Abstract
In higher plants, several lines of evidence suggest that long non-coding RNAs (lncRNAs) may play important roles in the regulation of various biological processes by regulating gene expression. In this study, we identified a total of 521 lncRNAs, classified as intergenic, intronic, sense, and natural antisense lncRNAs, from RNA-seq data of drought-exposed tomato leaves. A further 244 drought-responsive tomato lncRNAs were predicted to be putative targets of 92 tomato miRNAs. Expression pattern and preliminary functional analysis of potential mRNA targets suggested that drought-responsive tomato lncRNAs play important roles in a variety of biological processes via lncRNA–mRNA co-expression. Taken together, these data present a comprehensive view of drought-responsive tomato lncRNAs that serve as a starting point for understanding the role of long intergenic non-coding RNAs in the regulatory mechanisms underlying drought responses in crops.
1. Introduction
Because the world population is increasing, the global food demand is expected to approximately double by the year 2050 [1]. Meeting global food needs will require a substantial understanding of the climatic factors influencing agricultural production. Important in this regard is understanding how climate extremes caused by global warming impact crop yields. Drought, a recurring phenomenon with major impacts on natural systems, is one of the major widespread climatic extremes that negatively affect agricultural production [2,3]. Droughts led to global cereal (maize, rice, and wheat) production deficits of 10.1% on average during the past four decades [4]. Under drought conditions, crops display various physiological and biochemical responses, including stomatal movement (the opening or closing of stomata), morphological changes (repression of cell growth and development),and alteration in biosynthetic pathways, antioxidant pathways, and respiration pathways; all of these aid survival in this unfavorable climate [5]. Understanding drought-induced molecular and physiological mechanisms is necessary for successful yield protection in the context of drought.
Coding and non-coding genomic elements, including messenger RNA (mRNA) and long non-coding RNA (lncRNA), are the main subgroups of RNAs participating in transcription regulation. Generally, lncRNAs are defined as RNA transcripts characterized by a minimum length of 200 bp and lack of coding potential [6]. In plants, the majority of lncRNAs are transcribed by RNA polymerase II similar to mRNAs, although some lncRNAs are transcribed by RNA polymerase III or the plant-specific, DNA-dependent, RNA polymerases RNA polymerase IV and V [7]. Initially, lncRNAs were thought to represent transcriptional noise with low levels of evolutionary conservation [8]; nevertheless, a growing body of evidence suggests that lncRNAs play important roles in the regulation of various biological processes, including plant growth and development, epigenetic responses, and the responses to various stresses [6,9,10]. In Arabidopsis, more than 6500 lncRNAs were identified from a number of transcriptomic datasets, with either organ-specific or stress-induced expression profiles [9,11]. Extensive genome-wide identification of lncRNAs has been performed in some plants, including Populus tomentosa [12], Fragaria vesca [13], Cicer arietinum [14], Ginkgo biloba [15], and Zea mays [16]. Several reports also show that biotic or abiotic stress alters lncRNA expression in plants. For example, 664 and 98 drought-responsive lncRNAs were identified in maize [17] and rice [18], respectively. The expression of 1832 Arabidopsis long intergenic non-coding RNAs (lincRNAs), which are lncRNAs transcribed from intergenic regions of the genome, were significantly altered after drought, cold, salt and/or abscisic acid (ABA) treatment [9]. Among these, drought-induced lncRNA mediate plant tolerance to drought and salt stress by modulating the expression of genes involved in ABA signaling, water transport, and other stress-relief processes [19]. Overexpression of Arabidopsis long non-protein-coding RNA 536 resulted in visible differences when compared to wild-type plants under salt stress conditions [20]. Taken together, these data suggest that the identification of stress-responsive lncRNA provides an opportunity to increase our knowledge of the contribution of lncRNAs to the stress response and to explore lncRNAs as possible targets for improving plant tolerance to stress.
In this study, in order to investigate the regulation of tomato lncRNAs in response to drought stress, we analyzed transcriptome data obtained from drought-treated tomato leaf samples. Combined with bioinformatics approaches, we further analyzed the potential function of these lncRNAs and the relationship between tomato mRNAs, lncRNAs, and miRNAs. Taken together, our results will improve our understanding of lncRNA-mediated gene regulation in drought response.
2. Materials and Methods
2.1. Plant Material and Drought Treatment
Tomato (Solanum lycopersicum cv. Amoroso) was grown under controlled conditions (25 °C, 70% relative humidity, and light intensity of 1000 µmol m−2 s−1 for 12 h/day). For drought stress treatment, 4-week-old plants were subjected to water deprivation for 4 d, and non-treated plants were well-watered throughout the experiment. Leaves from drought-treated and control tomato plants were collected for total RNA isolation. The experiment was conducted with 3 replicates per treatment and 10 plants per replicate.
2.2. Transcriptome Data and Transcriptome Assembly
RNA-seq data from drought-treated tomato leaf samples were obtained from the National Agricultural Biotechnology Information Center (NABIC, http://nabic.rda.go.kr) with accession number NN-5505 [21]. Then, the quality of the raw data was controlled by using the FastQC tool and Trimmomatic v.0.33 as described by Eom et al. [5]. The clean reads were mapped to the tomato reference sequence (Tomato Genome version SL3.0 and Annotation ITAG3.10, https://solgenomics.net/organism/Solanum_lycopersicum/genome), using the HISAT2 aligner (http://ccb.jhu.edu/software/hisat2/index.shtml ). Then, these mapped reads were further assembled and merged using StringTie (https://ccb.jhu.edu/software/stringtie).
2.3. Long Non-Coding RNA Identification
LncRNAs were identified using the following workflow: Step 1, unknown transcripts with lengths longer than 200 nucleotides (nt), and FPKM (fragments per kilobase of transcript per million fragments mapped) more than 0.1 were selected as lncRNA candidates; Step 2, transcripts with ORFs (open reading frames) >300 bp and other non-coding RNAs (e.g., rRNAs, tRNAs, snoRNAs, snRNAs) were removed using gffcompare; Step 3, the coding potential of the remaining transcripts was evaluated using the coding potential calculator (CPC, CPC score <0) software (http://cpc.cbi.pku.edu.cn/), BlastX search against all plant protein sequences in the Swiss-Prot database, and PfamScan (protein family database, E-value < 0.001); Step 4, transcripts that passed these steps were annotated as lncRNAs; and Step 5, a long non-coding RNA Scan (lncRScan) program was used to categorize the identified lncRNAs as described by Sun et al. [22].
2.4. Prediction and Functional Annotation of Long Non-Coding RNA Targets
To explore whether lncRNAs function as miRNA decoys, all identified lncRNAs were analyzed for miRNA target sites using psRNATarget webserver (https://plantgrn.noble.org/psRNATarget/analysis). The interaction between tomato miRNAs and the identified lincRNAs was calculated using the following parameters: maximum expectation = 5 and allowed maximum energy to unpair the target site (UPE) = 25, as described by Hou et al. [23]. A lower UPE implies a higher possibility of establishing a contact between an miRNA and the target lncRNA. The interaction network of lincRNAs and miRNAs was drawn using Cytoscape software (Agilent Technologies Co., Santa Clara, CA, USA).
The potential target genes of drought-responsive lncRNAs were predicted using cis role analysis, as described by Wang et al. [15]. These lncRNA target genes were functionally annotated using Gene Ontology (GO) (p < 0.05) (http://geneontology.org/).
2.5. Quantitative Reverse Transcription–Polymerase Chain Reaction Analysis
The expression levels of selected miRNAs, lncRNAs and target genes were analyzed using quantitative reverse transcription–polymerase chain reaction (RT-qPCR). To analyze tomato miRNA expression, total RNA was extracted from drought-treated or non-treated tomato plants using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and treated with DNase I to remove any contaminating DNA. cDNA was synthesized using a Mir-X miRNA First-Strand Synthesis kit (Clontech Takara, Seoul, Korea) according to the manufacturer’s instructions. For analyzing the expression of lncRNAs and target genes, total RNA was extracted using the FavorPrep Plant Total RNA Mini Kit and reverse-transcribed into cDNA using the ReverTra Ace® qPCR RT Master Mix with qDNA Remover (TOYOBO, Co., Ltd., Osaka, Japan), according to the manufacturer’s instructions. RT-qPCR was performed using the SYBR® Green Real-Time PCR Master Mix (Toyobo, Co., Ltd., Osaka, Japan) in a CFX96TM real-time system (Bio-Rad, Hercules, CA, USA), with the following PCR conditions: 95 °C for 10 s, 40 cycles of 95 °C for 5 s, and 60 °C for 20 s. Sample cycle threshold (Ct) values were determined and standardized relative to the tomato ACTIN 4 gene (Solyc04g011500), and the 2−△△Ct method was used to calculate the relative expression of selected miRNAs, lncRNAs, and target genes. The specific primer pairs used in RT-qPCR are listed in Table S1.
2.6. Statistical Analysis
To determine the significance of differences between the groups, one-way analysis of variance (ANOVA) based on Duncan’s multiple range tests (p < 0.05) was used. All experiments were repeated at least three times, and the results were presented as mean ± standard error.
3. Results and Discussion
3.1. Identification and Characterization of Drought-Responsive Tomato Long Non-Coding RNAs
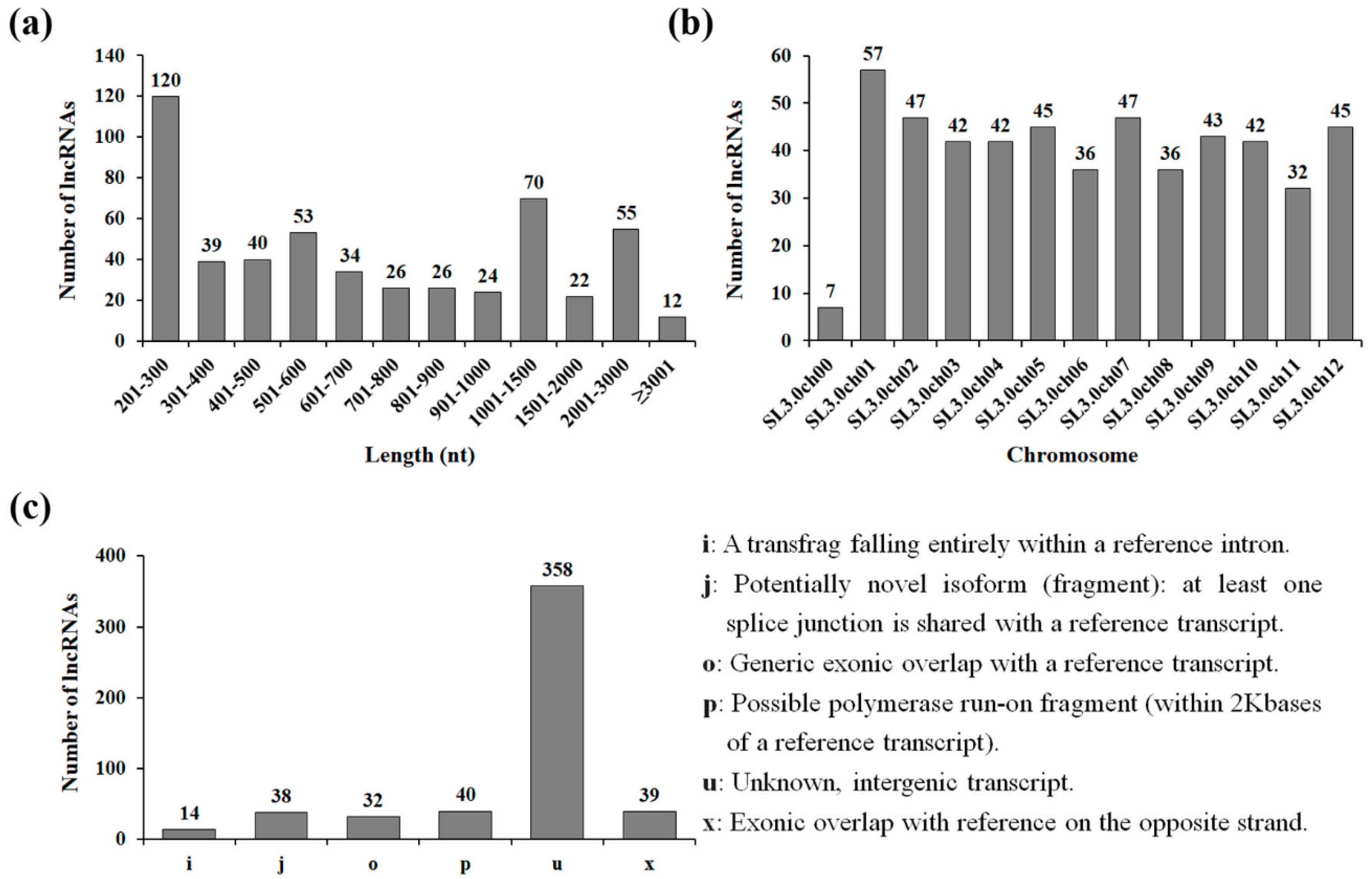
To understand the molecular mechanisms of tomato lncRNA responses to drought stress, the raw reads from RNA-seq data were analyzed. After removing low-quality reads, approximately 55 million paired-end clean reads were obtained from RNA-seq data generated from drought-treated tomato leaf samples (NN-5505) and were mapped and assembled as described in the methods section. To obtain confident lncRNA transcripts, we performed severalrounds of core filtering of transcripts with high sequence similarity to known proteins, protein-coding potentials, or open-reading frames. Using this comprehensive filtering pipeline, we finally identified 521 lncRNA expressed in drought-treated tomato leaves (Table S2). To characterize the basic genomic features of tomato lncRNAs, we analyzed their length and distribution on the chromosomes of tomato. The length of lncRNAs ranged from 201 to 6701 nt, with an average of 916 nt, and there were 362 lncRNAs with lengths varying from 200 to 1000 nt (Figure 1a). We also found that the drought-responsive lncRNAs were transcribed from all 12 chromosomes. Chromosome 1 had the highest number of lncRNA loci (57), followed by chromosome 2 (47) and chromosome 7 (47), whereas chromosome 11 had the lowest number of lncRNA loci (32) (Figure 1b).
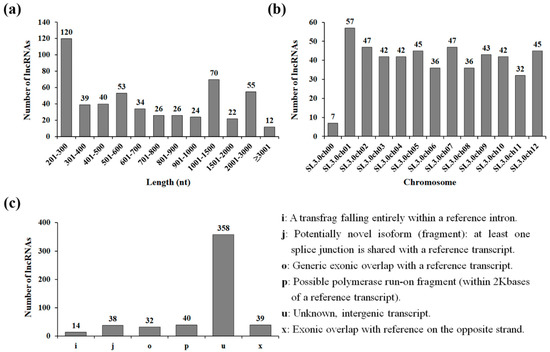
Figure 1.
Characteristics of drought-responsive tomato long non-coding RNAs (lncRNAs). (a) Length distribution of lncRNAs. (b) Chromosome-wise distribution of long intergenic non-coding RNAs (lincRNAs). (c) Classification of lincRNAs.
On the basis of the relationship with protein-coding genes and the genomic location, most plant lncRNAs have been classified as intergenic, intronic, sense, and natural antisense lncRNAs [24]. To characterize drought-responsive tomato lncRNAs, the identified lncRNAs were further classified into six categories by comparison with the known gene annotations. As shown in Figure 1c, 358 lncRNAs and 40 lncRNAs were assigned the ‘u’ classcode, defining the “unknown intergenic transcript” and the ‘p’ classcode, defining the “possible polymerase run-on fragment,” respectively. The identified lncRNAs from ‘u’ and ‘p’ classes were considered to be lincRNAs, as suggested by Wang et al. [25]. A total of 39 lncRNAs with the ‘x’ classcode had exonic overlap with references on the opposite strand, such as the natural antisense lncRNAs. A total of 14 lncRNAs with the ‘i’ classcode fell entirely within the reference intron, including the intronic lncRNAs. A total of 70 candidates in classes ‘j’ (potentially novel isoform) and ‘o’ (unknown, generic overlap with reference) were considered to be sense lncRNAs, suggesting that lincRNAs accounted for the largest proportion of drought-responsive tomato lncRNAs. Because thousands of lincRNAs have been identified in human and plant genomes [9,17,26], a few studies have revealed the function of lincRNAs, including transcriptional regulation by recruiting proteins for chromosome modification to specific loci, inhibition of the physical interaction between miRNAs and their target mRNAs, and controlling alternative splicing [27]. Although the physiological function of drought-responsive lincRNAs remains unclear, some drought-responsive Populus lincRNAs are thought to serve as putative targets of Populus miRNAs [28]. This suggests that miRNA–lncRNAs (such as lincRNAs) interactions might play key roles in drought stress tolerance.
3.2. Drought-Responsive Tomato Long Non-Coding RNA Transcripts as Potential Targets of Tomato miRNAs
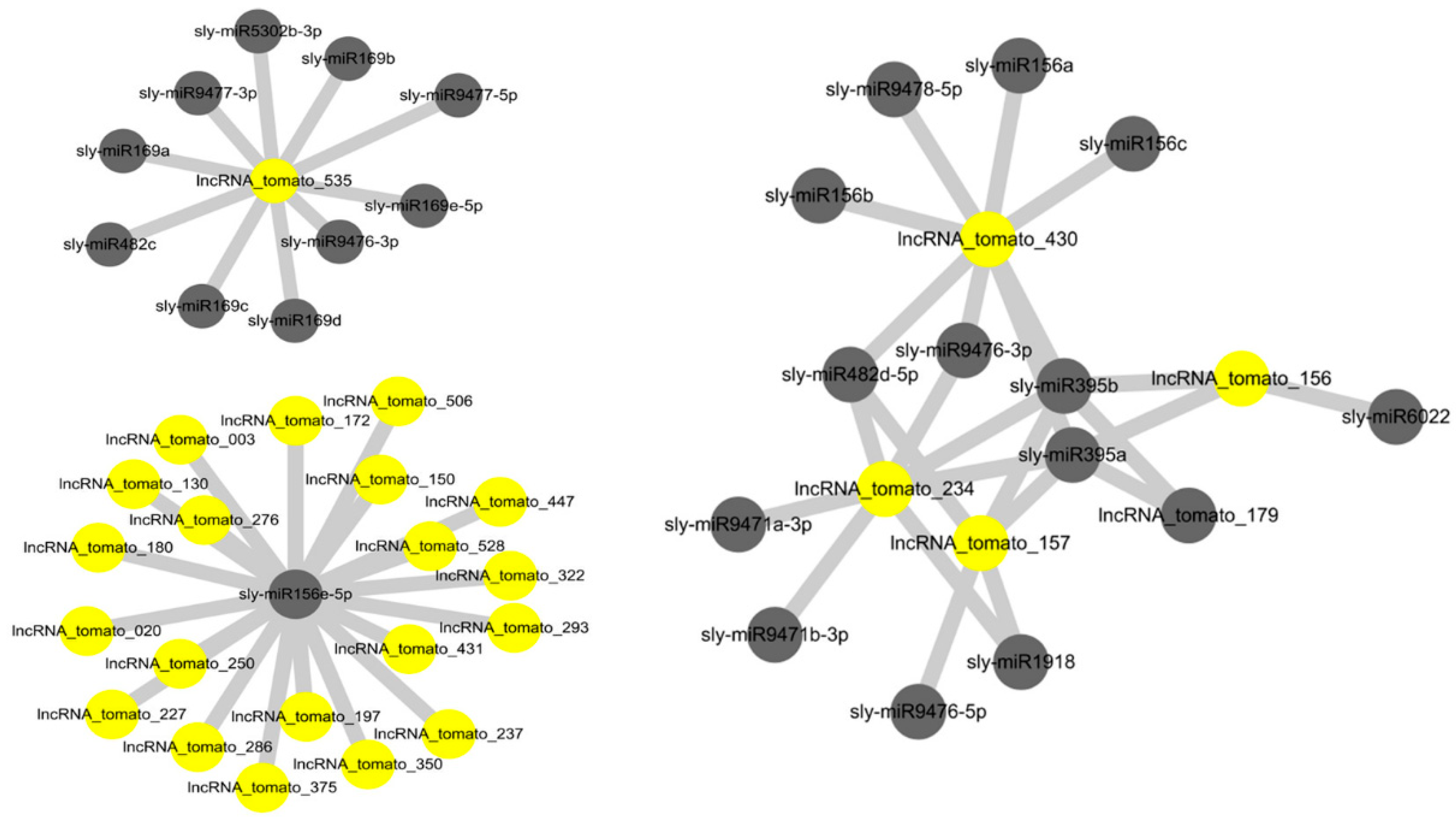
miRNAs and lncRNAs are two important types of non-coding RNA, and their interactions play important roles in various biological processes, including plant growth, development, and reproduction [29,30]. LncRNAs that interact with miRNA function as endogenous target mimics [27,31]. To investigate the interaction between our identified lncRNAs and tomato miRNAs, our identified lncRNAs as targets of tomato miRNAs were predicted using psRNATarget server [32]. An alignment of tomato miRNAs with 521 lncRNA suggested that a total of 244 drought-responsive tomato lncRNAs were putative targets of 92 tomato miRNAs (Table S3). As shown in Figure 2, multiple interaction patterns, including one lncRNA with many miRNAs, many lncRNAs with one miRNA, and many lncRNAs with many miRNAs were identified. This resulted in a total of 538 lncRNA–miRNA interactions (Table S3). The majority of miRNAs involved in interactions with lncRNAs were of the sly-miR156, sly-miR390, sly-miR482, sly-miR5302, and sly-miR9476 families. miR156 is one of the most conserved and highly expressed miRNAs in plants. In higher plants, overexpression of miR156 resulted in dramatic morphologic changes, including delayed flowering, increased root development, and enhanced biomass production, suggesting that miR156 has important regulatory functions in plant growth and development [33,34,35,36]. In addition, miR156 regulates tolerance to environmental stresses such as salt and drought stresses via downregulation of the SPL (squamosa promoter-binding protein-like) transcription factor family genes. These genes are master regulators of various biological processes, including vegetative to reproductive phase change, secondary metabolism, and stress responses [33,37,38].
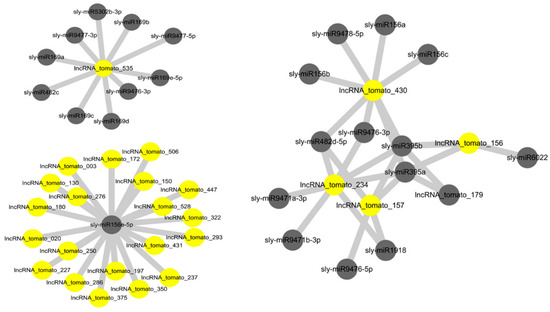
Figure 2.
LncRNA–miRNA interaction network. Yellow circle nodes represent lncRNAs, and gray circle nodes represent miRNAs. Examples of interactions are shown: one lncRNA with many miRNAs, one miRNA with many lncRNAs, and many lncRNAs with many miRNAs.
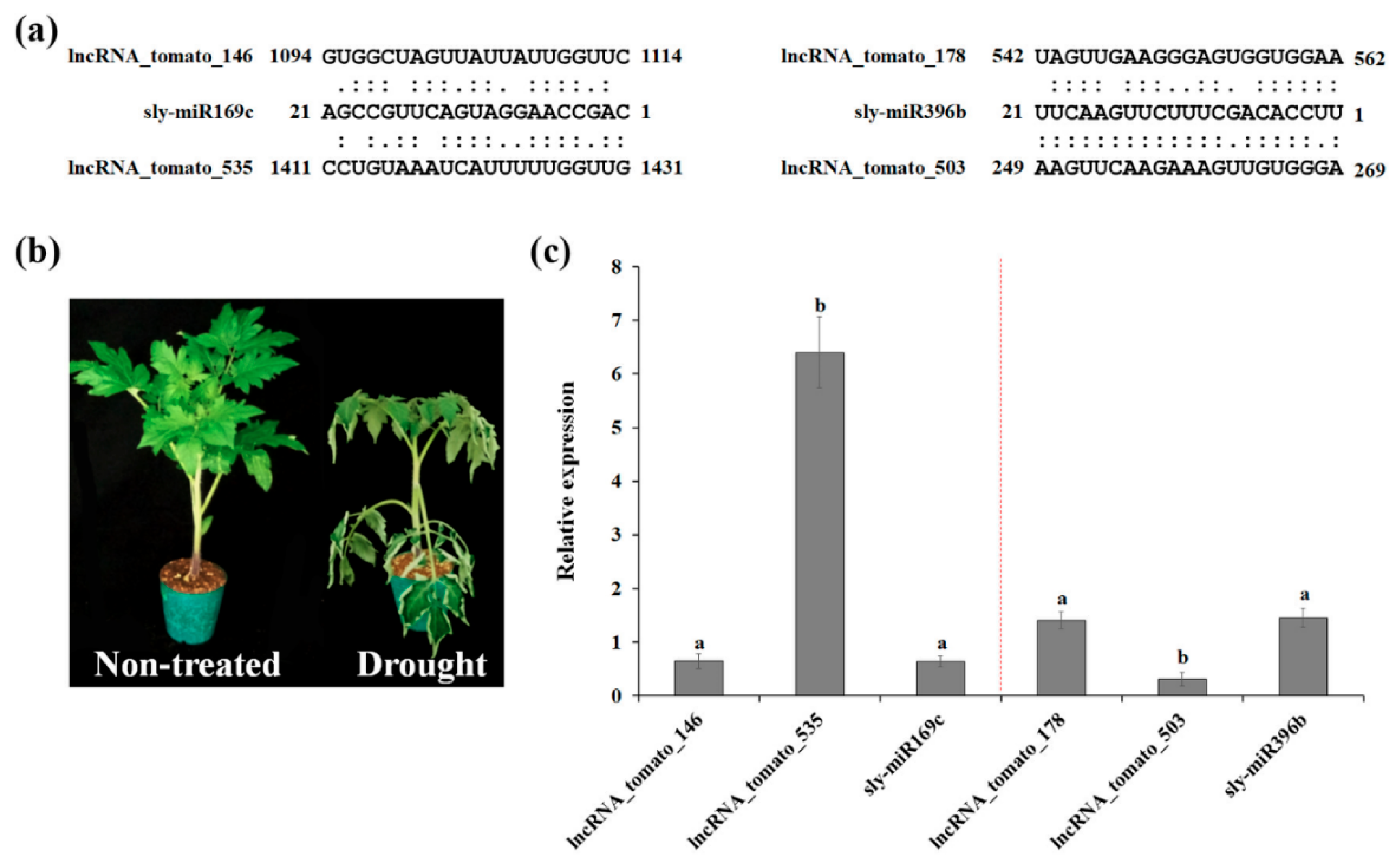
It has been shown that the expression of miR169 is regulated by CBF/DREB (C-repeat-binding factor/dehydration-responsive element binding factor) transcription factors [39,40]. In addition, overexpression of miR396 enhanced drought tolerance [41], suggesting the role of miR169 and miR396 in drought response. To investigate the relationship between miRNAs (miR169 and miR396) and their target lncRNAs, four lncRNA–miRNA pairs were selected and subjected to qRT-PCR analysis. Although current knowledge and data on miRNA–lncRNA interactions are still limited, accumulating evidence suggests that the interaction patterns of lncRNAs and miRNAs are closely related to their relative expression levels [42]. To investigate the interaction patterns of miRNAs and their target lncRNAs, four lncRNA–miRNA pairs were selected and subjected to RT-qPCR analysis. As shown in Figure 3, sly-miR169c was downregulated by drought stress, whereas the target lncRNA-tomato_535 was strongly induced by drought stress. In addition, sly-miR396b was upregulated, while lncRNA_tomato_503 was downregulated by drought stress. Sly-miR169c and sly-miR396b exhibited the same expression pattern as their other targets, lncRNA-tomato_146 and lncRNA-tomato_178, respectively, suggesting that lncRNA-tomato_146 and lncRNA-tomato_178 regulate the expression of sly-miR169c and sly-miR396b as target mimics under drought conditions, as suggested by Deng et al. [43]. As shown in Table S3, lncRNA-tomato_535 was predicted to be a putative target of sly-miR169, sly-miR482, sly-miR5302, sly-miR9476, and sly-miR9477 families, whereas one or two miRNA families targeted lncRNA-tomato_146, lncRNA-tomato_178, or lncRNA_tomato_503, suggesting that high expression of lncRNA-tomato_535 might be required for protecting mRNAs against miRNAs-induced degradation, as supported by Zhang et al. [44]. Taken together, these findings suggest that our identified lncRNAs regulate the expression of miRNA target genes by acting as miRNA targets or target mimics to downregulate miRNA activity.
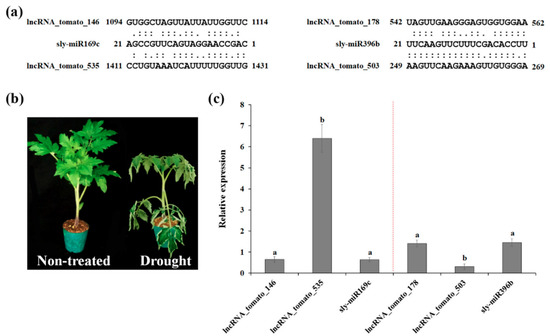
Figure 3.
Functional prediction of drought-responsive tomato lncRNAs as potential targets or target mimics of miRNAs. (a) The interaction miRNA–lncRNA was identified using psRNATarget webserver (https://plantgrn.noble.org/psRNATarget/analysis). (b) Phenotypes of tomato plants after exposure to drought stress for 4 days. (c) The relative expression levels of four lncRNA–miRNA pairs were validated by RT-qPCR. The expression levels of selected miRNAs and lncRNAs were calculated relative to their expression in the non-treated sample. Data are means ± standard error (SE). Values in the same column with different superscripted letters are significantly different (p < 0.05).
3.3. Functional Characterization of Drought-Responsive Tomato Long Non-Coding RNAs
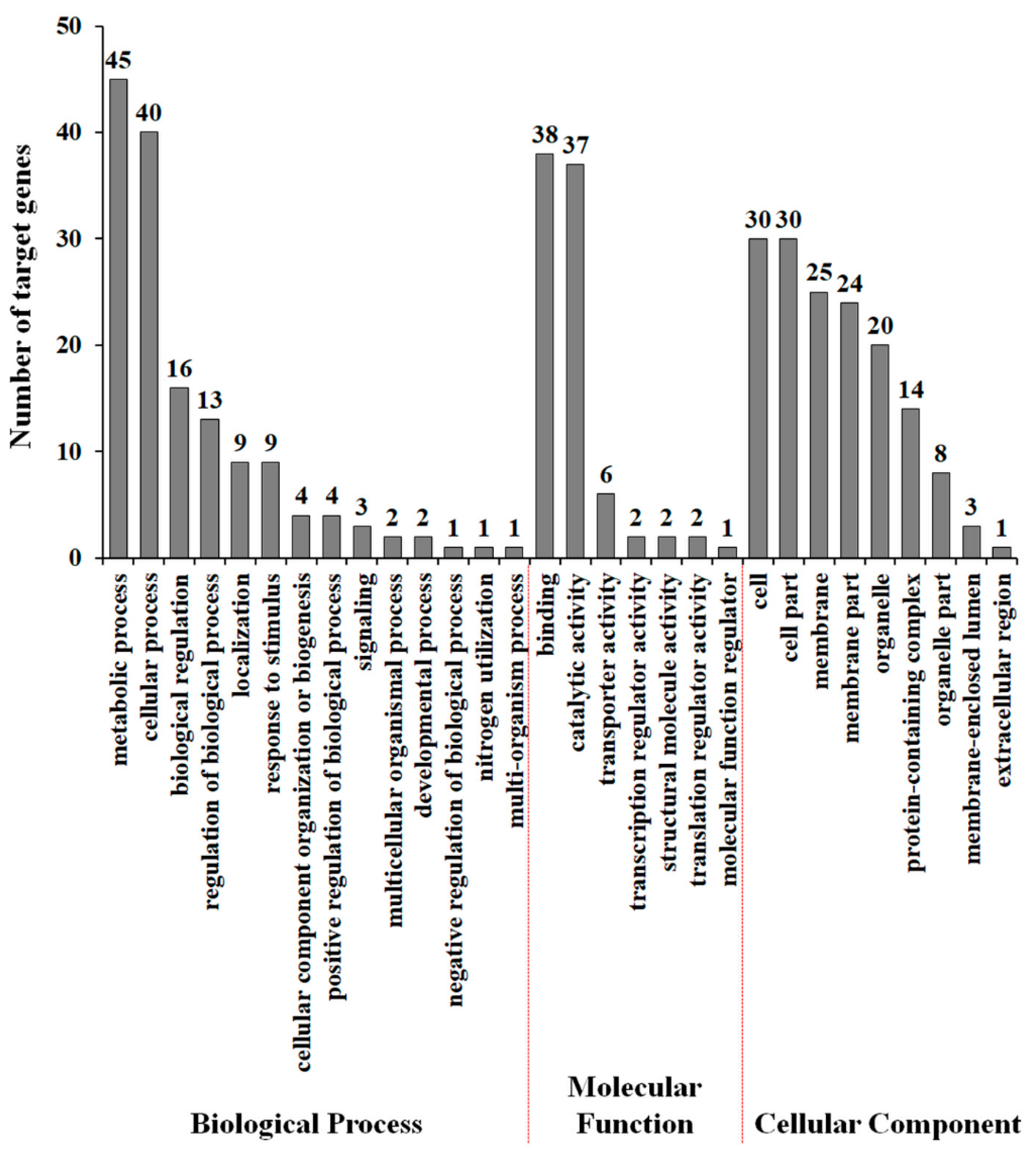
The transcription of some lncRNAs regulates the expression of neighboring protein-coding genes in cis via mechanisms including antisense-mediated repression, activation of divergent genes with bidirectional promoters, RNA-mediated enhancement, and genomic imprinting [45,46,47,48]. To identify potential mRNA targets of drought-responsive tomato lncRNA, we initially predicted potential mRNA targets by searching 100 kb upstream and downstream of each lncRNA. Then, we identified 183 target genes through complementary base pairing with lncRNAs. All target genes were aligned to GO terms, to predict and classify possible functions of drought-responsive tomato lncRNAs. As shown in Figure 4, metabolic process and cellular process were prominently represented under the biological process category. Furthermore, cell and cell part represented the majority of terms in the cellular component category, and the vast majority was related to binding and catalytic activity in the molecular function category. Furthermore, we also found GO terms related to stress tolerance, including response to stimulus (nine genes), signaling (three genes), and transporter activity (six genes), suggesting that some lncRNAs might contribute to drought stress tolerance via control of target gene expression.
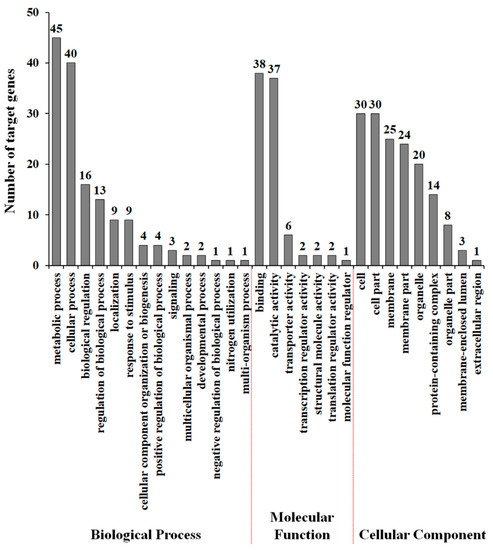
Figure 4.
Gene ontology classification of lncRNA target genes. The results are summarized in three main categories: biological process, molecular function, and cellular component.
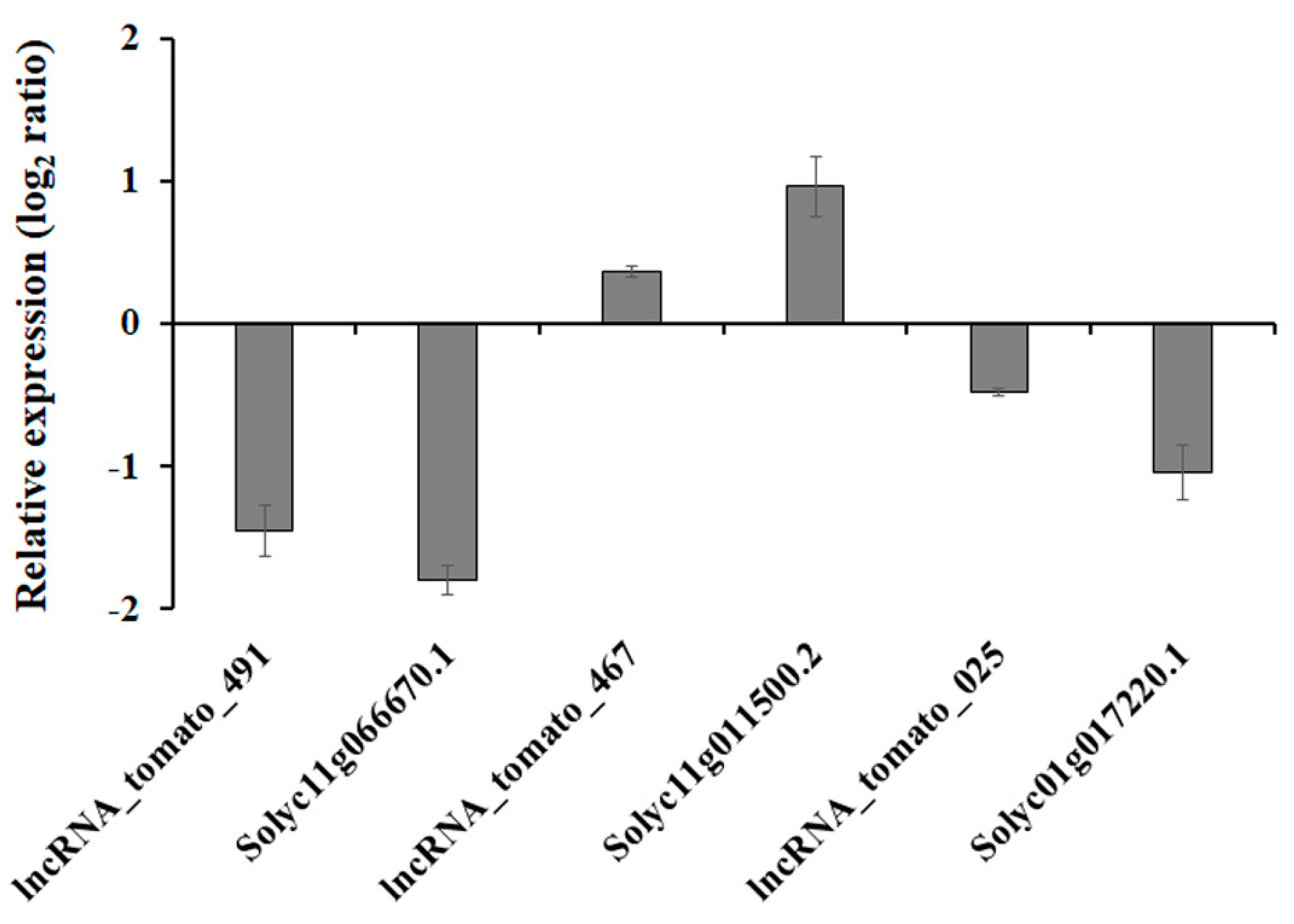
To determine the relationship between expression of drought-responsive tomato lncRNAs and their potential target genes, three lncRNA and target gene pairs were selected, and we analyzed expression patterns in response to drought stress. Under drought conditions, lncRNA_tomato_467 and its potential target gene associated with K+ channel, Solyc11g011500.2 [49], were upregulated, whereas other lncRNAs and their target genes were downregulated (Figure 5). This suggests that all tested pairs of lncRNAs and their putative target genes share similar transcription patterns and might be transcriptionally co-regulated. In guard cells, K+ channel activity mediated by either ABA-induced Ca+-dependent or -independent pathways was required for stomatal movements [50], indicating that drought-induced lncRNA_tomato_467 and Solyc11g011500.2 might play roles in stomatal movement via controlling K+ levels. It is known that ABA-mediated physiological processes, including closure of the stomata and acceleration of leaf senescence, are counteracted by cytokinins [51]. Trans-zeatin (tZ), an active cytokinin, is catalyzed by zeatin O-glucosyltransferase, and this process is required for protection of zeatin from cyctokinin oxidases/dehydrogenases [52]. In zeatin-O-glucosyltransferase-overexpressing plants, a delay in the accumulation of drought-induced ABA was observed, resulting in the delayed decrease of stomatal aperture in response to water deficit [53]. This suggests that the downregulation of zeatin o-glucosyltransferase, Solyc11g066670.1 [54], should be essential for ABA-induced stomatal closure in response to drought stress. In addition, drought-exposed plants exhibited a specific decrease in abundance of the ATP synthase complex, including the epsilon subunit of the chloroplast ATP-synthase-like Solyc01g017220.1 [55] due to water-deficit-induced biochemical limitation in photosynthesis, indicating that lncRNA_tomato_025 should be an important resource for improving chloroplast energy balance in response to drought stress. Taken together, these findings suggest that some drought-responsive lncRNAs act as transcriptional regulators of drought responses in tomato.
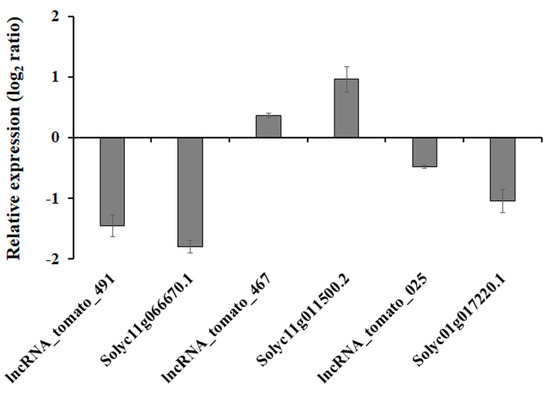
Figure 5.
Transcriptional changes of selected lncRNA–mRNA pairs during the response to drought stress. Transcript levels of the selected lncRNA–mRNA pairs were normalized to those of tomato actin and were expressed relative to the values in the non-treated sample. The level of expression is represented as the log2 ratio. Data are means ± SE.
4. Conclusions
We identified and characterized 521 putative lncRNAs expressed in drought-treated tomato leaves. Several drought-responsive lncRNAs acted as putative targets of tomato miRNAs. The prediction of lncRNA–mRNA interaction and GO enrichment analysis suggested that drought-responsive lncRNAs act as transcriptional regulators of genes involved in stress tolerance, including response to stimulus, signaling, and transporter activity. These findings provide valuable information for further characterization of lncRNA-mediated regulatory mechanisms underlying drought stress. Understanding the interactions of lncRNAs with other molecular elements is an interesting area that needs to be further developed to improve our knowledge of drought-induced molecular and physiological mechanisms.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/9/10/629/s1, Table S1: The primers of miRNAs, lncRNAs and target genes used for qRT-PCR analysis, Table S2: Details of drought-responsive lncRNAs, Table S3: List of miRNAs targeting the drought-responsive tomato lncRNAs.
Author Contributions
Conceptualization, S.H.E., H.J.L., and T.K.H.; methodology, S.H.E., H.J.L., S.K.K., and T.K.H.; formal analysis, S.H.E., H.J.L., and S.H.W.; investigation, S.H.E., H.J.L., J.H.L.; data curation, S.H.E., H.J.L., J.H.L.; writing-original draft preparation, S.H.E., H.J.L., and T.K.H.; writing-review and editing, S.H.E., H.J.L., J.H.L., S.H.W., S.K.K., and T.K.H.
Funding
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01261303)” Rural Development Administration, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Zipper, S.C.; Qiu, J.; Kucharik, C.J. Drought effects on US maize and soybean production: Spatiotemporal patterns and historical changes. Environ. Res. Lett. 2016, 11, 094021. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Eom, S.H.; Baek, S.A.; Kim, J.K.; Hyun, T.K. Transcriptome analysis in Chinese cabbage (Brassica rapa ssp. pekinensis) provides the role of glucosinolate metabolism in response to drought stress. Molecules 2018, 23, E1186. [Google Scholar] [CrossRef] [PubMed]
- Chekanova, J.A. Long non-coding RNAs and their functions in plants. Curr. Opin. Plant Biol. 2015, 27, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.J.; Böhmdorfer, G.; Wierzbicki, A.T. Analysis of long non-coding RNAs produced by a specialized RNA polymerase in Arabidopsis thaliana. Methods 2013, 63, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Liu, J.; Jung, C.; Xu, J.; Wang, H.; Deng, S.; Bernad, L.; Arenas-Huertero, C.; Chua, N.H. Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell 2012, 24, 4333–4345. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, H.; Sui, N. Regulation mechanism of long non-coding RNA in plant response to stress. Biochem. Biophys. Res. Commun. 2018, 503, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Lian, B.; Gu, H.; Li, Y.; Qi, Y. Global identification of Arabidopsis lncRNAs reveals the regulation of MAF4 by a natural antisense RNA. Nat. Commun. 2018, 9, 5056. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Quan, M.Y.; Zhang, D.Q. Genome-wide identification of novel long non-coding RNAs in Populus tomentosa tension wood, opposite wood and normal wood xylem by RNA-seq. Planta 2014, 241, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.Y.; Liu, Z.C. Global identification and analysis of long non-coding RNAs in diploid strawberry Fragaria vesca, during flower and fruit development. BMC Genom. 2015, 16, 815. [Google Scholar] [CrossRef] [PubMed]
- Khemka, N.; Singh, V.K.; Garg, R.; Jain, M. Genome-wide analysis of long intergenic non-coding RNAs in chickpea and their potential role in flower development. Sci. Rep. 2016, 6, 33297. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, X.; Jiang, H.; Lu, Z.; Cui, J.; Cao, F.; Jin, B. Genome-wide identification and characterization of novel lncRNAs in Ginkgo biloba. Trees 2018, 32, 1429–1442. [Google Scholar] [CrossRef]
- Li, L.; Eichten, S.R.; Shimizu, R.; Petsch, K.; Yeh, C.T.; Wu, W.; Chettoor, A.M.; Givan, S.A.; Cole, R.A.; Fowler, J.E.; et al. Genome-wide discovery and characterization of maize long non-coding RNAs. Genome Biol. 2014, 15, R40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Liao, J.Y.; Li, Z.Y.; Yu, Y.; Zhang, J.P.; Li, Q.F.; Qu, L.H.; Shu, W.S.; Chen, Y.Q. Genome-wide screening and functional analysis identify a large number of long noncoding RNAs involved in the sexual reproduction of rice. Genome Biol. 2014, 15, 512. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.J.; Jung, H.; Jeong, D.H.; Ha, S.H.; Choi, Y.D.; Kim, J.K. Transcriptome profiling of drought responsive noncoding RNAs and their target genes in rice. BMC Genom. 2016, 17, 563. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, H.; Cui, P.; Albesher, N.; Xiong, L. A nucleus-localized long non-coding RNA enhances drought and salt stress tolerance. Plant Physiol. 2017, 175, 1321–1336. [Google Scholar] [CrossRef]
- Ben Amor, B.; Wirth, S.; Merchan, F.; Laporte, P.; d’Aubenton-Carafa, Y.; Hirsch, J.; Maizel, A.; Mallory, A.; Lucas, A.; Deragon, J.M.; et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009, 19, 57–69. [Google Scholar] [CrossRef]
- Lee, H.J.; Eom, S.H.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Hyun, T.H. Genome-wide analysis of alternative splicing events during response to drought stress in tomato (Solanum lycopersicum L.). J. Hortic. Sci. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Bailey, T.L.; Perkins, A.C.; Tallack, M.R.; Xu, Z.; Liu, H. Prediction of novel long non-coding RNAs based on RNA-Seq data of mouse Klf1 knockout study. BMC Bioinform. 2012, 13, 331. [Google Scholar] [CrossRef]
- Hou, X.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Yan, N.; Zhang, Z. Genome-wide analysis of long non-coding RNAs in potato and their potential role in tuber sprouting process. Int. J. Mol. Sci. 2017, 19, E101. [Google Scholar] [PubMed]
- Yu, T.; Zhu, H. Long non-coding RNAs: Rising regulators of plant reproductive development. Agronomy 2019, 9, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, W.; Wang, Y. Genome-wide identification of long non-coding RNAs suggests a potential association with effector gene transcription in Phytophthora sojae. Mol. Plant Pathol. 2018, 19, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M. Functions of long intergenic non-coding (linc) RNAs in plants. J. Plant Res. 2017, 130, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Shuai, P.; Liang, D.; Tang, S.; Zhang, Z.; Ye, C.Y.; Su, Y.; Xia, X.; Yin, W. Genome-wide identification and functional prediction of novel and drought-responsive lincRNAs in Populus trichocarpa. J. Exp. Bot. 2014, 65, 4975–4983. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.D.; Sung, S. Long noncoding RNA: Unveiling hidden layer of gene regulatory networks. Trends Plant Sci. 2012, 17, 16–21. [Google Scholar] [CrossRef]
- Liu, X.; Hao, L.; Li, D.; Zhu, L.; Hu, S. Long non-coding RNAs and their biological roles in plants. Genom. Proteom. Bioinform. 2015, 13, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wang, Z.M.; Wang, M.; Wang, X.J. Widespread long noncoding RNAs as endogenous target mimics for microRNAs in plants. Plant Physiol. 2013, 161, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Shen, J.; Hou, X.; Yao, J.; Li, X.; Xiao, J.; Xiong, L. Gradual increase of miR156 regulates temporal expression changes of numerous genes during leaf development in rice. Plant Physiol. 2012, 158, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Sunkar, R.; Zhou, C.; Shen, H.; Zhang, J.Y.; Matts, J.; Wolf, J.; Mann, D.G.; Stewart, C.N., Jr.; Tang, Y.; et al. Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. Plant Biotechnol. J. 2012, 10, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.G.; Shan, J.X.; Shi, M.; Gao, J.P.; Lin, H.X. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014, 80, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Amyot, L.; Tian, L.; Xu, Z.; Gruber, M.Y.; Hannoufa, A. Ectopic expression of miR156 represses nodulation and causes morphological and developmental changes in Lotus japonicus. Mol. Genet. Genom. 2015, 290, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Feyissa, B.A.; Amyot, L.; Aung, B.; Hannoufa, A. MicroRNA156 improves drought stress tolerance in alfalfa (Medicago sativa) by silencing SPL13. Plant Sci. 2017, 258, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.R.; Bäurle, I. Arabidopsis miR156 regulates tolerance to recurring environmental stress through spl transcription factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Gill, S.S.; Tuteja, N. MicroRNAs as promising tools for improving stress tolerance in rice. Plant Signal. Behav. 2012, 7, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liang, R.; Ge, L.; Li, W.; Xiao, H.; Lin, H.; Ruan, K.; Jin, Y. Identification of drought-induced microRNAs in rice. Biochem. Biophys. Res. Commun. 2007, 354, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.X.; Yu, D.Q. Overexpression of Arabidopsis miR396 enhances drought tolerance in transgenic tobacco plants. Acta Bot. Yunnan. 2010, 31, 421–426. [Google Scholar] [CrossRef]
- Huang, Z.A.; Huang, Y.A.; You, Z.H.; Zhu, Z.; Sun, Y. Novel link prediction for large-scale miRNA-lncRNA interaction network in a bipartite graph. BMC Med. Genom. 2018, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhang, X.; Wang, W.; Yuan, R.; Shen, F. Identification of Gossypium hirsutum long non-coding RNAs (lncRNAs) under salt stress. BMC Plant Biol. 2018, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, L.; Li, J.; Le, T.D. LncmiRSRN: Identification and analysis of long non-coding RNA related miRNA sponge regulatory network in human cancer. Bioinformatics 2018, 34, 4232–4240. [Google Scholar] [CrossRef] [PubMed]
- Schor, I.E.; Bussotti, G.; Maleš, M.; Forneris, M.; Viales, R.R.; Enright, A.J.; Furlong, E.E.M. Non-coding RNA expression, function, and variation during Drosophila embryogenesis. Curr. Biol. 2018, 28, 3547–3561. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, B.S.; Gilchrist, D.A.; Nechaev, S.; Muse, G.W.; Burkholder, A.; Fargo, D.C.; Adelman, K. Bidirectional transcription arises from two distinct hubs of transcription factor binding and active chromatin. Mol. Cell 2015, 58, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Ørom, U.A.; Derrien, T.; Beringer, M.; Gumireddy, K.; Gardini, A.; Bussotti, G.; Lai, F.; Zytnicki, M.; Notredame, C.; Huang, Q.; et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010, 143, 46–58. [Google Scholar] [CrossRef]
- Camblong, J.; Iglesias, N.; Fickentscher, C.; Dieppois, G.; Stutz, F. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell 2007, 131, 706–717. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Liu, X.; Jiang, J. Comparative transcriptome profiling of two tomato genotypes in response to potassium-deficiency stress. Int. J. Mol. Sci. 2018, 19, E2402. [Google Scholar] [CrossRef]
- Shabala, S. Regulation of potassium transport in leaves: From molecular to tissue level. Ann. Bot. 2003, 92, 627–634. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot. 2006, 57, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Veach, Y.K.; Martin, R.C.; Mok, D.W.; Malbeck, J.; Vankova, R.; Mok, M.C. O-glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol. 2003, 131, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Havlová, M.; Dobrev, P.I.; Motyka, V.; Storchová, H.; Libus, J.; Dobrá, J.; Malbeck, J.; Gaudinová, A.; Vanková, R. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ. 2008, 31, 341–353. [Google Scholar] [CrossRef]
- Keshishian, E.A.; Hallmark, H.T.; Ramaraj, T.; Plačková, L.; Sundararajan, A.; Schilkey, F.; Novák, O.; Rashotte, A.M. Salt and oxidative stresses uniquely regulate tomato cytokinin levels and transcriptomic response. Plant Direct 2018, 2, e00071. [Google Scholar] [CrossRef] [PubMed]
- Hoshiyasu, S.; Kohzuma, K.; Yoshida, K.; Fujiwara, M.; Fukao, Y.; Yokota, A.; Akashi, K. Potential involvement of N-terminal acetylation in the quantitative regulation of the ε subunit of chloroplast ATP synthase under drought stress. Biosci. Biotechnol. Biochem. 2013, 77, 998–1007. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).