Closing Yield Gaps through Soil Improvement for Maize Production in Coastal Saline Soil
Abstract
1. Introduction
2. Materials and Methods
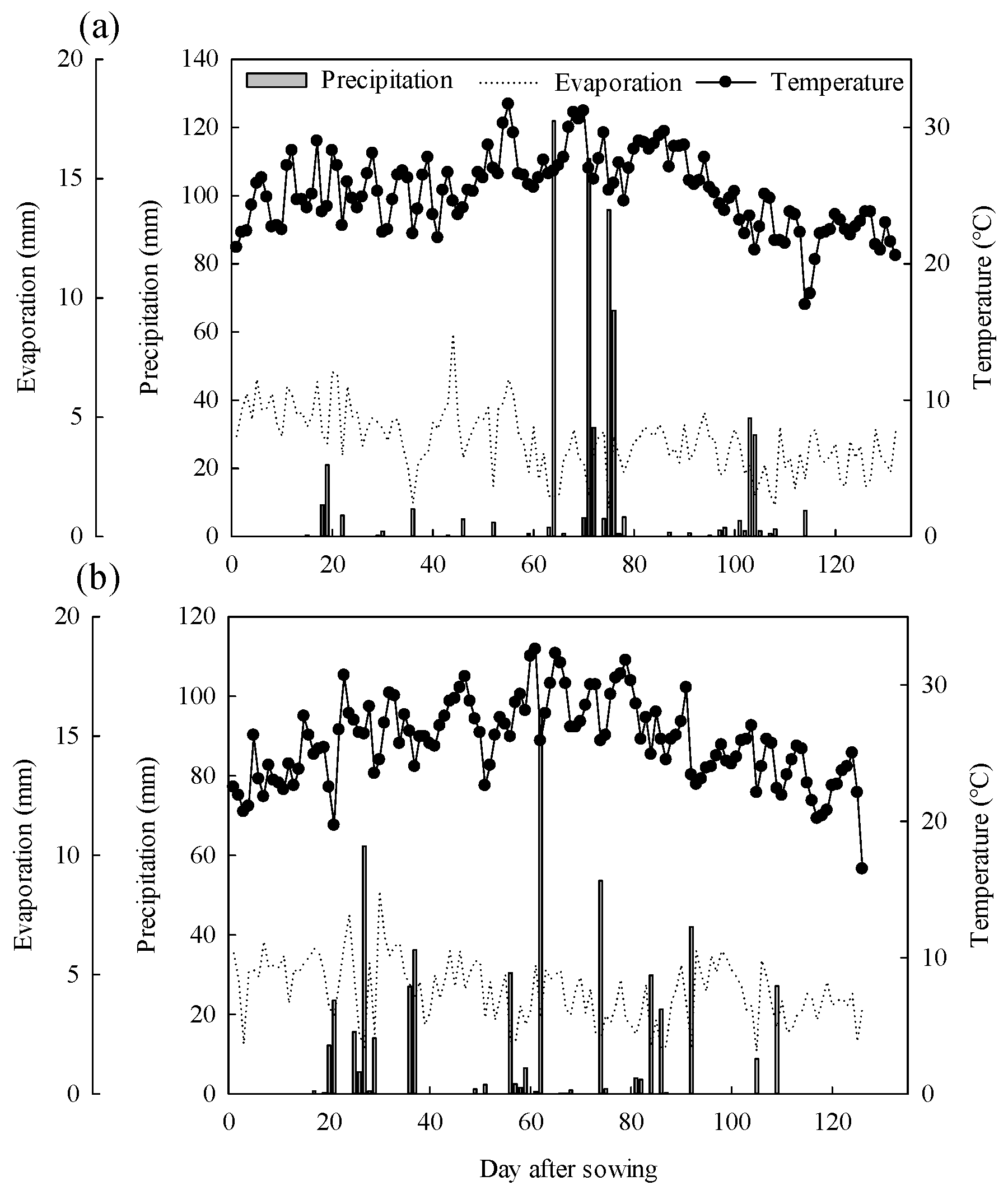
2.1. Experimental Sites
2.2. FGDG and FR
2.3. Experimental Design and Field Management
2.4. Sampling and Laboratory Procedures
2.5. Model Development and Preliminary Validation
2.6. Economic Analysis
2.7. Statistical Analysis
3. Results
3.1. Simulated Yield Potential, Grain Yield, and Economic Return
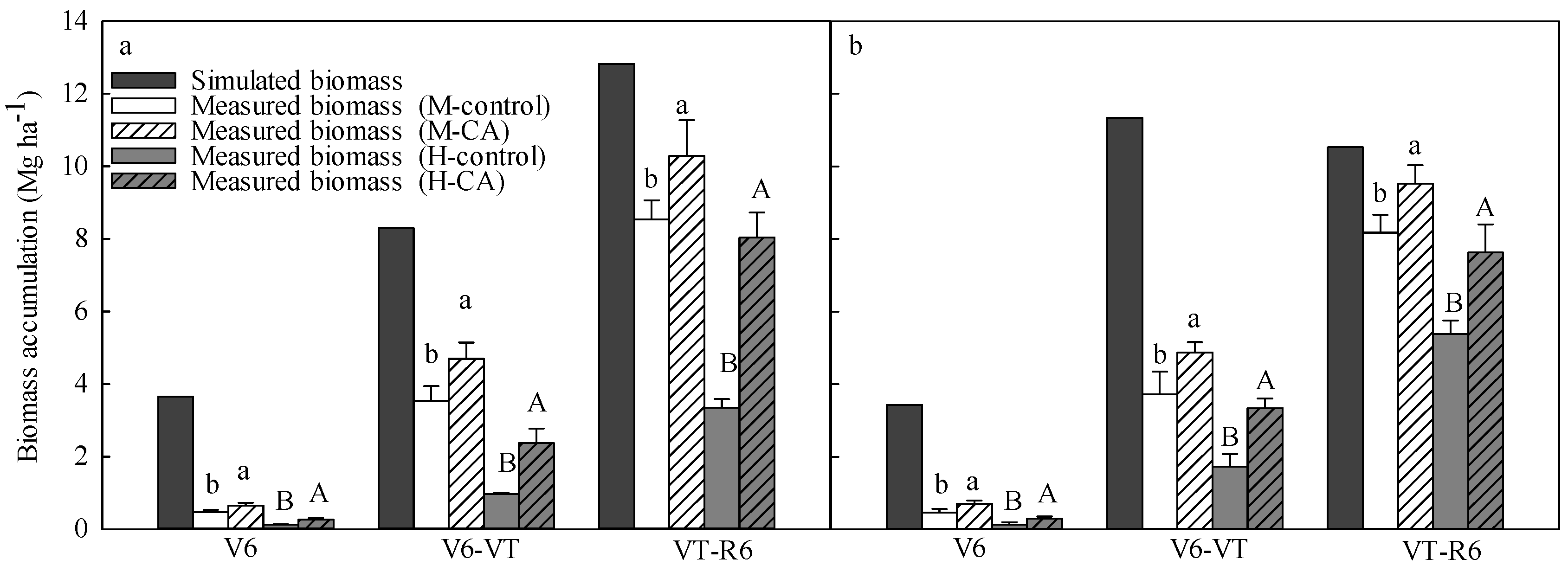
3.2. Simulated and Experimental Biomass Accumulation
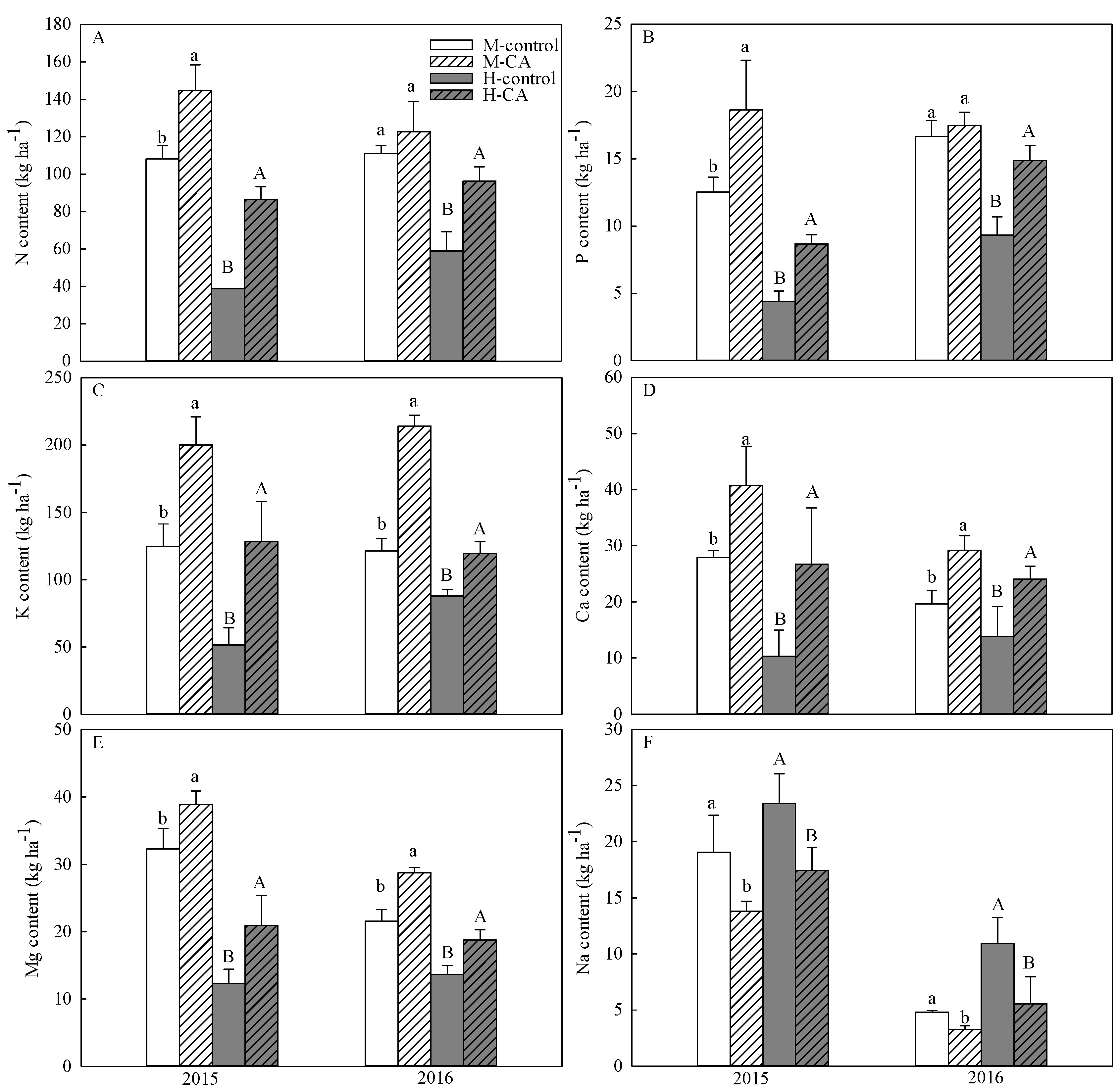
3.3. Ion Contents of Maize
3.4. Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, B.H.; Wu, L.; Chen, X.P.; Meng, Q.F. Quantifying the potential yield and yield gap of Chinese wheat production. Agron. J. 2016, 108, 1890. [Google Scholar] [CrossRef]
- Cao, H.Z.; Li, Y.N.; Chen, G.F.; Chen, D.D.; Qu, H.R.; Ma, W.Q. Identifying the limiting factors driving the winter wheat yield gap on smallholder farms by agronomic diagnosis in North China Plain. J. Integr. Agric. 2019, 18, 2–14. [Google Scholar] [CrossRef]
- Lobell, D.B.; Cassman, K.G.; Field, C.B. Crop yield gaps: Their importance, magnitudes, and causes. Annu. Rev. Environ. Resour. 2009, 34, 179–204. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.C.; Cui, Z.L.; Chen, X.P.; Xie, J.G.; Hou, Y.P. Closing the yield gap and achieving high N use efficiency and low apparent N losses. Field Crops Res. 2017, 209, 39–46. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, G.; Wang, J.; Cheng, J.; Meng, Y.; Dong, C.; Lei, T. Restoration and reutilization evaluation of coastal saline-alkaline degraded lands in Yellow River Delta. Transact. Chin. Soc. Agric. Eng. 2009, 25, 306–311, (in Chinese with English abstract). [Google Scholar]
- Fan, X.M.; Liu, G.H.; Tang, Z.P.; Shu, L.C. Analysis on main contributors influencing soil salinization of Yellow River Delta. J. Soil Water Conserv. 2010, 24, 139–144, (in Chinese with English abstract). [Google Scholar]
- Singh, K. Microbial and enzyme activities of saline and sodic soils. Land Degrad. Dev. 2016, 27, 706–718. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, G.; Gao, M.; Chang, C. Spatial variability of soil salinity in coastal saline soil at different scales in the Yellow River Delta, China. Environ. Monit. Assess. 2017, 189, 80. [Google Scholar] [CrossRef]
- Qadir, M.; Ghafoor, A.; Murtaza, G. Amelioration strategies for saline soils: A review. Land Degrad. Dev. 2000, 11, 501–521. [Google Scholar] [CrossRef]
- Kobayashi, O.; Higuchi, K.; Miwa, E.; Tadano, T. Growth injury induced by high pH in rice and tomato. Soil Sci. Plant Nutr. 2010, 56, 407–411. [Google Scholar] [CrossRef]
- George, E.; Horst, W.; Neumann, E. Calcareous and alkaline soils. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 444–455. [Google Scholar]
- Higuchi, K.; Ono, K.; Araki, S.; Nakamura, S.; Uesugi, T.; Makishima, T.; Ikari, A.; Hanaoka, T.; Sue, M. Elongation of barley roots in high pH nutrient solution is supported by both cell proliferation and differentiation in the root apex. Plant Cell Environ. 2017, 40, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.P.; Zhang, Q.; Liang, L.; Zhang, X.Z.; Wang, Y.L.; Guo, C.X.; Guo, J.L. Remediation of heavily saline-sodic soil with flue gas desulfurization gypsum in arid-inland China. Soil Sci. Plant Nutr. 2018, 64, 526–534. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Farsiani, A.; Ghobadi, M.E. Effects of PEG and NaCl stress on two cultivars of corn (Zea mays L.) at germination and early seedling stages. World Acad. Sci. Eng. Technol. 2009, 57, 382–385. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hiyane, R.; Hiyane, S.; Tang, A.C.; Boyer, J.S. Sucrose feeding reverses shade-induced kernel losses in maize. Ann. Bot. 2010, 106, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S. Salt resistance of crop plants: Physiological characterization of a multigenic trait. Mol. Physiol. Basis Nutr. Effic. Crops 2011, 13, 443–455. [Google Scholar]
- Sun, X.L. Research on the Irrigation and Drainage Pattern of Modern Yellow River Delta Area Based on RS. Master’s Thesis, China University of Petroleum, Beijing, China, 2008. (in Chinese with English abstract). [Google Scholar]
- Rasouli, F.; Pouya, A.K.; Karimian, N. Wheat yield and physico-chemical properties of a sodic soil from semi-arid area of Iran as affected by applied gypsum. Geoderma 2013, 193, 246–255. [Google Scholar] [CrossRef]
- Chun, S.; Nishiyama, M.; Matsumoto, S. Sodic soils reclaimed with by-product from flue gas desulfurization: Corn production and soil quality. Environ. Pollut. 2001, 114, 453–459. [Google Scholar] [CrossRef]
- Mahmoodabadi, M.; Yazdanpanah, N.; Sinobas, L.R.; Pazira, E.; Neshat, A. Reclamation of calcareous saline sodic soil with different amendments (I): Redistribution of soluble cations within the soil profile. Agric. Water Manag. 2013, 120, 30–38. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Li, S.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in north China: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, e89185. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.E.; Wolkowski, R.P. In-season effect of flue gas desulfurization gypsum on soil physical properties. J. Environ. Qual. 2014, 43, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.B.; Dick, W.A. Sustainable uses of FGD gypsum in agricultural systems: Introduction. J. Environ. Qual. 2014, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Yang, P.L.; Lin, H.; Ren, S.M.; He, X. Effects of sodic soil reclamation using flue gas desulphurization gypsum on soil pore characteristics, bulk density, and saturated hydraulic conductivity. Soil Sci. Soc. Am. J. 2014, 78, 1201–1213. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; US Department of Agriculture Soil Conservation Service: Washington, DC, USA, 1999.
- Smagin, A.V.; Sadovnikova, N.B.; Kirichenko, A.V.; Egorov, Y.V.; Vityazev, V.G.; Bashina, A.S. Dependence of the Osmotic Pressure and Electrical Conductivity of Soil Solutions on the Soil Water Content. Eurasian Soil Sci. 2018, 51, 1462–1473. [Google Scholar] [CrossRef]
- Shahid, S.A.; Rahman, K. Soil Salinity Development, Classification, Assessment and Management in Irrigated Agriculture. Handbook of Plant and Crop Stress, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 23–39. [Google Scholar]
- Eshel, G.; Levy, G.J.; Mingelgrin, U.; Singer, M.J. Critical evaluation of the use of laser diffraction for particle-size distribution analysis. Soil Sci. Soc. Am. J. 2004, 68, 736–743. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. US Dep. Agric. Circ. 1954, 939, 1954. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate uptake by rye. Soil Sci. Soc. Am. J. 1952, 48, 31–36. [Google Scholar]
- Kim, H.S.; Kim, K.R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.H. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef]
- Li, P.Y.; Zeng, Y.; Xie, Y.; Li, X.; Yan, K.; Wang, Y.B.; Xie, T.H.; Zhang, Y.K. Effect of pretreatment on the enzymatic hydrolysis of kitchen waste for xanthan production. Bioresour. Technol. 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.Y.; Liu, Y.M.; Cui, Z.L.; Chen, X.P.; Zou, C.Q. Zinc uptake and accumulation in winter wheat relative to changes in root morphology and mycorrhizal colonization following varying phosphorus application on calcareous soil. Field Crops Res. 2016, 197, 74–82. [Google Scholar] [CrossRef]
- Yang, H.S.; Dobermann, A.; Cassman, K.G.; Walters, D.T. Features, applications, and limitations of the Hybrid-Maize simulation model. Agron. J. 2006, 98, 737–748. [Google Scholar] [CrossRef]
- Liu, B.H.; Chen, X.P.; Meng, Q.F.; Yang, H.S.; Wart, J.V. Estimating maize yield potential and yield gap with agro-climatic zones in China—Distinguish irrigated and rainfed conditions. Agric. Forest Meteorol. 2017, 239, 108–117. [Google Scholar] [CrossRef]
- Van Wart, J.; Kersebaum, K.C.; Peng, S.; Milner, M.; Cassman, K.G. Estimating crop yield potential at regional to national scales. Field Crops Res. 2013, 143, 34–43. [Google Scholar] [CrossRef]
- Meng, Q.F.; Hou, P.; Wu, L.; Chen, X.P.; Cui, Z.L.; Zhang, F.S. Understanding production potentials and yield gaps in intensive maize production in China. Field Crops Res. 2013, 143, 91–97. [Google Scholar] [CrossRef]
- Hou, P.; Cui, Z.L.; Bu, L.D.; Yang, H.S.; Zhang, F.S.; Li, S.K. Evaluation of a modifed Hybrid-Maize model incorporating a newly developed module of plastic film mulching. Crop Sci. 2014, 54, 2796–2804. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P. Potential flue gas desulfurization gypsum utilization in agriculture: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 1969–1978. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, C.H.; Xu, X.C.; Li, Y.J. Amelioration of alkali soil using flue gas desulfurization byproducts: Productivity and environmental quality. Environ. Pollut. 2008, 151, 200–204. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J.; Chaba, G.D.; Poss, J.A.; Shannon, M.C. Salt sensitivity of corn at various growth stages. Irrig. Sci. 1983, 4, 45–57. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Yan, P.; Yue, S.C.; Meng, Q.F.; Pan, J.X.; Ye, Y.L.; Chen, X.P.; Cui, Z.L. An understanding of the accumulation of biomass and nitrogen is benefit for Chinese maize production. Agron. J. 2016, 108, 895. [Google Scholar] [CrossRef]
- Lizaso, J.I.; Ruiz-Ramos, M.; Rodríguez, L.; Gabaldon-Leal, C.; Oliveira, J.A.; Lorite, I.J.; Sánchez, D.; García, E.; Rodríguez, A. Impact of high temperatures in maize: Phenology and yield components. Field Crops Res. 2018, 216, 129–140. [Google Scholar] [CrossRef]
- Li, R.; Liu, P.; Dong, S.; Zhang, J.; Zhao, B. Increased maize plant population induced leaf senescence, suppressed root growth, nitrogen uptake, and grain yield. Agron. J. 2019, 111, 1–11. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Dynamic remobilization of leaf nitrogen components in relation to photosynthetic rate during grain filling in maize. Plant Physiol. Biochem. 2018, 129, 27–34. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Panda, R.K.; Chakraborty, A.; Halder, D. Enhancing grain yield, biomass and nitrogen use efficiency of maize by varying sowing dates and nitrogen rate under rainfed and irrigated conditions. Field Crops Res. 2018, 221, 339–349. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Z.; Qin, J.; Ma, Z.; Zhang, Y.; Zhang, L. The potential of residues of furfural and biogas as calcareous soil amendments for corn seed production. Environ. Sci. Pollut. Res. 2016, 23, 6217–6226. [Google Scholar] [CrossRef]
- Cui, Z.L.; Chen, X.P.; Zhang, F.S. Development of regional nitrogen rate guidelines for intensive cropping systems in China. Agron. J. 2013, 105, 1411–1416. [Google Scholar] [CrossRef]
- Ju, X.T.; Gu, B.J.; Wu, Y.Y.; Galloway, J.N. Reducing China’s fertilizer use by increasing farm size. Glob. Environ. Chang. 2016, 41, 26–32. [Google Scholar] [CrossRef]
| Characteristics | Moderate Salinity Level | High Salinity Level | ||
|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | |
| Sand (%) | 69.1 | 89.4 | 73.5 | 81.5 |
| Silt (%) | 28.0 | 9.21 | 24.0 | 16.8 |
| Clay (%) | 2.86 | 1.43 | 2.45 | 1.72 |
| pH | 8.19 | 8.33 | 8.10 | 8.35 |
| EC (dS m−1) | 3.91 | 4.05 | 6.30 | 6.25 |
| Organic carbon (g kg−1) | 4.33 | 0.62 | 2.99 | 0.75 |
| Total N (g kg−1) | 0.67 | 0.36 | 0.53 | 0.22 |
| Olsen-P (mg kg−1) | 4.98 | 1.95 | 6.72 | 1.85 |
| Exchangeable K (mg kg−1) | 77 | 25 | 110 | 50 |
| Exchangeable Ca (mg kg−1) | 3760 | 2701 | 3567 | 3409 |
| Exchangeable Na (mg kg−1) | 426 | 270 | 777 | 1094 |
| Exchangeable Mg (mg kg−1) | 467 | 211 | 527 | 515 |
| Yield (Mg ha−1) | The Ratio of YP (%) | Ear Number (104 ha−1) | Grain Number per Ear | Grain Weight (g 1000−1) | Economic Return ($ ha−1) | |
|---|---|---|---|---|---|---|
| 2015 Moderate | ||||||
| control | 5.58 | 38.2 | 6.42 | 420 | 269 | 1351 |
| CA | 7.53 | 51.6 | 6.56 | 528 | 283 | 1489 |
| LSD0.05 | 1.51 | ns | 48.2 | ns | ||
| 2015 High | ||||||
| control | 2.05 | 14.0 | 4.25 | 329 | 207 | 496.2 |
| CA | 4.23 | 29.0 | 5.94 | 451 | 264 | 690.3 |
| LSD0.05 | 2.08 | 1.11 | 121 | 33.3 | ||
| 2016 Moderate | ||||||
| control | 7.88 | 49.9 | 7.73 | 400 | 295 | 1907 |
| CA | 8.31 | 52.6 | 7.96 | 508 | 290 | 1678 |
| LSD0.05 | 0.42 | ns | 50.4 | ns | ||
| 2016 High | ||||||
| control | 4.42 | 28.0 | 5.86 | 304 | 230 | 1070 |
| CA | 6.78 | 42.9 | 8.06 | 401 | 281 | 1307 |
| LSD0.05 | 1.61 | 1.63 | 96.4 | 37.9 |
| ANOVA | Sum of Squares | Degrees of Freedom | Mean of the Squares | F Value | p Value |
|---|---|---|---|---|---|
| Yield (Mg ha−1) | |||||
| Year | 24.018 | 1.000 | 24.018 | 52.70 | <0.0001 |
| Salinity levels | 52.330 | 1.000 | 52.330 | 114.82 | <0.0001 |
| Treatment | 17.943 | 1.000 | 17.943 | 39.37 | <0.0001 |
| Ear number (104 ha−1) | |||||
| Year | 15.589 | 1.000 | 15.589 | 66.68 | <0.0001 |
| Salinity levels | 7.772 | 1.000 | 7.772 | 33.24 | <0.0001 |
| Treatment | 6.793 | 1.000 | 6.793 | 29.06 | <0.0001 |
| Grains per ear | |||||
| Year | 5097.7 | 1.000 | 5097.7 | 3.64 | 0.0744 |
| Salinity levels | 51729 | 1.000 | 51729 | 36.98 | <0.0001 |
| Treatment | 70790 | 1.000 | 70790 | 50.60 | <0.0001 |
| Grain weight (g 1000−1) | |||||
| Year | 2070.2 | 1.000 | 2070.2 | 6.32 | 0.0231 |
| Salinity levels | 9067.6 | 1.000 | 9067.6 | 27.66 | <0.0001 |
| Treatment | 4987.2 | 1.000 | 4987.2 | 15.22 | 0.0013 |
| Soil pH | SOC (g kg−1) | Soil Ca2+ (g kg−1) | Soil Na+ (g kg−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | |
| Moderate | ||||||||
| control | 8.17 | 8.23 | 5.05 | 2.52 | 3.31 | 3.04 | 0.46 | 0.29 |
| CA | 8.08 | 8.12 | 5.76 | 2.46 | 4.24 | 3.08 | 0.38 | 0.25 |
| LSD0.05 | 0.06 | 0.08 | 0.64 | ns | 0.15 | ns | ns | ns |
| High | ||||||||
| control | 8.33 | 8.33 | 3.93 | 1.97 | 3.53 | 2.97 | 1.42 | 0.82 |
| CA | 8.26 | 8.29 | 4.85 | 2.82 | 4.33 | 3.29 | 0.66 | 0.54 |
| LSD0.05 | 0.05 | ns | 0.86 | 0.59 | 0.26 | 0.27 | 0.23 | 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Jiang, X.; Xue, Y.; Li, Z.; Yu, B.; Xu, L.; Lu, X.; Miao, Q.; Liu, Z.; Cui, Z. Closing Yield Gaps through Soil Improvement for Maize Production in Coastal Saline Soil. Agronomy 2019, 9, 573. https://doi.org/10.3390/agronomy9100573
Zhang J, Jiang X, Xue Y, Li Z, Yu B, Xu L, Lu X, Miao Q, Liu Z, Cui Z. Closing Yield Gaps through Soil Improvement for Maize Production in Coastal Saline Soil. Agronomy. 2019; 9(10):573. https://doi.org/10.3390/agronomy9100573
Chicago/Turabian StyleZhang, Jishi, Xilong Jiang, Yanfang Xue, Zongxin Li, Botao Yu, Liming Xu, Xingchen Lu, Qi Miao, Zitong Liu, and Zhenling Cui. 2019. "Closing Yield Gaps through Soil Improvement for Maize Production in Coastal Saline Soil" Agronomy 9, no. 10: 573. https://doi.org/10.3390/agronomy9100573
APA StyleZhang, J., Jiang, X., Xue, Y., Li, Z., Yu, B., Xu, L., Lu, X., Miao, Q., Liu, Z., & Cui, Z. (2019). Closing Yield Gaps through Soil Improvement for Maize Production in Coastal Saline Soil. Agronomy, 9(10), 573. https://doi.org/10.3390/agronomy9100573





