O2 in one min. Peroxidase (POD, EC 1.11.1.7) activity was assayed using the guaiacol test. The enzyme unit was calculated as the enzyme protein required for the formation of 1 μmol tetra–guaiacol for 1 min. The total superoxide dismutase (SOD, EC 1.15.1.1) activity was determined using the mono–formazan formation test. One unit of SOD was defined as the amount of enzyme required to induce a 50% inhibition of nitro blue tetrazolium (NBT) reduction as measured at 560 nm compared with control samples without enzyme aliquot. The concentration of H2O2 was determined using KI at 508 nm. Lipid peroxidation was estimated from the amount of malondialdehyde (MDA) formed in a reaction mixture containing thio-barbituric acid (Sigma–Aldrich, Munich, Germany) at 532 nm. The MDA levels were calculated from a 1,1,3,3–tetraethoxypropane (Sigma–Aldrich, Munich, Germany) standard curve [35].
2.8. Mineral Nutrient Analysis
For the determination of the plant nutritional status, 250 mg of dried leaf material was ashed in a muffle furnace at 500 °C for 5 h. After cooling, the samples were extracted twice with 2.5 mL of 3.4 M HNO3 until dryness to precipitate SiO2. The ash was dissolved in 2.5 mL of 4 M HCl, subsequently diluted ten times with hot deionized water, and boiled for 2 min. After the addition of a 0.1 mL cesium chloride/lanthanum chloride buffer to the 4.9 mL ash solution, Fe, Mn, and Zn concentrations were measured by atomic absorption spectrometry (AAS, UNICAM 939, Offenbach/Main, Germany) [36].
2.9. Silicon Determination
Dry leaf material (0.2 g) was microwave digested with 3 mL concentrated HNO3 + 2 mL H2O2 for 1 h. Samples were diluted with circa 15 mL deionized H2O and transferred into 25 mL plastic flasks; 1 mL concentrated Hydrofluoric acid was added and left overnight. After the addition of 2.5 mL 2% (w/v) H3BO3, the flask volume was adjusted to 25 mL with deionized H2O, and Si was determined by ICP–OES (Vista−PRO, Varian Inc., Palo Alto, USA) [36].
2.10. Statistical Analyses
A primary statistical analysis was carried out using the Sigma Plot 11.0 software Systat Software Inc. San Jose, USA. Experimental data were checked for normality using the Shapiro–Wilk test. Where necessary, data were transformed through standard methods to meet the requirements of statistical analysis. In a second analytical step, a so-called insert-and-absorb algorithm was used to truthfully present all relevant significant differences for the main factors and interactions between the main factors. The algorithm was implemented using the SAS 9.4 macro% (Multi factors)based on the work of Piepho, 2012 [37]. The %MULT macro uses output generated from the MIXED, GLIMMIX, or GENMOD procedures. It allows up to three by-variables for factorial experiments but can process the least squares means for one effect only. If Least Squares Means (LSMEANS) are needed for several effects, the linear model procedure must be run several times, each time using only one LSMEANS statement with only one effect. It means each level of one main factor (e.g. IR) was compared separately for each level of the remaining two factors (e.g. AMF and Si) as pairwise comparisons. In our three-factorial analyses (IR, Si and AMF factors), the main effects of the experiment (IR, AMF, Si, IR×AMF, IR×Si, Si×AMF, IR×Si×AMF) were compared using a proc mixed model (MIXED procedures) in the SAS environment at a significance level of α = 0.05. LSMEANS of the main and interaction effects were determined.
3. Results
3.1. Effect of Si and Inoculation with AMF on Plants Biomass And Root Colonization
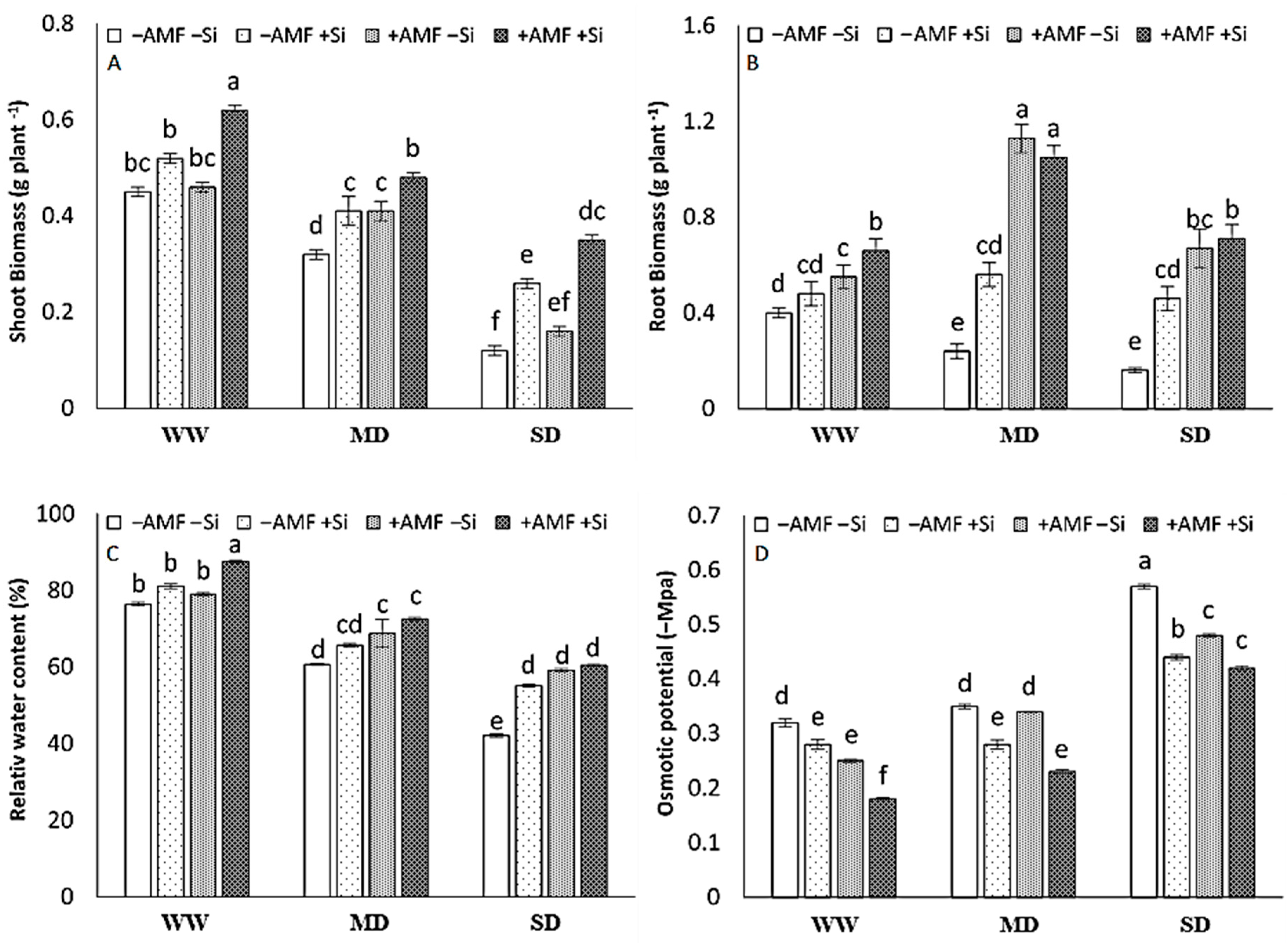
Mycorrhization or Si as single treatments did not significantly affect shoot biomass under well-watered (WW) conditions while the combination of both treatments resulted in a higher shoot biomass suggesting a synergistic effect between AMF and Si. In the plants exposed to mild drought (MD) and severe drought (SD) stresses, in contrast, Si and AMF as single treatments increased the shoot biomass; however, the effect of AMF was not significant in SD plants (Figure 1A). Root biomass was increased by the AMF treatment under WW conditions. Under MD and SD, in comparison, the effect of both Si and AMF as single treatments was significant on root biomass; the effect of AMF was much higher than Si particularly under MD (Figure 1B).
Figure 1.
The biomass of shoot (A) and root (B), the leaf relative water content (RWC) (C), and the osmotic potential (D) in strawberry plants at harvest after six experimental weeks under three irrigation regimes (IR): well-watered (WW), mild drought (MD), and severe drought (SD) without (−AMF) or with inoculation with arbuscular mycorrhizal fungus (+AMF) Rhizophagus clarus (Walker & Schüßler), in the absence (−Si) or presence of silicon (+Si, 3 mmol L−1 Na2SiO3). The bars show the treatment means (4 replicates) ±SE of the mean. The interactions among the main factors are in the table (F); *** p < 0.001, ** p < 0.01, * p < 0.05, and ns is not significant (Tukey test, alpha = 0.05).
The relative water content decreased with the severity of D. Under WW conditions, there was no significant effect of Si or AMF as single treatments on RWC while the combination of both treatments resulted in higher RWC. In MD and SD plants, in contrast, the effect of single treatments was mainly significant (Figure 1C). The osmotic potential of the leaves and roots was affected by an inverse trend of RWC (Figure 1D). There was a significant interaction among the three main factors including IR, Si, and AMF on the shoot and root biomass, RWC, and osmotic potential where all decreased with the severity of D (IR factor) but were modified by Si and AMF applications (Figure 1).
There was a low colonization percentage detectable even in –AMF plants, which might be caused by carryover of some fungal populations from the field-grown mother plants or from the peat culture substrate used for the preculture (Table 1). Interestingly, D decreased the hyphal and arbuscular colonization rates (%) in the –AMF plants while not influencing them in the +AMF ones (Table 1). The pairwise comparison indicated that the hyphal colonization percentage of +AMF plants was increased by Si under all IR treatments. The same was true for the frequency of arbuscules that was significant even for the –AMF plants under MD and SD conditions. The frequency of vesicles increased in the –AMF plants under SD conditions. In the +AMF plants, a significant effect was observed under both MD and SD conditions. Si did not affect this parameter. Interestingly, inoculation with AMF decreased the frequency of vesicles in the WW while it increased in the MD and SD plants (Table 1).

Table 1.
The root colonization rate (%) in strawberry plants at harvest after six experimental weeks under three irrigation regimes (IR): well-watered (WW), mild drought (MD), and severe drought (SD) without (−AMF) or with inoculation with arbuscular mycorrhizal fungus (+AMF) Rhizophagus clarus (Walker & Schüßler), in the absence (−Si) or presence of silicon (+Si, 3 mmol L−1 Na2SiO3). The numbers show the treatment means (4 replicates) ±SE of the mean. Means with the same letters are not significantly different. The interactions among the main factors include *** p < 0.001, ** p < 0.01, * p < 0.05, and ns as not significant (Tukey test, alpha = 0.05).
3.2. Effect of Si and Inoculation with AMF on the Leaf Gas Exchange Parameters
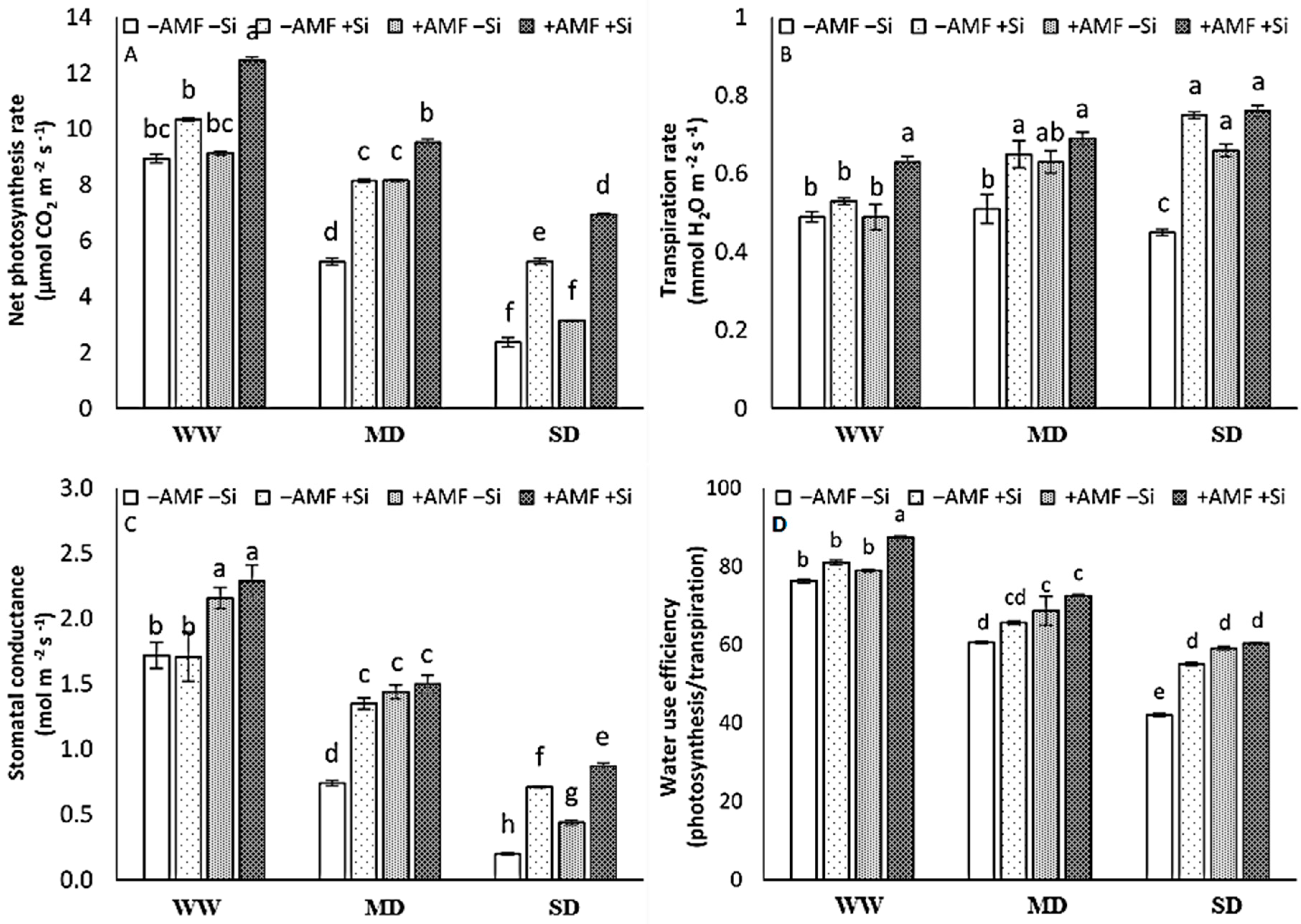
The single application of Si or AMF did not influence the rate of photosynthesis under WW conditions. A significant effect of Si as the single treatment, however, was observed under the MD and SD conditions, and a significant AMF effect was observed under MD conditions. The combined application of Si and AMF, in contrast, increased the rate of photosynthesis under WW, MD, and SD conditions, and the highest photosynthesis rate was obtained under the combination of both treatments with a significant difference with each single treatment (Figure 2A). In the absence of AMF and Si treatments, SD decreased the transpiration rate. This parameter increased by AMF only under SD conditions and by Si under MD and SD conditions. Under WW conditions, in contrast, the rate of transpiration was only increased by the combined application of Si and AMF (Figure 2B). The stomatal conductance showed a similar pattern to the rate of photosynthesis (Figure 2C). The sater use efficiency (WUE) decreased by D irrespective of the AMF or Si treatments. Significant effects of the single treatments were observed in the SD plants for Si and in both MD and SD plants for AMF, and the highest value of WUE was obtained in the combination of both treatments (Figure 2D). There was a significant interaction among the three main factors on photosynthetic activity, transpiration rate, and stomatal conductance. There was not any three-way interaction evident for water use efficiency. Significant differences were observed in IR, AMF, Si, and IR×Si (Figure 2).
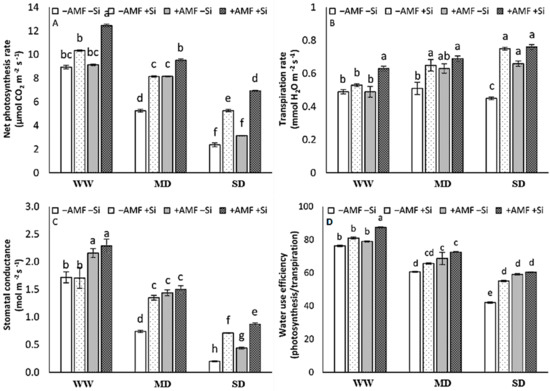
Figure 2.
The net photosynthesis rate (A), transpiration rate (B), stomatal conductance (C), and water use efficiency (D) of strawberry plants at harvest after six experimental weeks under three irrigation regimes (IR): well-watered (WW), mild drought (MD), and severe drought (SD) without (−AMF) or with inoculation with arbuscular mycorrhizal fungus (+AMF) Rhizophagus clarus (Walker & Schüßler), in the absence (−Si) or presence of silicon (+Si, 3 mmol L−1 Na2SiO3). The bars show the treatment means (4 replicates) ±SE of the mean. The interactions among the main factors are in the table (F); *** p < 0.001, ** p < 0.01, * p < 0.05, and ns is not significant (Tukey test, alpha = 0.05).
3.3. Effect of Si and Inoculation with AMF on the Concentrations of Osmolytes
Under WW conditions, there was no effect of either AMF or Si on the proline concentrations (Table 2). Under MD and SD, in comparison, both Si and AMF treatments decreased leaf proline concentrations; a synergistic effect, however, was observed only under SD conditions (Table 2). The opposite trend of the proline concentration was observed in the root under SD, which was increased by Si and AMF applications where the combined application was not significantly different from the single application. There was a significant interaction among the three main factors including IR, Si, and AMF on the leaf proline concentration (Table 2). D conditions decreased the concentration of proteins while increased the concentration of free amino acids (AA) in leaf and root tissues. The application of Si in the −AMF plants increased leaf protein concentrations under D (but not under WW) conditions while decreasing that of free AA. In the roots, in contrast, both protein and free AA concentrations increased by Si in the −AMF plants under SD conditions. Similar to Si as a single treatment, AMF application as a single treatment decreased the concentration of free AA in the leaves while increased that in the roots under SD conditions (Table 2). The total free AA concentration of the leaf was significantly affected by all two-way and three-way interactions, while there was not IR×AMF interaction regarding leaf protein concentration. For the roots, there was only an interaction of AMF and Si factors on protein concentrations and of IR and Si on free AA concentration (Table 2).

Table 2.
The concentrations of proline (µg g −1 FW), total free amino acids (AA, µg g −1 FW), total soluble proteins (mg g −1 FW), and soluble sugars (mg g −1 FW) in the leaf and roots of strawberry plants at harvest after six experimental weeks under three irrigation regimes (IR): well-watered (WW), mild drought (MD), and severe drought (SD) without (−AMF) or with inoculation with arbuscular mycorrhizal fungus (+AMF) Rhizophagus clarus (Walker & Schüßler), in the absence (−Si) or presence of silicon (+Si, 3 mmol L−1 Na2SiO3). The numbers show the treatment means (4 replicates) ±SE of the mean. Means with the same letters are not significantly different. Interactions among the main factors are indicated as *** p < 0.001, ** p < 0.01, and * p < 0.05 and ns as not significant (Tukey test, alpha = 0.05).
The concentration of soluble sugars increased under D conditions in both leaves and roots irrespective the AMF and Si treatments. Upon the application of Si and AMF, the soluble sugars concentration decreased in the leaves under MD and SD conditions while increased in the roots of SD plants. The lowest and the highest concentrations of soluble sugars was observed in the leaves and roots in the +AMF+Si plants, respectively. Under WW conditions, the effects of Si and AMF as single treatments were not statistically significant in the leaves and of Si in the roots. There was a three-way interaction among the main factors on shoot sugar concentrations (Table 2).
3.4. Effect of Si and Inoculation with AMF on the Function of Enzymatic Antioxidant Defense
The activity of CAT, SOD, and POD in the leaves and the activity of CAT and SOD in the roots were increased under D conditions irrespective the Si and AMF treatments (Table 3). The highest activity of antioxidative enzymes was observed in the combination of Si and AMF treatments (+AMF+Si). A significant effect of Si and AMF as single treatments was found in SD plants for all analyzed antioxidative enzymes while this effect in the leaves was not significant for POD in MD and for SOD and POD in WW plants. Among all analyzed leaf antioxidative enzymes, only SOD was significantly affected by a three-way interaction. In the roots, the effect of AMF on the CAT and SOD activity was higher than Si as single treatments. There was a significant interaction of the three main factors in CAT but not SOD activities of the root. The activity of root SOD was affected only by IR×AMF (Table 3).

Table 3.
The activity of catalase (CAT, µmol mg −1 protein min −1), superoxide dismutase (SOD, Unit mg −1 protein min −1), and peroxidase (POD, (µmol tetra guaicol mg −1 protein min −1) and the concentration of malondialdehyde (MDA, nmol g−1 FW) in the leaf and the activity of CAT and SOD and the concentration of hydrogen peroxide (H2O2, µmol g −1 FW) in the roots of strawberry plants at harvest after six experimental weeks under three irrigation regimes (IR): well-watered (WW), mild drought (MD), and severe drought (SD) without (−AMF) or with inoculation with arbuscular mycorrhizal fungus (+AMF) Rhizophagus clarus (Walker & Schüßler), in the absence (−Si) or presence of silicon (+Si, 3 mmol L−1 Na2SiO3). The numbers show the treatment means (4 replicates) ±SE of the mean. Means with the same letters are not significantly different. Interactions among the main factors are indicated as *** p < 0.001, ** p < 0.01, and * p < 0.05 and ns as not significant (Tukey test, alpha = 0.05).
In the absence of Si and AMF, MDA concentration as an indicator of damage to the membrane increased with increasing severity of D. Both Si and AMF treatments decreased the concentration of the leaf MDA that was observed only in D plants. AMF was more effective than Si as single treatment on the reduction of MDA concentrations; the lowest value was observed in +AMF+Si plants. A significant three-way interaction affected the leaf MDA (Table 3).
D treatment led to the accumulation of H2O2 in the roots that increased with increasing severity of stress. Si treatment decreased H2O2 concentration that was significant only in the D treatments. AMF inoculation caused a significant reduction of the H2O2 concentration only under D treatment that was significant in SD plants. The H2O2 concentration of the root was decreased by a significant interaction among IR, Si, and AMF factors (Table 3).
3.5. Effect of Si and Inoculation with AMF on the Leaf Concentrations of Nutrients and Si
The Si concentration significantly decreased in SD plants and increased by Si application in the presence or absence of AMF under WW and D conditions (Table 4). The effect of AMF on Si concentration was significant only in +Si plants under WW and in −Si ones under SD conditions (Table 4). The interaction effects between two (IR×AMF, IR×Si, and AMF×Si) and among three main factors (IR×AMF×Si) on Si concentration were significant (Table 4).

Table 4.
The concentrations of Si (%), Zn (µg g −1 DW), Mn (µg g −1 DW), Fe (µg g −1 DW), and Cu (µg g −1 DW) in the leaf of strawberry plants at harvest after six experimental weeks under three irrigation regimes (IR): well-watered (WW), mild drought (MD), and severe drought (SD) without (−AMF) or with inoculation with arbuscular mycorrhizal fungus (+AMF) Rhizophagus clarus (Walker & Schüßler), in the absence (−Si) or presence of silicon (+Si, 3 mmol L−1 Na2SiO3). The numbers show the treatment means (4 replicates) ±SE of the mean. Means with the same letters are not significantly different. Interactions among the main factors are indicated as *** p < 0.001, ** p < 0.01, and * p < 0.05 and ns as not significant (Tukey test, alpha = 0.05).
A significant effect of D on the leaf concentrations of Mn, Fe, and Cu was observed only in the SD treatment while leaf Zn concentration decreased under both MD and SD conditions (Table 4). Si and AMF treatments alone or in combination did not influence the concentrations of Mn, Fe, and Cu. However, Si and AMF significantly increased the leaf Zn concentration under MD conditions (Table 4). Furthermore, significant two-way (IR×Si) and three-way (IR×AMF×Si) interactions were observed for the leaf Zn concentration (Table 4)
4. Discussion
Our results showed that the application of Si and AMF in strawberry might alleviate the adverse effects of D stress in a synergistic manner. Different mechanisms could be involved in this synergistic effect, including Si-mediated improvement of the carbon supply for fungi and likely an increase in the formation of arbuscules. Our results also provide evidence for the effect of Si and AMF on the improvement of strawberry growth under optimum growth conditions through an elevated photosynthesis and water use efficiency.
4.1. Effect of Si and AMF on Growth and Photosynthesis of Plants under Water Stress
Biomass production, water content, and photosynthetic activity of leaves decreased under D conditions in the strawberry plants of this work. Both the Si and AMF treatments alleviated the effects of D and increased leaf water content and photosynthesis rate, leading to a higher biomass production. The observations of gas exchange parameters indicated a D-induced decrease in CO2 assimilation caused by the closure of stomata. The application of Si and AMF increased net photosynthesis rate through an elevation of stomatal conductance. Our results on the effect of Si are in agreement with those of Ma, 2004 [38] for cucumber, Chen et al. 2011 [14] for rice, and Pilon et al. 2013 [39] for potato. Further research has shown that AMF significantly increased leaf area, carboxylation efficiency, chlorophyll content, net photosynthetic rate, and the photochemical efficiency of PS II under water stress [40,41]. Although an improved stomatal conductance upon Si and AMF treatments resulted also in a higher transpiration rate, a greater stimulation of photosynthetic capacity than water loss led to higher water use efficiency in +AMF and +Si plants.
Despite lower photosynthesis activity, soluble sugars accumulated in the leaves of D plants following an impaired growth. It has been stated that water stress triggers sugar accumulation and leads to an adjustment of the rate of photosynthesis [42]. This accumulation of soluble sugars under water stress, in turn, causes an impaired plant metabolism by changing either the composition or the translocation of sugars in the leaves [43]. In the leaves, the concentration of soluble sugars decreased by AMF and Si treatments most likely because of the growth resumption and consumption of carbohydrates for biomass production. Thus, Si and AMF may modulate the accumulation of soluble sugars in water-stressed leaves in a negative feedback mechanism of biochemical limitations.
The same effect of D on the soluble sugars concentration was observed in the roots. However, in contrast to the leaves, the soluble sugars concentration in the roots increased by AMF and Si treatments. This increase may be resulted from an improved net CO2 assimilation and/or allocation of photosynthates to the roots and may, in turn, contribute to the stimulation of root growth under these conditions. Considering the osmotic effect of soluble carbohydrates, elevated soluble sugars pool may also improve root water uptake capacity from a dry substrate (see below).
4.2. Effect of Si and AMF on the Water Status and Concentration of Organic Osmolytes
The accumulation of organic osmolytes leading to an osmotic gradient with the environment, as a common response in plants under water stress [44], was observed in the strawberry plants in this work for proline, free AA, and soluble sugars, concomitant with the reduction of osmotic potential. The alleviating effect of AMF and Si, however, was not mediated by an osmo-adjustment, and the concentration of organic osmolytes rather decreased in the leaves of +AMF and +Si plants. These results suggest that the Si-mediated increase in leaf water uptake was not due to an increase in the osmotic driving force in strawberry plants under water stress. An increase in the leaf RWC was achieved apparently by an increased capacity for water uptake that in turn hindered triggering the stomatal closure and allowed the maintenance of a high photosynthetic capacity for supporting growth and dry matter production. Increasing levels of organic compounds under osmotic stress are usually thought to adversely affect growth because of the cost associated with their synthesis [45]. Thus, the method of stress alleviation of AMF and Si for an increase in water uptake capacity may be less expensive than the strategy of osmo-adjustment. This result is in contrast with our previous observation on tobacco plants showing a Si-mediated improvement of plant water status through the leaf accumulation of organic osmolytes including soluble sugars, free amino acids, and proline [13].
In contrast to the leaves, the root concentration of organic osmolytes increased by AMF and Si treatments, suggesting a different strategy for the adjustment of the water economy triggered by AMF and Si in the roots than in the leaves of strawberry. In tomatos, water stress did not change the root osmotic potential in Si-treated plants [46], and in cucumbers, the role of the osmotic driving force in the Si-mediated enhancement of water uptake was genotype-dependent [47]. Collectively, these results suggest different strategies for the improvement of water content and uptake capacity under osmotic stress in Si-treated plants depending on plant organ, species, and genotypes. There are reports on the increased root hydraulic conductance by Si, and the increase was attributed to the Si-mediated upregulation of transcription of some aquaporin genes [48].
Under D conditions, proline accumulated in the leaves while the application of AMF and Si reduced leaf proline concentrations. The accumulation of proline in the leaves under water stress is a well-documented phenomenon, but the role of proline in osmotolerance remains controversial. In some studies, the accumulation of proline has been correlated with stress tolerance [49], but other researchers suggest that proline accumulation is a symptom of stress impairment rather than stress tolerance [50]. Our results support the view that proline accumulation under stress is a symptom of stress and, thus, the Si-mediated reduction of proline concentrations is a sign of stress alleviation. Similarly, the AMF-mediated reduction of the proline concentration suggests that the AMF colonization of plants, to an extent, mitigated the effects of drought stress and reduced proline concentrations in leaves. These results are in agreement with a previous report [51].
An inhibited formation of proteins from amino acids, which could be judged by the accumulation of free AA concomitant with a reduced protein concentration, was observed under water stress of leaves. Both AMF and Si treatments caused the reduction of the free AA pool associated with an increase in soluble proteins. The accumulation of proteins helps the plant to maintain the water-status of leaves, reduce negative effects from active and reactive oxygen species [52] under severe and long-term drought, and maintain the water-status of leaves [10].
4.3. Effect of Si and AMF on the Antioxidative Defense System
Water stress caused the activation of antioxidative defense enzymes in the leaves and roots. However, this activation was not obviously sufficient for the protection of the plants against ROS that was reflected well in the increasing MDA concentrations in the D plants. The application of AMF and Si to the D plants similarly increased the activity of antioxidative defense enzymes (particularly of SOD). However, compared to the stress-induced activation of enzymes, it led to a decline of stress metabolites (MDA, H2O2). It may be suggested that AMF and Si contributed to the alleviation of oxidative damage not only by an elevated capacity of defense system but also through less production of the stress metabolites. It has been frequently shown that plants with higher root colonization with AMF exhibit greater enzymatic and non-enzymatic antioxidative defense systems activity [21,35] than non-inoculated plants. A clear biochemical link between Si and antioxidative capacity in stressed plants, however, has not yet been found. It has been argued that the biochemical enhancement of antioxidant defense mechanisms is a beneficial, physical result of Si-deposition in the cell membrane [4]. Several investigators argue that the Si-induced increases in the activity of antioxidant enzymes and the levels of non-enzymatic antioxidative substances in plants exposed to abiotic stress lead to an implication of Si in the plant metabolism [46,47,53]. According to Ma and Yamaji, 2006 [54], the Si-mediated increase in antioxidant defense abilities is a beneficial result of Si rather than a direct effect.
4.4. Effect of Si and AMF on Plants Nutrients Uptake
Water stress reduced the nutritional status of plants, causing deficiencies in Zn, Mn, Cu, and Fe, particularly in more severely stressed plants, but was already partially detectable under MD conditions. The application of AMF and Si led to an improved micronutrient status, equaling or even exceeding the critical deficiency thresholds of Fe, Zn, and Mn. Maksimovi´c et al., 2012 [55] and Pavlovic et al., 2013 [56] found that Si application increased the uptake of Zn and Fe at low concentrations on the rhizoplane. In this work, the effect of Si on nutrient acquisition under D stress was more pronouncedly observed for Zn than other micronutrients. This effect is likely mediated by stimulation of root growth [57] that increases the spatial availability of Zn for plants [58] or by an enhanced concentrations of low molecular weight organic compounds by Si (e.g., citrate) that might contribute to metal uptake and transport from root to shoot, thereby diminishing deficiency symptoms [59]. The higher Zn uptake after the application of Si under D conditions is also likely to result from the effect of the Si on Zn transporters. It has been observed that Si increases the expression levels of the Fe transporters (IRT1 and IRT2) [56] belonging to the ZIP (Zrt/IRT-like protein) family that include also Zn transporters. A limited Zn/Mn availability in the D plants of this work disbalanced Zn/Mn-dependent ROS detoxification systems produced excessive ROS accumulation and caused oxidative damage. The excessive production of ROS can promote oxidative degradation of indoleacetic acid, as was demonstrated in Zn-deficient maize plants under cold stress, which is restored by the Si application [60]. Auxin deficiency is an important factor for growth limitation in Zn-deficient plants [60]. Regarding the role of AMF, plants with a higher root colonization by AMF are more efficient in the uptake and translocation of macro- and micronutrients to the shoot than non-inoculated plants [61,62].
4.5. A Synergistic Effect of Si and AMF
The synergistic effects of Si and AMF as a combined treatment (+AMF+Si) on the low-Si medium used as growth substrate in this work may partly be related to the contribution of AMF to Si uptake observed in this work and in other works [63,64,65,66], the Si-induced stimulation in root growth that in turn promotes AMF colonization in the combination treatment, and the effect of Si on an increase in the root soluble sugars pool, which is important for supporting AMF entry, and further establishment in the roots are other probable mechanisms. The mycorrhizal association is completely dependent on the organic carbon supply from their photosynthetic partner since 4 to 20% of the C fixed through photosynthesis is transferred to the AM fungi [67]. Similarly, the Si-induced increase in the percentage of arbuscules formation observed in this work may result from the improved root growth, the enhancement of nutrients uptake and transfer within the plant, and the induced photosynthesis rate that provides more carbon sources for the fungi partner. A significant increase in the percentage of arbuscule formation in response to Si added to a sand substrate has been reported for Banana [65]. In contrast to our results, in a report on the effect of Si on mycorrhizal chickpea [66], an increase was observed in the salinity tolerance by both Si and AMF, but a synergistic effect was not detected.
Another possible explanation for the synergistic effect of AMF and Si is a Si-induced alteration of the AMF-hosts metabolism. In another report, the authors reported an enhanced metabolism of phenolic compounds (flavonoid-type phenolics) influenced by Si [68]. Phenolic compounds such as flavonoids may play a role in facilitating the interactions between fungus and host [69] and have some positive effects on fungal growth parameters, e.g., hyphal growth and branching, germination of spores [70], and formation of secondary spores. Moreover, they play a role during the fungal invasion and arbuscule formation inside the root [71]. The recent identification of strigolactones as host-recognition signals for AM fungi, however, raises the question about the role of flavonoids as general signaling molecules in AMF-plant interactions [72].
4.6. Effect of Si and AMF on Plants Growth in the Absence of Stress
In the well-watered (WW) strawberry plants grown as unstressed controls, Si treatment caused a significant increase in the shoot growth, where the highest biomass production of the shoot was observed in the +AMF+Si treatment. This Si effect under WW conditions disagrees with some of the previous reports [4] describing the beneficial effects of Si on plant growth only under stress conditions. The Si application has been frequently related to the stimulation of enzymatic defense strategies involved in the detoxification of ROS [12]. However, the lower growth of –Si plants under WW conditions in our experiment was not associated with significant changes in the physiological stress indicators, such as MDA and proline. Furthermore, the positive effects of Si on plant growth under WW conditions could also not be attributed to the increased concentrations of the micronutrients. Even in −Si control plants, the nutritional status exceeded the critical levels reported for the respective micronutrient deficiencies. The unexpected positive effects of Si supplementation on the growth of WW plants may be attributed to a significant improvement of the leaf photosynthesis and water content. Considering a higher leaf area in the +Si plants, it is expected that the photosynthesis of the whole strawberry plants is considerably higher than the –Si ones. Improved Si supply may increase the physical stability of the leaves, leading to a more horizontal orientation of the leaves and thereby improving photosynthetic efficiency as previously reported for cucumber [73]. A recent unified model, so-called apoplastic obstruction hypothesis (74), argued for a fundamental role of Si as an extracellular prophylactic agent as opposed to an active cellular agent. In this model, Si, rather than being involved directly in the regulation of gene expression and metabolism, regulates plant metabolism through a cascading effect [74]. Here in our work, the highest growth improvement was observed in the WW plants under the combination of Si with AMF treatments because a Si-induced shoot growth was associated with an AMF-mediated increase in the root growth. The soil-free culture systems that are based on perlite or vermiculite and are being widely used in horticultural practices and are characterized by low plant availability of Si [75]. Thus, the significant effect of Si supplementation in plants cultivated on these potting substrates, in contrast to the soil-grown plants, could be related to supply of plants with Si and meeting their requirement at least in the accumulator species.
5. Conclusions
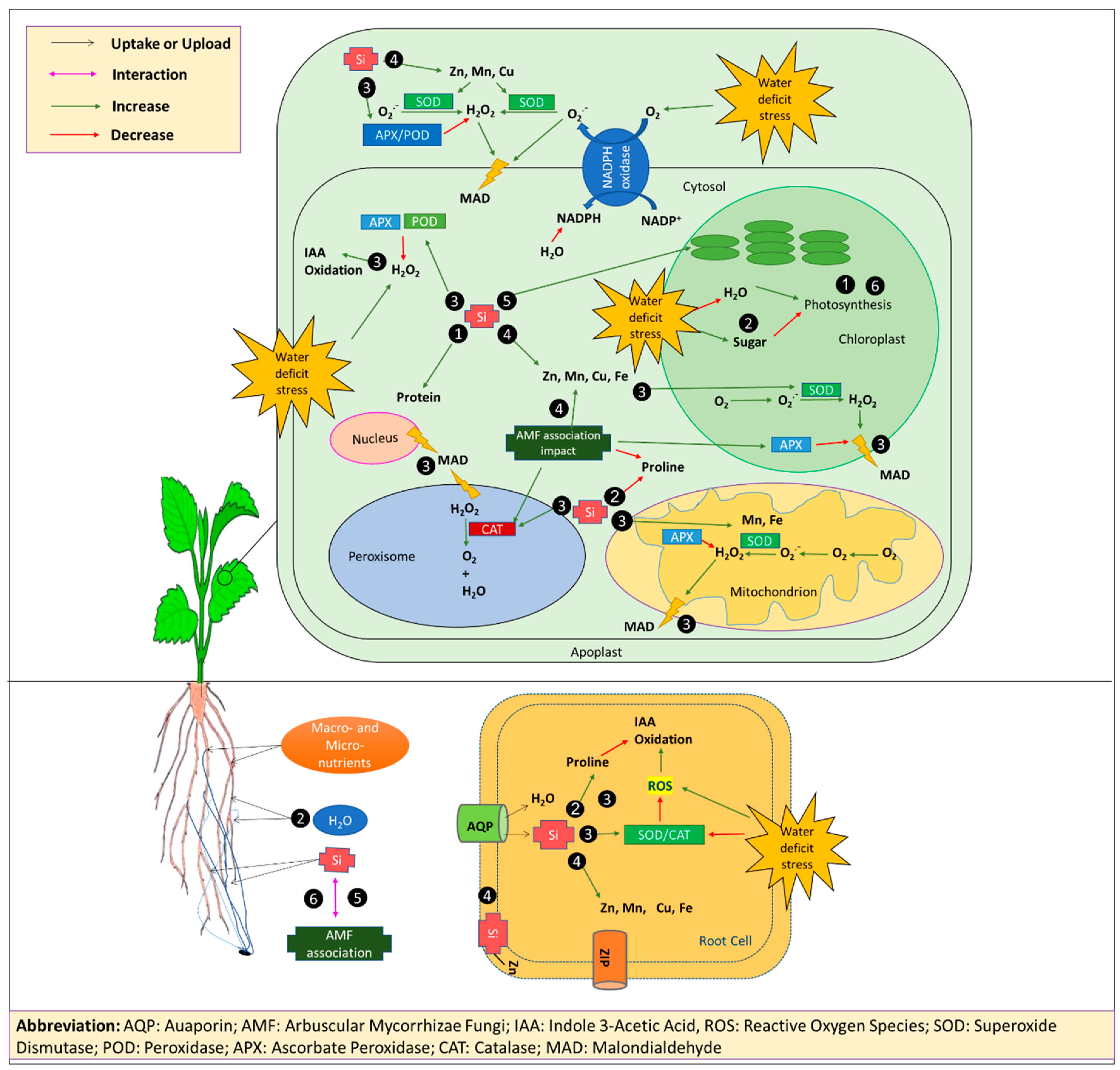
The findings of the present study suggest that the major factors determining the sensitivity of strawberry plants to D stress are a reduction of micronutrients uptake, particularly Zn, a reduced photosynthesis rate and protein level, a ROS overproduction, and the consequent membrane damage. In this context, the protective effects of Si and AMF treatments seems to be related to an improved micronutrients status, an increased expression of the enzymatic antioxidative defense system, and an elevated water uptake capacity and use efficiency. Our results indicate that Si and AMF alleviated water stress in a synergistic manner. The AMF colonization and formation of fungal structures were increased by Si, and, in turn, Si uptake was increased upon mycorrhization. Other probable interactions at the metabolic levels need to be elucidated. A conceptual model of these proposed roles of Si and AMF, mediating D tolerance in strawberry plants is presented in Figure 3. Our results provide a theoretical basis for the application of Si fertilizers and AMF in water-conserving irrigation systems for strawberry cultivation under field conditions and for greenhouse production, particularly in the soil-free culture systems.
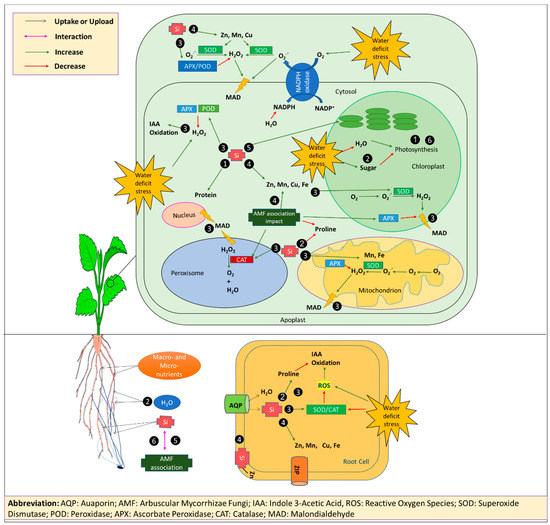
Figure 3.
A conceptual model representing the effect of Si and AMF in drought-stressed strawberry plants reverting plant performance to well-watered conditions. Si and AMF 1) enhanced growth and photosynthesis of plants, 2) regulated the water status and concentration of organic osmolytes, 3) promoted the antioxidative defense system, 4) increased plants nutrients uptake, 5) had synergistic effects, and 6) enhanced plant growth even in the absence of stress. Abbreviations: AQP: Aquaporin, AMF: Arbuscular Mycorrhizae Fungi, IAA: Indole 3-Acetic Acid, ROS: Reactive Oxygen Species, SOD: Superoxide Dismutase, POD: Peroxidase, APX: Ascorbate Peroxidase, CAT: Catalase, MAD: Malondialdehyde, NADPH: nicotinamide adenine dinucleotide phosphate hydrogen, NADP+: Nicotinamide adenine dinucleotide phosphate.
Author Contributions
R.H. and N.M. conceived and designed the experiments. N.M. conducted the experiments, performed the analyses, and collected the data. N.A., R.H., and G.N. provided the facilities and advised on the preparation of materials. N.M. wrote the manuscript. N.M. and T.E.H. did the statistics evaluations. G.N. and R.H. read and edited the manuscript. All authors approved the final manuscript.
Funding
This research was funded by the University of Tabriz, Iran [Ph.D project].
Acknowledgments
The University of Tabriz, Iran, is greatly appreciated for its financial support. Thanks to Zarrin Eshaghi (Payame Noor University, Mashhad) for giving support to the analytical facilities and to Filippo Capezzone (Hohenheim University, Biostatistics department) for the support with SAS and statistical analyses. Very special thanks to Hans Lambers (University of Western Australia) for reviewing the manuscript.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Etesami, H.; Jeong, B.R. Silicon (Si) Review and Future Prospects on the Action Mechanisms in Alleviating Biotic and Abiotic Stresses in Plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.; Brown, P.; Cakmak, I.; Ma, J.F.; Rengel, Z.; Zhao, F. Chapter 8—Beneficial Elements Marschner, Petra BT. In Marschner’s Mineral. Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012; pp. 249–269. ISBN 9780123849052. [Google Scholar]
- Liang, Y.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of Silicon–mediated Alleviation of Abiotic Stresses in Higher Plants: A Review. Environ. Pollut. 2007, 147, 4228. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant Activity of Silicon in Horticulture. Sci. Hort. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Basu, S.; Ramegoda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Res 2016, 5, F1000 Faculty Rev-1554. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R. Chapter 1—Reactive Oxygen Species and Photosynthesis. In Oxidative Damage to Plants, Antioxidant Networks and Signaling; Ahmad, P., Ed.; Elsevier: San Diego, CA, USA, 2014; pp. 1–63. ISBN 978-0-12-799963-0. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, S.; Kumar, R. Redox Biology Reactive Oxygen Species Signaling and Stomatal Movement: Current Updates and Future Perspectives. Redox Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Lelarge–Trouverie, C.; Mhamdi, A. The Metabolomics of Oxidative Stress. Phytochemistry 2014, 112, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of Osmoprotectants in Improving Salinity and Drought Tolerance in Plants: A Review. Rev. Environ. Sci. Biol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, Physiological and Biochemical Responses of Plants to Drought Stress. Afr. J. Agric Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Liu, P.; Wang, W.; Cao, D.; Deng, X.; Zhang, S. Silicon-mediated Changes in Polyamine and 1-aminocyclopropane-1-carboxylic Acid are Involved in Silicon-induced Drought Resistance in Sorghum bicolor L. Plant. Physiol. Biochem. 2014, 80, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, E.; Li, J. Silicon Effects on Photosynthesis and Antioxidant Parameters of Soybean Seedlings under Drought and Ultraviolet-B Radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Hajiboland, R.; Cheraghvareh, L.; Poschenrieder, C. Improvement of Drought Tolerance in Tobacco (Nicotiana rustica L.) Plants by Silicon. J. Plant Nutr. 2017, 40, 1661–1676. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon Alleviates Drought Stress of Rice Plants by Improving Plant Water Status, Photosynthesis and Mineral Nutrient Absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Lux, A.; Luxová, M.; Hattori, T.; Inanaga, S.; Sugimoto, Y. Silicification in Sorghum (Sorghum bicolor) Cultivars with Different Drought Tolerance. J. Plant Physiol. 2002, 115, 87–92. [Google Scholar] [CrossRef]
- Ming, D.F.; Pei, Z.F.; Naeem, M.S.; Gong, H.J.; Zhou, W.J. Silicon alleviates PEG-induced Water-deficit Stress in Upland Rice Seedlings by Enhancing Osmotic Adjustment. J. Agron. Crop. Sci. 2012, 198, 14–26. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M. Mechanisms of Silicon–mediated Alleviation of Drought and Salt Stress in Plants: A Review. Environ. Sci. Pollut. Res. Int. 2015, 22, 15416–15431. [Google Scholar] [CrossRef]
- Willis, A.; Rodrigues, B.F.; Harris, P.J.C. The Ecology of Arbuscular Mycorrhizal Fungi. CRC Crit. Rev. Plant Sci. 2013, 32, 1–20. [Google Scholar] [CrossRef]
- Abdel, A.; Abdel, H.; Hashem, A.; Rasool, S.; Fathi, E.; Allah, A. Arbuscular Mycorrhizal Symbiosis and Abiotic Stress in Plants: A Review. J. Plant Biol. 2016, 59, 407. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami-Rad, S.; Bastani, S. Phenolics Metabolism in Boron Deficient Tea (Camellia sinensis (L.) O. Kuntze) Plants. Acta Biol. Hung. 2013, 64, 196–206. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced Tolerance to Drought Stress in Citrus: A Review. Sci. Hort. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Krishna, H.; Das, B.; Attri, B.L.; Grover, M.; Ahmed, N. Suppression of Botryosphaeria Canker of Apple by Arbuscular Mycorrhizal Fungi. Crop. Prot. 2010, 29, 1049–1054. [Google Scholar] [CrossRef]
- Boyer, L.R.; Brain, P.; Xu, X.M.; Jeffries, P. Inoculation of Drought-stressed Strawberry with a Mixed Inoculum of Two Arbuscular Mycorrhizal Fungi: Effects on Population Dynamics of Fungal Species in Roots and Consequential Plant Tolerance to Water Deficiency. Mycorrhiza 2015, 25, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Mycorrhizal Stimulation of Leaf Gas Exchange in Relation to Root Colonization, Shoot Size, Leaf Phosphorus and Nitrogen: A Quantitative Analysis of the Literature Using Meta-Regression. Front. Plant Sci. 2016, 7, 1084. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.A.; Smith, S.E. What is the Significance of the Arbuscular Mycorrhizal Colonization of Many Economically Important Crop Plants? Plant Soil 2011, 348, 63–79. [Google Scholar] [CrossRef]
- Ouellette, S.; Goyette, M.H.; Labbé, C.; Laur, J.; Gaudreau, L.; Gosselin, A.; Dorais, M.; Deshmukh, R.K.; Bélanger, R.R. Silicon Transporters and Effects of Silicon Amendments in Strawberry under High Tunnel and Field Conditions. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Galletta, G.J. Foliar Application of Potassium Silicate Induces Metabolic Changes in Strawberry Plants. J. Plant Nutr. 1998, 21, 157–167. [Google Scholar] [CrossRef]
- Nicolson, T.H.; Schenck, N.C. Endogonaceous Mycorrhizal. Endophytes in Florida. Mycologia 1979, 71, 178–198. [Google Scholar] [CrossRef]
- Merryweather, J.W.; Fitter, A.H. A Modified Method for Elucidating the Structure of the Fungal Partner in a Vesicular Arbuscular Mycorrhiza. Mycol. Res. 1991, 95, 1435–1437. [Google Scholar] [CrossRef]
- Giovanetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method which Gives an Objective Measure of Colonization of Roots by Vesicular Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C. The Determination of Amino Acids with Ninhydrin. Analyst 1955, 80, 209–213. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water–stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with Arbuscular Mycorrhizal Fungi Improves Salinity Tolerance of Tomato (Solanum lycopersicum L.) Plants. Plant Soil 2010, 1, 313–327. [Google Scholar] [CrossRef]
- VDLUFA Method Book VII Environmental Analysis; VDLUVA-Verlag: Darmstadt, Germany, 2011; p. 690. ISBN 978-3-941273-10-8.
- Piepho, H.P. A SAS Macro for Generating Letter Displays of Pairwise Mean Comparisons. Com. Biom. Crop. Sci. 2012, 7, 4–13. [Google Scholar]
- Ma, J.F. Role of Silicon in Enhancing the Resistance of Plants to Biotic and Abiotic Stresses. J. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Pilon, C.; Soratto, R.P.; Moreno, L.A. Effects of Soil and Foliar Spplication of Soluble Silicon on Mineral Nutrition, Gas Exchange, and Growth of Potato Plants. J. Crop. Sci. 2013, 53, 1605–1614. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X. Arbuscular Mycorrhizal Fungi Influence Growth, Osmotic Adjustment and Photosynthesis of Citrus under Well–watered and Water Stress Conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Wang, C.Y.; Chen, H.; Tang, M. Effects of Arbuscular Mycorrhizal Fungi on Photosynthesis, Carbon Content, and Calorific Value of Black Locust Seedlings. Photosynthetica 2014, 52, 247–252. [Google Scholar] [CrossRef]
- McCormick, A.J.; Cramer, M.D.; Watt, D.A. Regulation of Photosynthesis by Sugars in Sugarcane Leaves. J. Plant Physiol. 2008, 165, 1817.e29. [Google Scholar] [CrossRef]
- Silva, E.N.; Ribeir, R.V.; Ferreira-Silva, S.L.; Vieira, S.; Ponte, L.F.; Silveira, J.G. Coordinate Changes in Photosynthesis, Sugar Accumulation and Antioxidative Enzymes Improve the Performance of Jatropha curcas Plants under Drought Stress. Biomass Bioenergy 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A.; Foolad, M.R. Drought Tolerance: Roles of Organic Osmolyts, Growth Regulators, and Mineral Nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar] [CrossRef]
- Munns, R. Comparative Physiology of Salt and Water Stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon Enhances Water Stress Tolerance by Improving Root Hydraulic Conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Xu, X.B.; Hu, Y.H.; Han, W.H.; Yin, J.L.; Li, H.L.; Gong, H.J. Silicon Improves Salt Tolerance by Increasing Root Water Uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yin, L.N.; Deng, X.P.; Wang, S.W.; Tanaka, K.; Zhang, S.Q. Aquaporin-mediated Increase in Root Hydraulic Conductance is Involved in Silicon-induced Improved Root Water Uptake under Osmotic Stress in Sorghum bicolor L. J. Exp. Bot. 2014, 65, 4747–4756. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Wu, Q.S.; Huang, Y.M.; Ni, Q.D.; He, X.H. Mycorrhizal Mediated Lower Proline Accumulation in Poncirus trifoliata under Drought Derives from the Integration of Inhibition of Proline Synthesis with Increase of Proline Degradation. PLoS ONE 2013, 8, e80568. [Google Scholar] [CrossRef] [PubMed]
- Crusciol, C.C.; Pulz, A.L.; Lemos, L.B.; Soratto, R.P.; Lima, G.P.P. Effects of Silicon and Drought Stress on Tuber Yield and Leaf Biochemical Characteristics in Potato. Crop. Sci. 2009, 49, 949–954. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity Stress Alleviation Using Arbuscular Mycorrhizal Fungi. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Martinelli, T.; Whittaker, A.; Bochicchio, A.; Vazzana, C.; Suzuki, A.; Masclaux-Daubresse, C. Amino acid Pattern and Glutamate Metabolism during Dehydration Stress in the “Resurrection” Plant Sporobolus stapfianus: A Comparison Between Desiccation-sensitive and Desiccation-tolerant Leaves. J. Exp. Bot. 2007, 58, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gong, H. Beneficial Effects of Silicon on Salt and Drought Tolerance in Plants. Agron. Sustain. Dev 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maksimović, J.D.; Mojović, M.; Maksimović, V.; Römheld, V.; Nikolic, M. Silicon Ameliorates Manganese Toxicity in Cucumber by Decreasing Hydroxylradical Accumulation in the Leaf Apoplast. J. Exp. Bot. 2012, 63, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, J.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y.; et al. Silicon Alleviates Iron Deficiency in Cucumber by Promoting Mobilization of Iron in the Root Apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Inanaga, S.; Tanimoto, E.; Lux, A.; Luxová, M.; Sugimoto, Y. Silicon-induced Changes in Viscoelastic Properties of Sorghum Root Cell Walls. Plant Cell Physiol. 2003, 44, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Rengel, Z. Availability of Mn, Zn and Fe in the Rhizosphere. J. Soil. Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Hernandez-apaolaza, L. Can Silicon Partially Alleviate Micronutrient Deficiency in Plants? A Review. Planta 2014, 240, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon Improves Chilling Tolerance during Early Growth of Maize by Effects on Micronutrient Homeostasis and Hormonal Balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H.; Bangerth, F. Effect of Zinc Nutritional Status on Growth, Protein Metabolism and Levels of Indole-3-acetic Acid and Other Phytohormones in Bean (Phaseolus vulgaris L.). J. Exp. Bot. 1989, 40, 405–412. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular Mycorrhizal Fungi Act as Biostimulants in Horticultural Crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the Role of Fungal Symbionts in Plant Abiotic Stress Tolerance. Plant Signal. Behav. 2011, 6, 175–19164. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K. Mineral Acquisition by Arbuscular Mycorrhizal Plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Anda, O.C.C.; Opfergelt, S.; Declerck, S. Silicon Acquisition by Bananas (c.V. Grande Naine) is Increased in Presence of the Arbuscular Mycorrhizal Fungus Rhizophagus irregularis MUCL 41833. Plant Soil 2016, 409, 77–85. [Google Scholar] [CrossRef]
- Garg, N.; Bhandari, P. Silicon Nutrition and Mycorrhizal Inoculations Improve Growth, Nutrient Status, K+/Na+ Ratio and Yield of Cicer arietinum L. Genotypes under Salinity Stress. Plant Growth Regul. 2016, 78, 371–387. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; p. 800. ISBN 9780123705266. [Google Scholar]
- Rodrigues, F.A.; McNally, D.J.; Datnoff, L.E.; Jones, J.B.; Labbé, C.; Benhamou, N.; Menzies, J.G.; Bélanger, R.R. Silicon Enhances the Accumulation of Diterpenoid Phytoalexins in Rice: A Potential Mechanism for Blast Resistance. Phytopathology 2004, 94, 177–183. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signalling Molecules in Plant-microbe Symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.P.; Vierheilig, H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant–Fungus Interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The Tole of Flavonoids in Rootrhizosphere Signalling: Opportunities and Challenges for Improving Plant-microbe Interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The Role of Flavonoids in the Establishment of Plant Roots Endosymbioses with Arbuscular Mycorrhiza Fungi, Rhizobia and Frankia Bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef]
- Botta, A.; Rodrigues, F.A.; Sierras, N.; Marin, C.; Cerda, J.M.; Brossa, R. Evaluation of Armurox® (cComplex of Peptides with Soluble Silicon) on Mechanical and Biotic Stresses in Gramineae. In Proceedings of the 6th International Conference on Silicon in Agriculture, Stockholm, Sweden, 26–30 August 2014. [Google Scholar]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The Controversies of Silicon’s Role in Plant Biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Reddy, S. Time to Say Sí to Silicon—And Bring Back the Missing Element in Soilless Growing. Available online: http://www.sungro.com/time-say-si-silicon-bring-back-missing-element-soilless-growing (accessed on 12 May 2014).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).