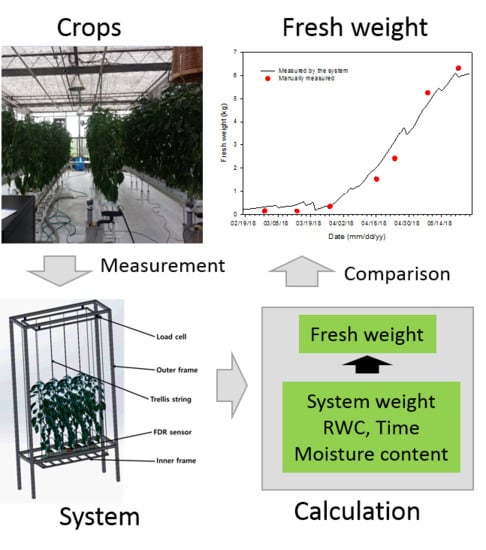
Nondestructive and Continuous Fresh Weight Measurements of Bell Peppers Grown in Soilless Culture Systems
Abstract
1. Introduction
2. Materials and Methods
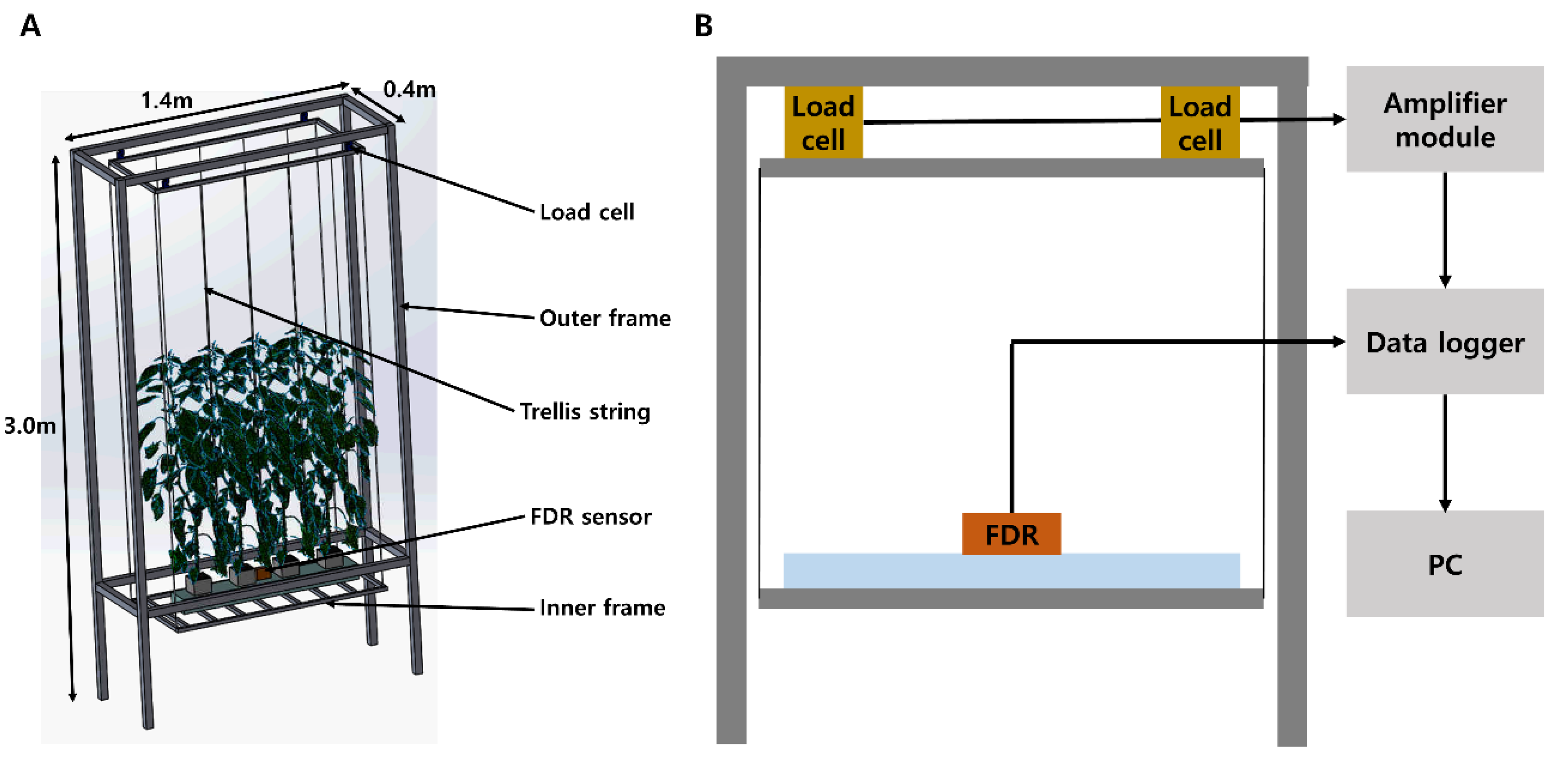
2.1. Fresh Weight Measurement System in a Soilless Culture Environment
2.2. Growth Conditions
2.3. Fresh Weight Calculation
2.4. Growth Survey and Verification
2.5. Statistical Analysis
3. Results and Discussion
3.1. Diurnal Change in RWC
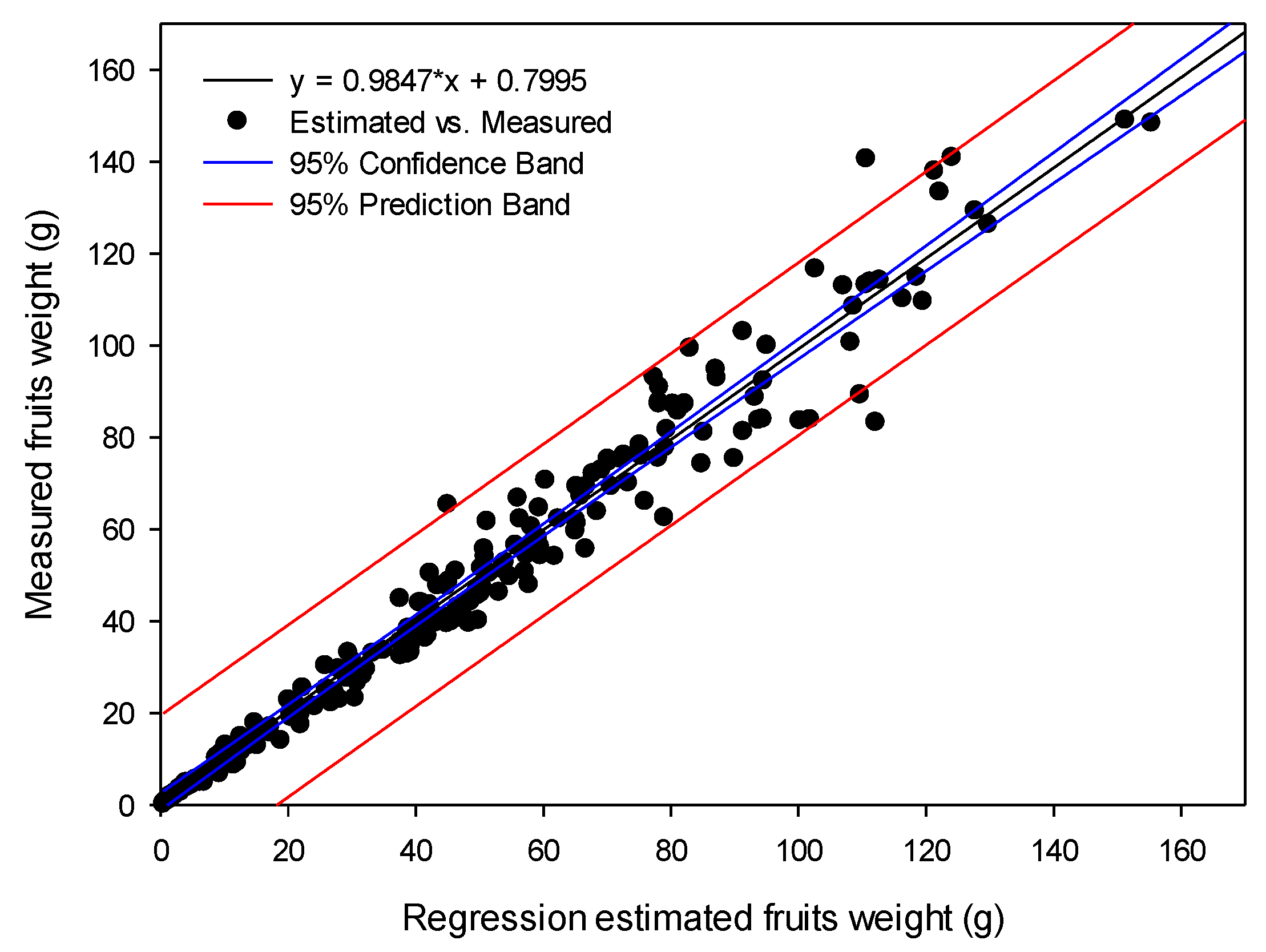
3.2. Weight-Moisture Content Calibration and Fresh Weight Calculation
3.3. Destructive Crop Growth Survey
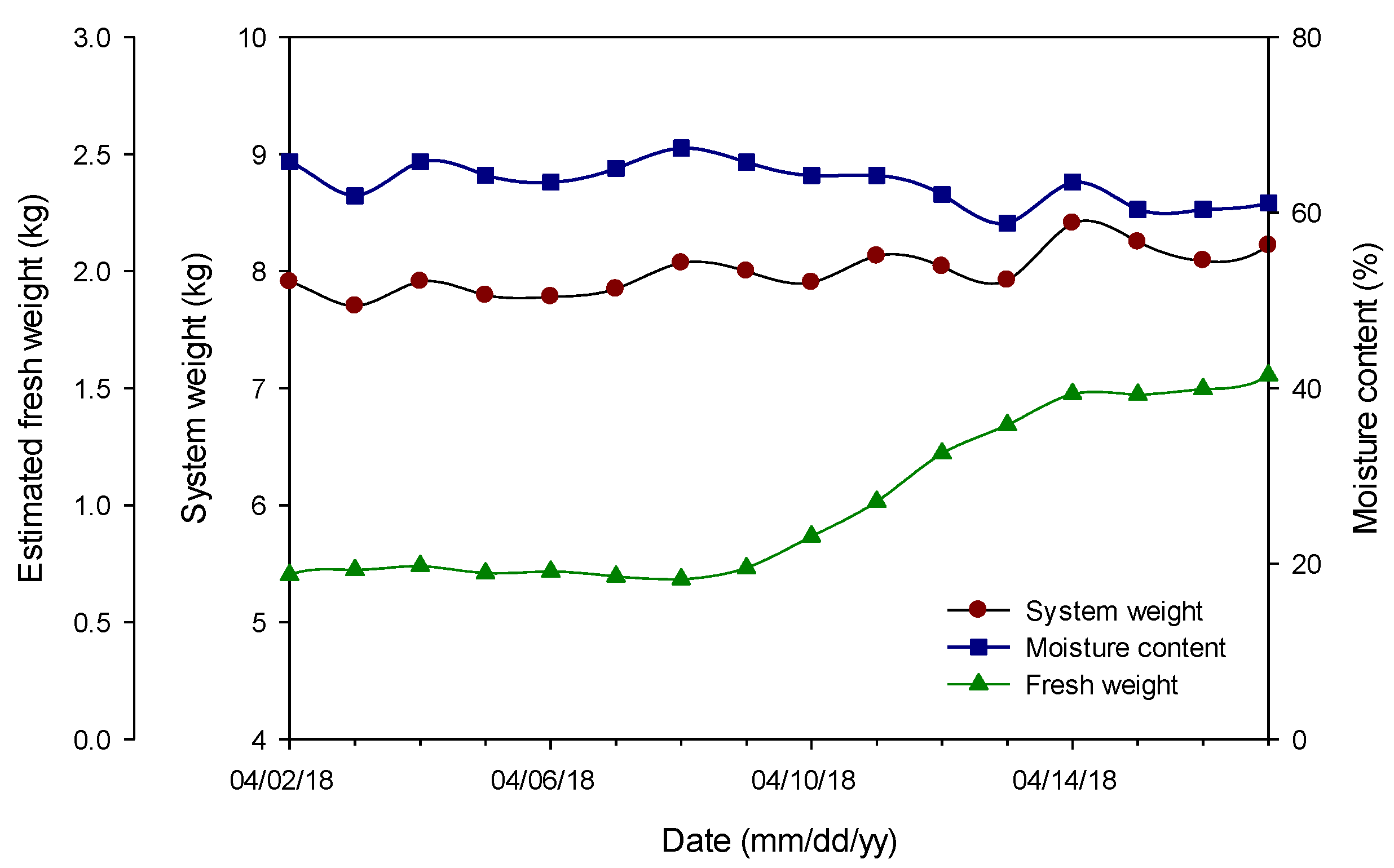
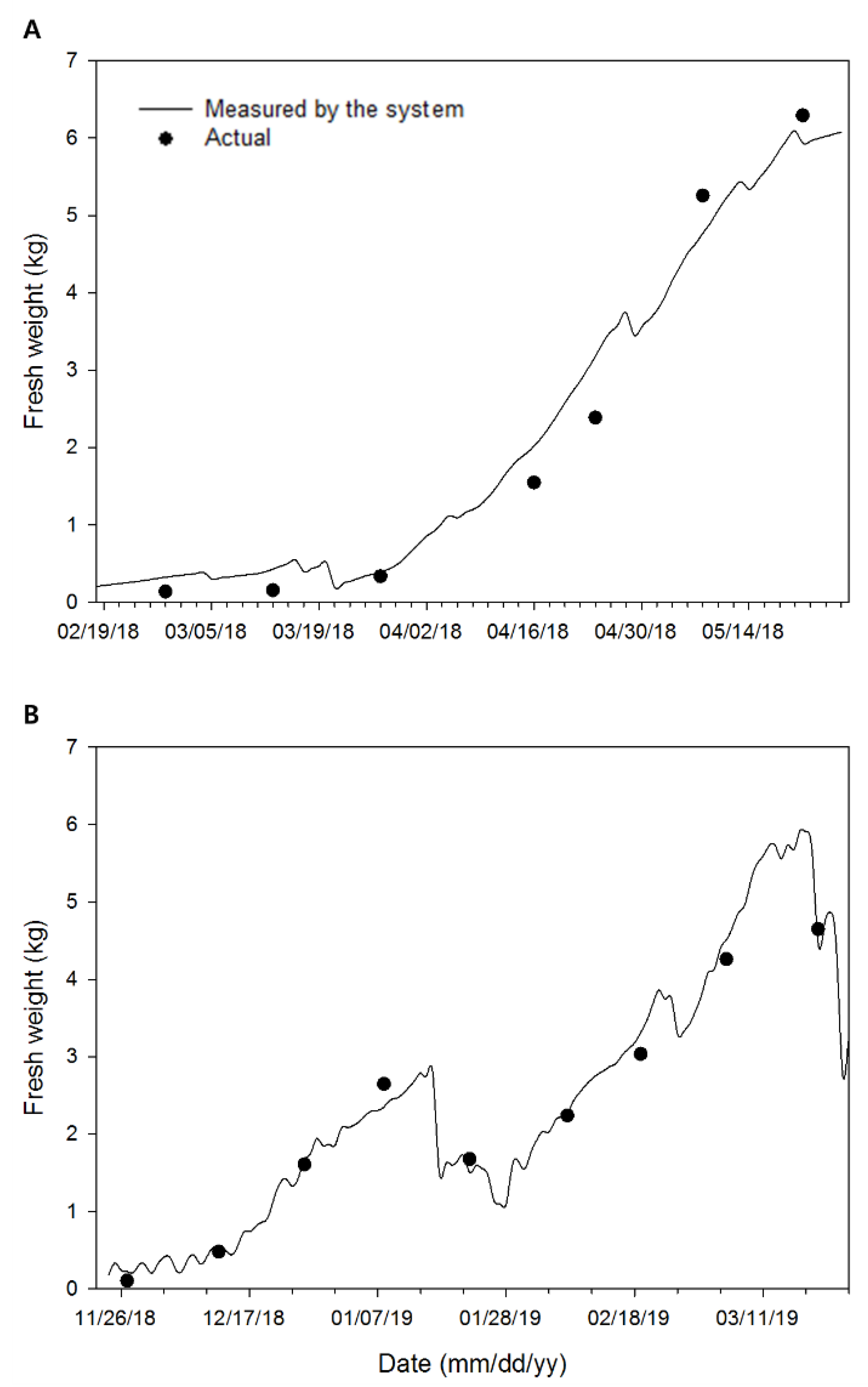
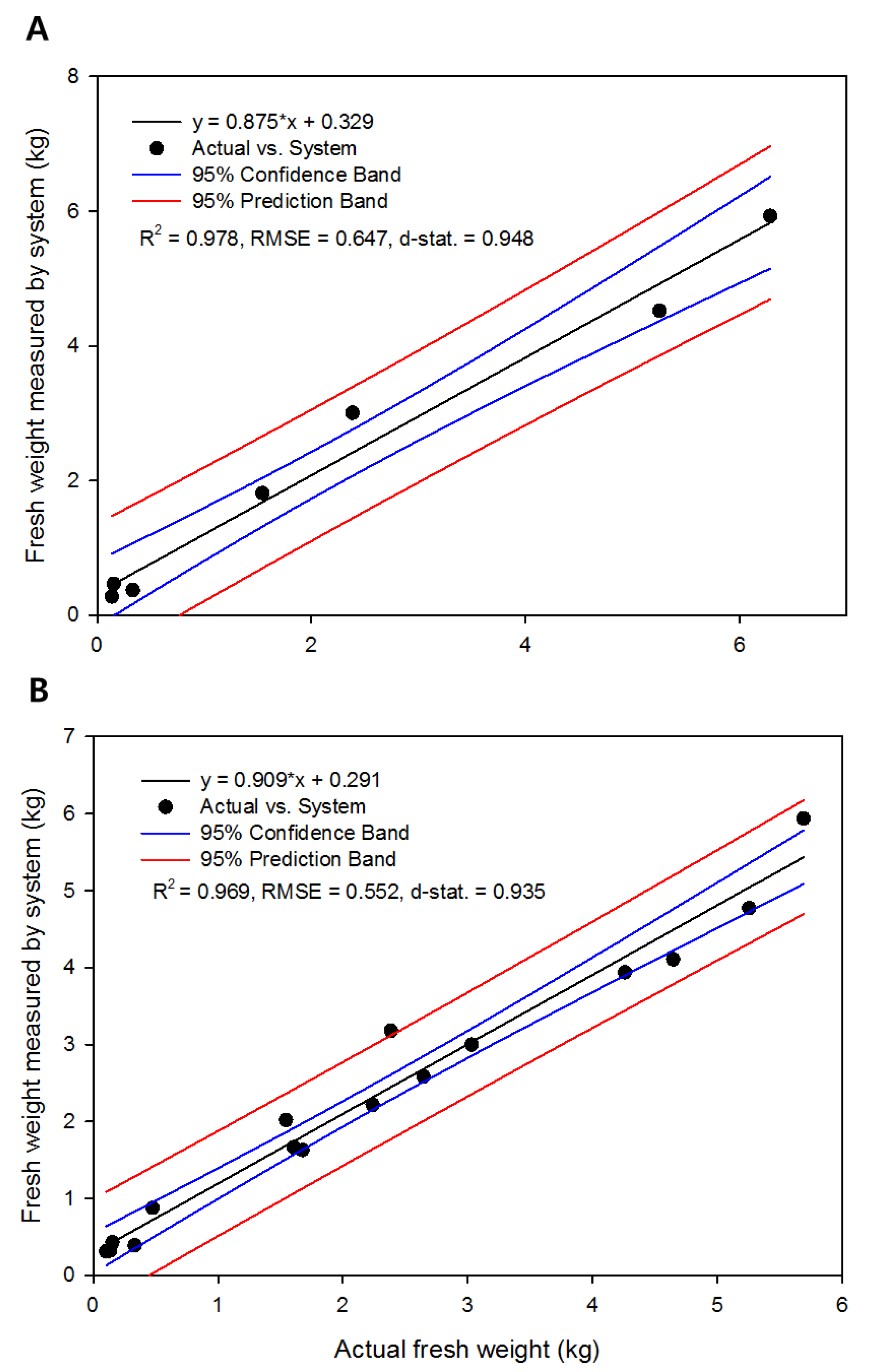
3.4. Continuous Changes in Fresh Weight and Its Verification
3.5. Applicability and Limitations of the Developed System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Castro, S.M.; Saraiva, J.A.; Lopes-da-Silva, J.A.; Delgadillo, I.; van Loey, A.; Smout., C.; Hendrickx, M. Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chem. 2008, 107, 1436–1449. [Google Scholar] [CrossRef]
- Frans, M.; Moerkens, R.; Van Gool, S.; Sauviller, C.; Van Laethem, S.; Luca, S.; Aerts, R.; Ceusters, J. Modelling greenhouse climate factors to constrain internal fruit rot (Fusarium spp.) in bell pepper. J. Plant Dis. Prot. 2018, 125, 425–432. [Google Scholar] [CrossRef]
- Wyenandt, C.A.; Kline, W.L.; Ward, D.L.; Brill, N.L. Production system and cultivar effects on the development of skin separation or “silvering” in bell pepper fruit. HortTechnology 2017, 27, 37–44. [Google Scholar] [CrossRef]
- Ngouajio, M.; Auras, R.; Fernandez, R.T.; Rubino, M.; Counts, J.W.; Kijchavengkul, T. Field performance of aliphatic-aromatic copolyester biodegradable mulch films in a fresh market tomato production system. HortTechnology 2008, 18, 605–610. [Google Scholar] [CrossRef]
- Sezen, S.M.; Yazar, A.; Eker, S. Effect of drip irrigation regimes on yield and quality of field grown bell pepper. Agric. Water Manag. 2006, 81, 115–131. [Google Scholar] [CrossRef]
- Shin, J.H.; Son, J.E. Development of a real-time irrigation control system considering transpiration, substrate electrical conductivity, and drainage rate of nutrient solutions in soilless culture of paprika (Capsicum annuum L.). Eur. J. Hortic. Sci. 2015, 80, 271–279. [Google Scholar] [CrossRef]
- Heuvelink, E. Evaluation of a dynamic simulation model for tomato crop growth and development. Ann. Bot. 1999, 83, 413–422. [Google Scholar] [CrossRef]
- Jones, J.W.; Dayan, E.; Allen, L.H.; Van Keulen, H.; Challa, H. A dynamic tomato growth and yield model (TOMGRO). Trans. ASAE. 1991, 34, 663–672. [Google Scholar] [CrossRef]
- Katsoulas, N.; Peponakis, K.; Ferentinos, K.P.; Kittas, C. Calibration of a growth model for tomato seedlings (TOMSEED) based on heuristic optimisation. Biosyst. Eng. 2015, 140, 34–47. [Google Scholar] [CrossRef]
- Martínez-Ruiz, A.; López-Cruz, I.L.; Ruiz-García, A.; Pineda-Pineda, J.; Prado-Hernández, J.V. HortSyst: A dynamic model to predict growth, nitrogen uptake, and transpiration of greenhouse tomatoes. Chil. J. Agric. Res. 2019, 79, 89–102. [Google Scholar] [CrossRef]
- Chen, W.T.; Yeh, Y.H.F.; Liu, T.Y.; Lin, T.T. An automated and continuous plant weight measurement system for plant factory. Front. Plant Sci. 2016, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, W.; Ahn, T.; Shin, J.; Son, J.E. Precise, real-time measurement of the fresh weight of lettuce with growth stage in a plant factory using a nutrient film technique. Hortic. Sci. Technol. 2016, 34, 77–83. [Google Scholar]
- Baas, R.; Slootweg, C. On-line acquisition of plant related and environmental parameters (plant monitoring) in gerbera: Determining plant responses. Acta Hortic. 2004, 654, 139–146. [Google Scholar] [CrossRef]
- Helmer, T.; Ehret, D.L.; Bittman, S. CropAssist, an automated system for direct measurement of greenhouse tomato growth and water use. Comput. Electron. Agric. 2005, 48, 198–215. [Google Scholar] [CrossRef]
- Ahmad, U.; Subrata, D.M.; Arif, C. Speaking plant approach for automatic fertigation system in greenhouse. arXiv 2013, arXiv:1303.1869. [Google Scholar]
- Ehret, D.L.; Hill, B.D.; Helmer, T.; Edwards, D.R. Neural network modeling of greenhouse tomato yield, growth and water use from automated crop monitoring data. Comput. Electron. Agric. 2011, 79, 82–89. [Google Scholar] [CrossRef]
- Story, D.; Kacira, M. Design and implementation of a computer vision-guided greenhouse crop diagnostics system. Mach. Vis. Appl. 2015, 26, 495–506. [Google Scholar] [CrossRef]
- Jovicich, E.; Cantliffe, D.J.; Stoffella, P.J.; Haman, D.Z. Bell pepper fruit yield and quality as influenced by solar radiation-based irrigation and container media in a passively ventilated greenhouse. HortScience 2007, 42, 642–652. [Google Scholar] [CrossRef]
- Ueda, M.; Nakamura, Y. Chemical basis of plant leaf movement. Plant Cell Physiol. 2007, 48, 900–907. [Google Scholar] [CrossRef]
- Luis, G.; Marco, G. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Roger, M.J.R., Ed.; Kluwer Academic Publishers: New York, NY, USA, 2001; Volume 14, pp. 207–212. [Google Scholar]
- Willmott, C.J.; Robeson, S.M.; Matsuura, K. A refined index of model performance. Int. J. Climatol. 2012, 32, 2088–2094. [Google Scholar] [CrossRef]
- Himeno, S.; Azuma, W.; Gyokusen, K.; Ishii, H.R. Leaf water maintains daytime transpiration in young Cryptomeria japonica trees. Tree Physiol. 2017, 37, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Klepper, B.; Browning, V.D.; Taylor, H.M. Stem diameter in relation to plant water status. Plant Physiol. 1971, 48, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, V.; Balasubramanian, V.; Sinha, S.K. Diurnal fluctuations in relative water content, nitrate reductase and proline content in water-stressed and non-stressed wheat. Physiol. Plant. 1977, 40, 69–71. [Google Scholar] [CrossRef]
- Klein, T.; Cohen, S.; Paudel, I.; Preisler, Y.; Rotenberg, E.; Yakir, D. Diurnal dynamics of water transport, storage and hydraulic conductivity in pine trees under seasonal drought. iForest Biogeosci. For. 2016, 9, 710. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbó, M.; Bota, J.; Galmés, J.; Henkle, M.; Martínez-Cañellas, S.; Medrano, H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2006, 172, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, C.I.; Sarr, B.; Brou, C.; Diouf, O.; Diop, N.N.; Roy-Macauley, H. Selection of cowpea genotypes in hydroponics, pots, and field for drought tolerance. Crop Sci. 2003, 43, 1114–1120. [Google Scholar] [CrossRef]
- Kang, S.; van Iersel, M.W.; Kim, J. Plant root growth affects FDR soil moisture sensor calibration. Sci. Hortic. 2019, 252, 208–211. [Google Scholar] [CrossRef]
- Skierucha, W.; Wilczek, A. A FDR sensor for measuring complex soil dielectric permittivity in the 10–500 MHz frequency range. Sensors 2010, 10, 3314–3329. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Katsoulas, N.; Kittas, C. Effect of irrigation frequency on growth and production of a cucumber crop under soilless culture. Emir. J. Food Agric. 2017 29, 863–871. [CrossRef]
- Marcelis, L.F.M.; Heuvelink, E.; Baan Hofman-Eijer, L.R.; Den Bakker, J.; Xue, L.B. Flower and fruit abortion in sweet pepper in relation to source and sink strength. J. Exp. Bot. 2004, 55, 2261–2268. [Google Scholar] [CrossRef]
- Gijzen, H.; Heuvelink, E.; Challa, H.; Marcelis, L.F.M.; Dayan, E.; Cohen, S.; Fuchs, M. HORTISIM: A model for greenhouse crops and greenhouse climate. Acta Hortic. 1997, 456, 441–450. [Google Scholar] [CrossRef]
- Avotins, A.; Gruduls, J.; Apse-Apsitis, P.; Potapovs, A. Crop weight measurement sensor for IoT based industrial greenhouse systems. Agron. Res. 2018, 16, 952–957. [Google Scholar]
- Muangprathub, J.; Boonnam, N.; Kajornkasirat, S.; Lekbangpong, N.; Wanichsombat, A.; Nillaor, P. IoT and agriculture data analysis for smart farm. Comput. Electron. Agric. 2019, 156, 467–474. [Google Scholar] [CrossRef]
- O′Grady, M.J.; O′Hare, G.M. Modelling the smart farm. Inf. Process. Agric. 2017, 4, 179–187. [Google Scholar] [CrossRef]
- Kumar, R.; Chhabra, S.; Verma, A.S.; Gupta, A. Analysis of load cell. Int. J. Appl. Eng. Res. 2018, 13, 274–277. [Google Scholar]




| DAT | Fresh Weight (g, A) | Dry Weight (g, B) | A/B |
|---|---|---|---|
| 10 | 0.098 ± 0.021z | 0.013 ± 0.001 | 0.133 |
| 30 | 1.206 ± 0.327 | 0.165 ± 0.06 | 0.137 |
| 75 | 1.935 ± 0.420 | 0.248 ± 0.024 | 0.128 |
| 113 | 1.117 ± 0.246 | 0.147 ± 0.013 | 0.132 |
| Growth Period | DAT | Root Dry Weight (g) | Root Fresh Weight (g) | Leaf Fresh Weight (g) | Stem Fresh Weight (g) | Fruit Fresh Weight (g) |
|---|---|---|---|---|---|---|
| 1 | 20 | 0.650 z | 4.912 | 19.779 ± 11.586 y | 35.864 ± 4.191 | 1.738 ± 0.953 |
| 34 | 0.647 | 4.866 | 22.402 ± 3.986 | 10.0398 ± 2.373 | 1.182 ± 0.311 | |
| 48 | 1.407 | 10.582 | 40.066 ± 18.726 | 30.479 ± 11.999 | 1.964 ± 0.626 | |
| 68 | 4.793 | 36.037 | 165.687 ± 15.148 | 124.935 ± 13.171 | 59.295 ± 82.027 | |
| 76 | 7.256 | 54.555 | 298.869 ± 45.735 | 197.085 ± 13.425 | 45.603 ± 25.274 | |
| 91 | 7.267 | 54.638 | 301.019 ± 64.667 | 195.685 ± 49.313 | 762.398 ± 31.465 | |
| 104 | 7.293 | 54.837 | 312.207 ± 25.668 | 199.720 ± 8.823 | 1003.479 ± 221.610 | |
| 2 | 29 | 0.635 | 4.500 | 13.31825 ± 4.645 | 7.395 ± 2.624 | 0.568 ± 0.122 |
| 44 | 1.155 | 9.329 | 59.598 ± 18.169 | 47.498 ± 5.462 | 27.732 ± 29.648 | |
| 58 | 3.907 | 30.057 | 132.838 ± 37.745 | 99.262 ± 12.357 | 139.211 ± 42.562 | |
| 71 | 4.793 | 40.556 | 176.768 ± 29.590 | 139.951 ± 14.957 | 304.009 ± 101.153 | |
| 85 | 6.921 | 53.239 | 201.902 ± 41.542 | 151.156 ± 18.901 | 12.534 ± 6.236 | |
| 101 | 7.161 | 55.077 | 258.571 ± 75.537 | 196.274 ± 32.862 | 49.459 ± 21.397 | |
| 113 | 7.250 | 56.669 | 340.491 ± 67.764 | 200.019 ± 27.662 | 160.932 ± 53.152 | |
| 127 | 10.391 | 79.931 | 421.408 ± 67.072 | 301.940 ± 24.236 | 262.037 ± 71.329 |
| Value | Temperature | CO2 Concentration | Radiation | Relative Humidity |
|---|---|---|---|---|
| Average a | 0.510 ** | 0.157 | 0.545 ** | 0.639 ** |
| Maximum b | −0.187 * | 0.179 | 0.547 ** | 0.692 ** |
| Minimum c | 0.061 | −0.288 ** | − | 0.353 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.W.; Son, J.E. Nondestructive and Continuous Fresh Weight Measurements of Bell Peppers Grown in Soilless Culture Systems. Agronomy 2019, 9, 652. https://doi.org/10.3390/agronomy9100652
Lee JW, Son JE. Nondestructive and Continuous Fresh Weight Measurements of Bell Peppers Grown in Soilless Culture Systems. Agronomy. 2019; 9(10):652. https://doi.org/10.3390/agronomy9100652
Chicago/Turabian StyleLee, Joon Woo, and Jung Eek Son. 2019. "Nondestructive and Continuous Fresh Weight Measurements of Bell Peppers Grown in Soilless Culture Systems" Agronomy 9, no. 10: 652. https://doi.org/10.3390/agronomy9100652
APA StyleLee, J. W., & Son, J. E. (2019). Nondestructive and Continuous Fresh Weight Measurements of Bell Peppers Grown in Soilless Culture Systems. Agronomy, 9(10), 652. https://doi.org/10.3390/agronomy9100652