Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Application of Sodium Azide Treatments
2.2. Soil Preparation for Potted Field Experiment
2.3. Sowing of Seeds in Nursery Beds, Transplanting into Experimental Pots, and Acclimatization
2.4. Application of Flooding or Waterlogging Conditions
2.5. Growth Parameters Measured
2.6. Soil Chemical Analyses
2.7. Soil Microflora Counts
2.8. Root Anatomy
2.9. Antioxidant Enzyme Assays
2.10. RNA Isolation, cDNA Synthesis, and Quantitative RT-PCR
2.11. Statistical Analysis
3. Results
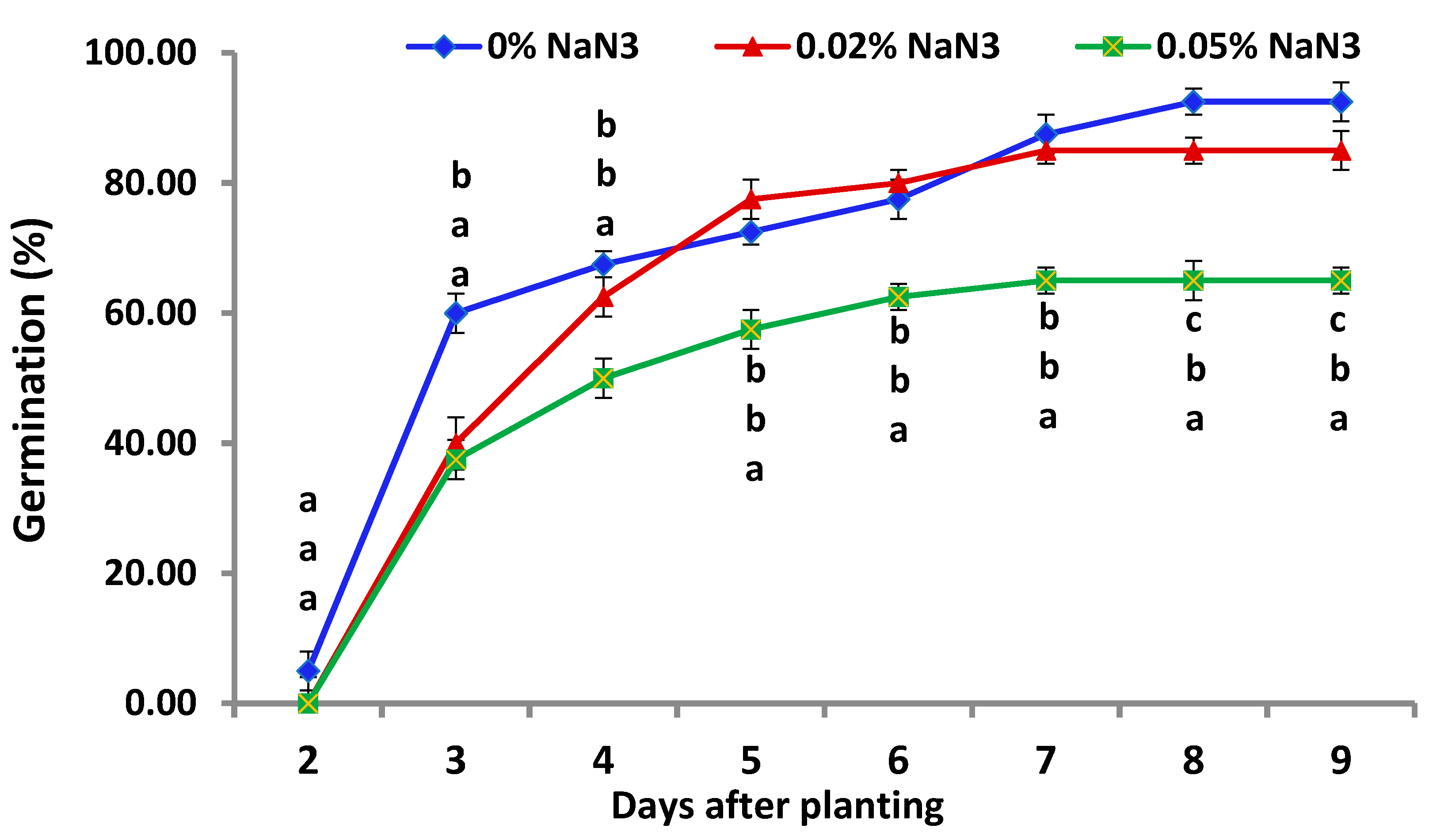
3.1. Germination of NaN3-Treated Seeds
3.2. Plant Height
3.3. Stem Girth
3.4. Number of Leaves Formed, Number of Adventitious Roots Produced, and Percentage of Survival of Plants
3.5. Number of Flower Buds, Flowers, and Fruits Produced
3.6. Soil Microflora Counts
3.7. Soil Chemical Analysis
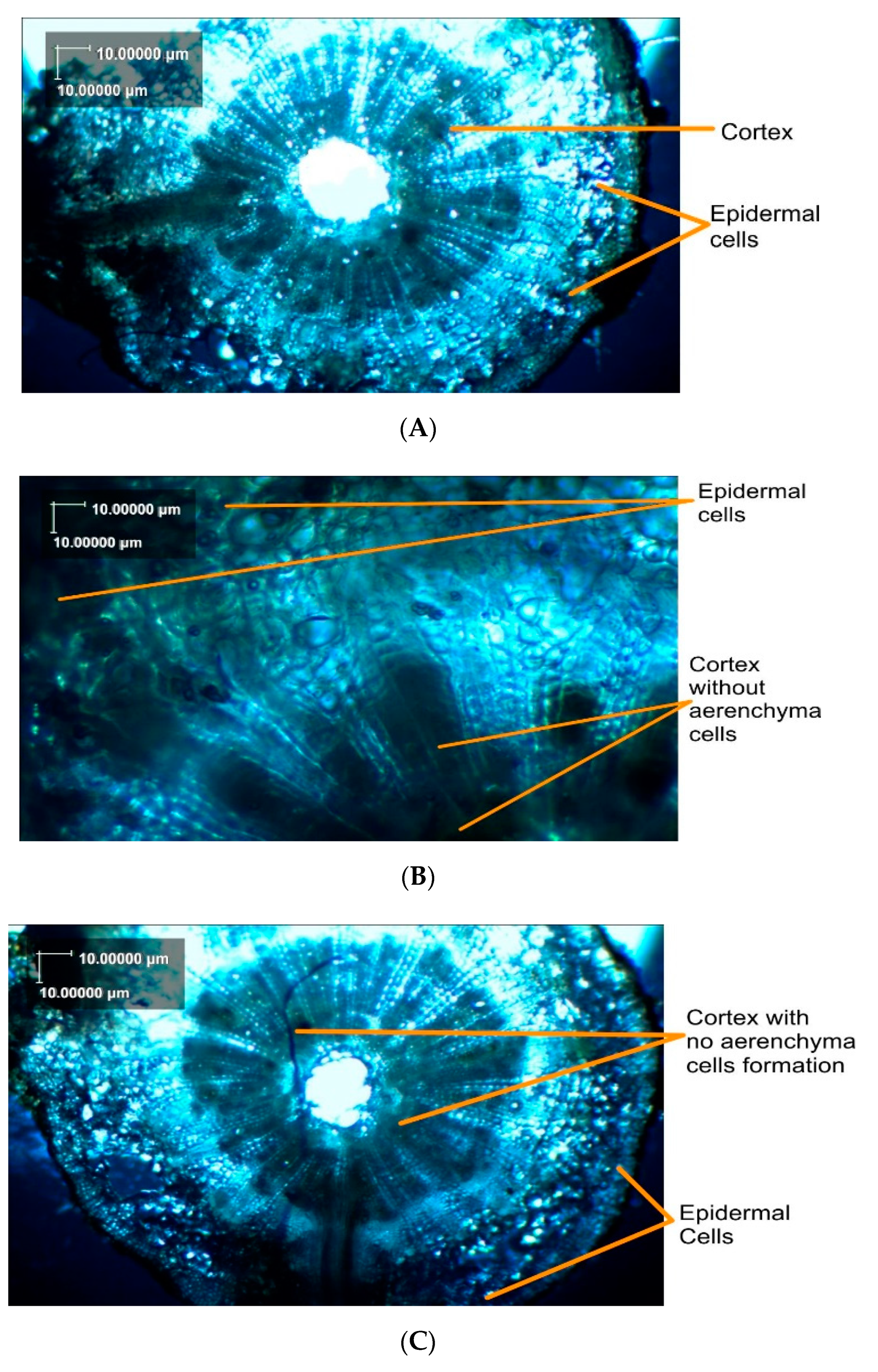
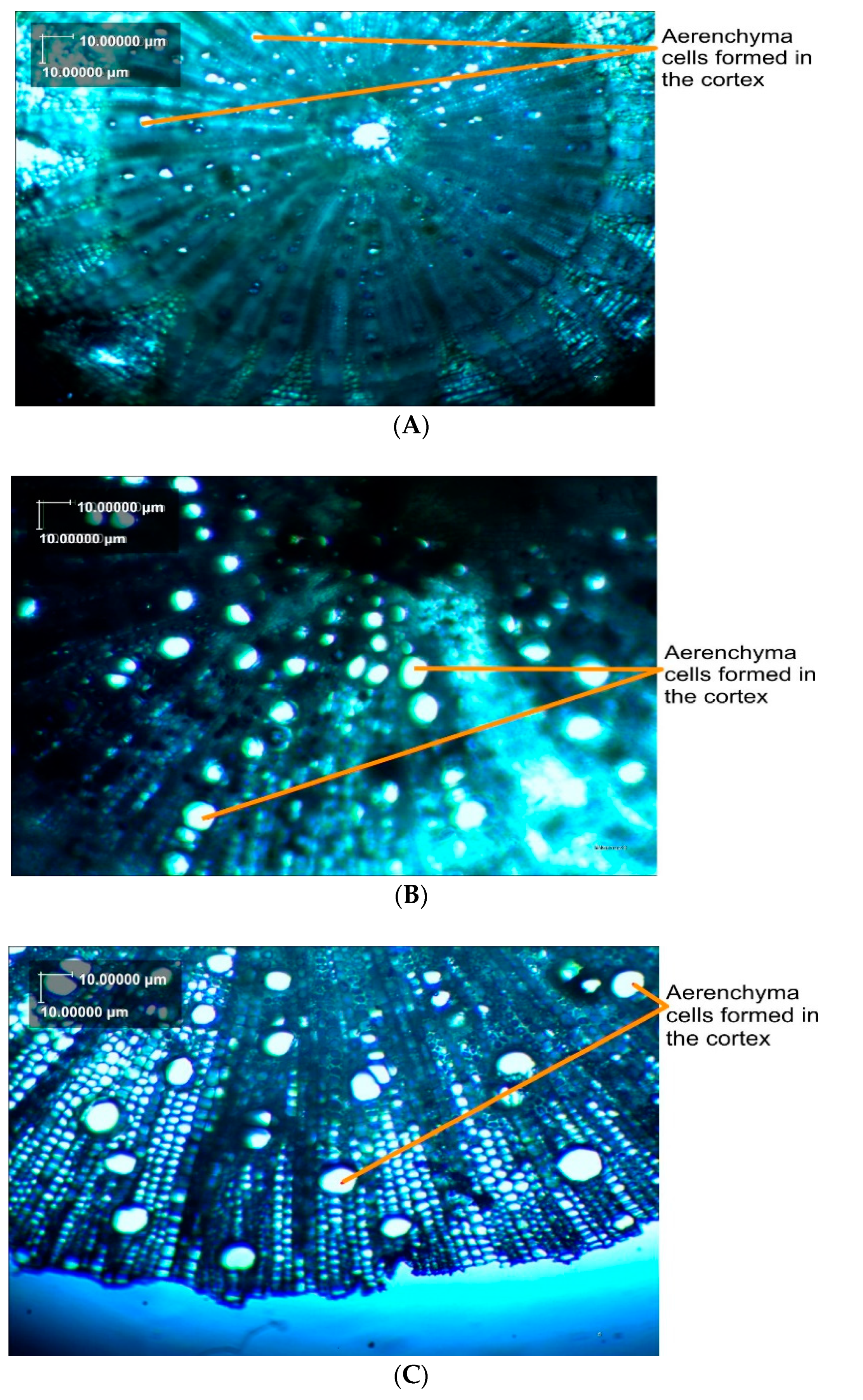
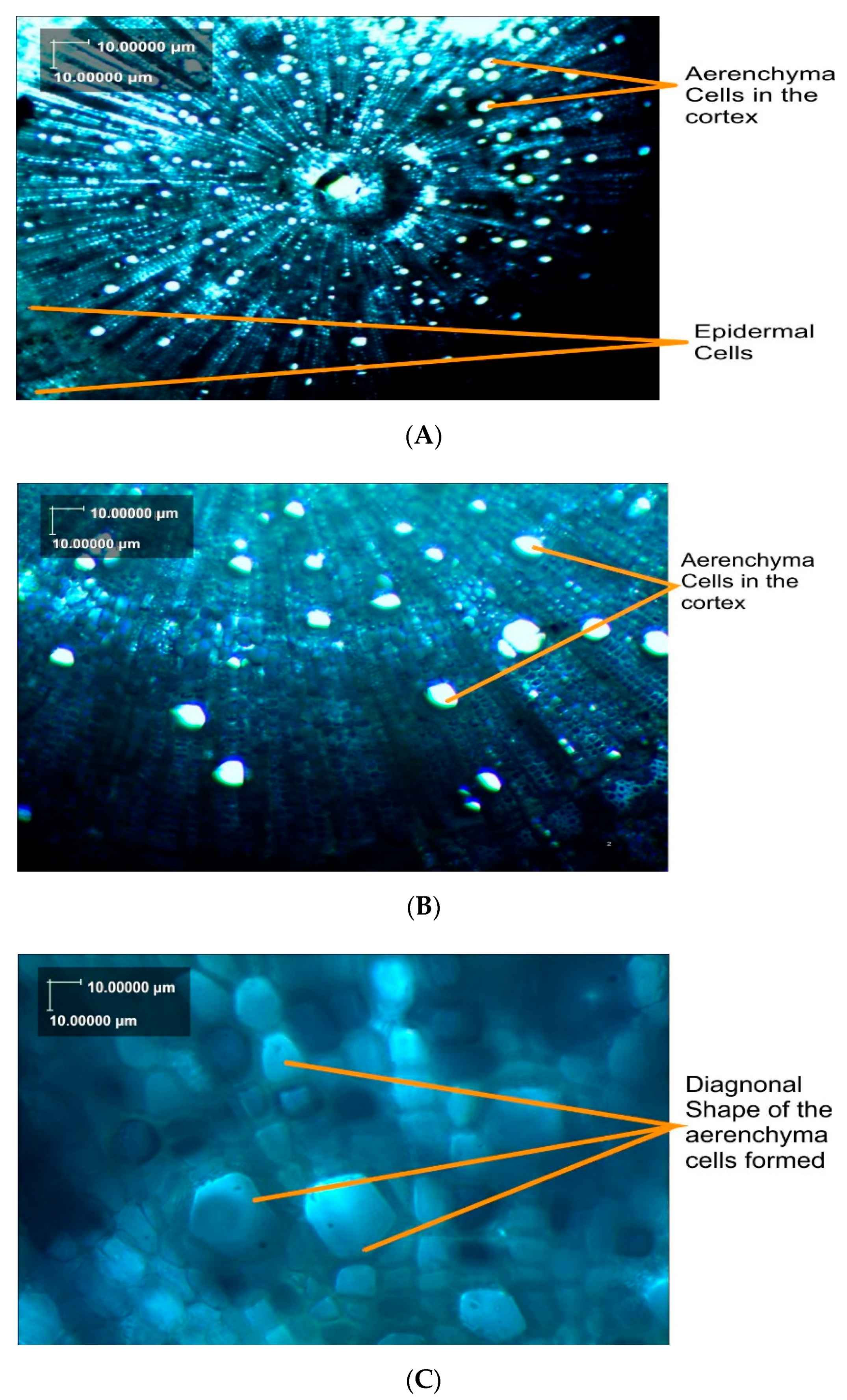
3.8. Anatomy of Okra Roots
3.9. Antioxidant Enzymes Activity and Gene Expression Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Bey, F. Nutritional Quality and Health Benefits of Okra (Abelmoschus esculentus): A Review. Int. J. Nut. Food Sci. 2015, 4, 208–215. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of Genetic Variation and Enhancement of Salt Tolerance in French Pea. Int. J. Mol. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Ahmad, M. Overexpression of AtWRKY30 Transcription Factor Enhances Heat and Drought Stress Tolerance in Wheat (Triticum aestivum L.). Genes 2019, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic transformation and hairy root induction enhance the antioxidant potential of Lactuca serriola L. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of StDREB2 Transcription Factor Enhances Drought Stress Tolerance in Cotton (Gossypium barbadense L.). Genes 2019, 10, 142. [Google Scholar] [CrossRef]
- Vwioko, E.; Adinkwu, O.; El-Esawi, M.A. Comparative physiological, biochemical and genetic responses to prolonged waterlogging stress in okra and maize given exogenous ethylene priming. Front. Physiol. 2017, 8, 632. [Google Scholar] [CrossRef]
- Heschbach, C.; Mult, S.; Kreuzwieser, J.; Kopriva, S. Influence of anoxia on whole plant sulphur nutrition of flooding tolerant poplar (Populus tremula × P. alba). Plant Cell Environ. 2005, 28, 167–175. [Google Scholar] [CrossRef]
- Herrera, A.; Tezara, W.; Marin, O.; Rengifo, E. Stomatal and non-stomatal limitations of photosynthesis in trees of a tropical seasonally flooded forest. Physiol. Plant. 2008, 134, 41–48. [Google Scholar] [CrossRef]
- Syversten, J.P.; Zablotowicz, R.M.; Smith, M.L. Soil-temperature and flooding effects on two species of citrus. 1. Plant growth and hydraulic conductivity. Plant Soil 1983, 72, 3–12. [Google Scholar]
- Setter, T.L.; Waters, I.; Sharma, S.K.; Singh, K.N.; Kulshreshtha, N.; Yaduvanshi, N.P.S.; Ram, P.C.; Singh, B.N.; Rane, J.; McDonald, G.; et al. Review of wheat improvement for waterlogging tolerance in Australia and India: The importance of anaerobiosis and element toxicities associated with different soils. Ann. Bot. 2009, 103, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Polanco, M.; Senorans, J.; Zwiazek, J.J. Role of adventitious roots in water relations of tamarack (Larix laricina) seedlings exposed to flooding. BMC Plant Biol. 2012, 12, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Yamauchi, T.; Colmer, T.; Nakazono, M. Aerenchyma Formation in Plants. In Low-Oxygen Stress in Plants, Oxygen Sensing and Adaptive Responses to Hypoxia, 1st ed.; Van Dongen, J.T., Licausi, F, Eds.; Plant Cell Monographs; Springer: New York, NY, USA, 2014; Volume 21, pp. 247–265. [Google Scholar]
- Janda, T.; Szalai, G.; Tari, I.; Paldi, E. Hydroponic treatments with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar] [CrossRef]
- Rajasekaran, L.R.; Blake, T.J. New plant growth regulators protect photosynthesis and enhance growth under drought of jack pine seedlings. J. Plant Growth Reg. 1999, 18, 171–181. [Google Scholar] [CrossRef]
- Gondor, O.K.; Pál, M.; Darkó, É.; Janda, T.; Szalai, G. Salicylic Acid and Sodium Salicylate Alleviate Cadmium Toxicity to Different Extents in Maize (Zea mays L.). PLoS ONE 2016, 11, e0160157. [Google Scholar] [CrossRef]
- Vwioko, E.D. Performance of soybean (Glycine max L.) in salt-treated soil environment following salicylic acid mitigation. NISEB J. 2013, 13, 44–49. [Google Scholar]
- Jackson, M.B. Ethylene-promoted elongation: An adaptation to submergence stress. Ann. Bot. 2008, 101, 229–248. [Google Scholar] [CrossRef]
- Vidoz, M.L.; Loreti, E.; Mensuali, A.; Alpi, A.; Perata, P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J. 2011, 63, 551–562. [Google Scholar] [CrossRef]
- Sasidharan, R.; Voesenek, L.A.C.J. Ethylene-mediated acclimations to flooding stress. Plant Physiol. 2015, 169, 3–12. [Google Scholar] [CrossRef]
- Gnanamurthy, S.; Dhanavel, D.; Girija, M.; Pavadai, P.; Bharathi, T. Effect of chemical mutagenesis on quantitative traits of maize (Zea mays (L.). Int. J. Res. Bot. 2012, 2, 34–36. [Google Scholar]
- Shagufta, B.; Aijaz, A.W.; Irshad, A.N. Mutagenic sensitivity of gamma rays, EMS and sodium azide in Trigonella foenumgraecum L. Sci. Res. Rep. 2013, 3, 20–26. [Google Scholar]
- Vwioko, D.E.; Onobun, E. Vegetative response of ten accessions of Abelmoschus esculentus (L) Moench. treated with sodium azide. J. Life Sci. Res. Dis. 2015, 2, 13–24. [Google Scholar]
- Al-Qurainy, F. Effects of sodium azide on growth and yield traits of Eruca sativa (L.). World Appl. Sci. J. 2009, 7, 220–226. [Google Scholar]
- Zuzana, K.; Katarína, R.; Elena, Z.; Maria, L.B.; Ján, B. Sodium azide induced morphological and molecular changes in persimmon (Diospyros lotus L.). Agriculture 2012, 58, 57–64. [Google Scholar]
- Adamu, A.K.; Aliyu, H. Morphological effects of sodium azide on tomato (Lycopersicon esculentum Mill.). Sci. World J. 2007, 2, 9–12. [Google Scholar]
- Gruszka, D.; Szarejko, L.; Maluszynski, M. Sodium azide as a mutagen. In Plant Mutation Breeding and Biotechnology; Shu, Q., Forster, B.P., Nakagawa, H., Eds.; CABI Publishing Company: Wallingford, UK, 2012; pp. 159–166. [Google Scholar]
- Kravchik, M.; Bernstein, N. Effects of salinity on the transcriptome of growing maize leaf cells points at differential involvement of the antioxidative response in cell growth restriction. BMC Genom. 2013, 16, 14–24. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidant and stress tolerance: A review. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Haq, I.U.; Memon, S.; Gill, N.P.; Rajput, M.T. Regeneration of plantlets under NaCl-stress from NaN3 treated sugarcane explants. Afr. J. Biotechnol. 2011, 10, 16152–16156. [Google Scholar]
- El Kaaby, E.A.J.; Al-Ajeel, S.A.; Al-Anny, J.A.; Al-Aubaidy, A.A.; Ammar, K. Effect of the chemical mutagen sodium azide on plant regeneration of two tomato cultivars under salinity stress condition in vitro. J. Life Sci. 2015, 9, 25–31. [Google Scholar] [CrossRef][Green Version]
- Kuasha, M.; Nasiruddin, K.M.; Hassan, L. Effects of sodium azide on callus in sugarcane. Discovery 2016, 52, 1683–1688. [Google Scholar]
- Salim, K.; Fahad, A.-Q.; Firoz, A. Sodium azide: A chemical mutagen for enhancement of agronomic traits of crop plants. Int. J. Sci. Tech. 2009, 4, 1–21. [Google Scholar]
- Ademoroti, C.A. Standard Methods for Water and Effluent Analysis, 1st ed.; Foludex Press Ltd.: Ibadan, Nigeria, 1996. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Blum, U. Benefits of citrate over EDTA for extracting phenolics from soils and plant debris. J. Chem. Ecol. 1997, 23, 347–362. [Google Scholar] [CrossRef]
- Appiah, M.R.; Ahenkorah, Y. Determination of available sulphate in some soils of Ghana considering five extraction methods. Biol. Fertil. Soils 1989, 8, 80–86. [Google Scholar] [CrossRef]
- Ben Mussa, S.A.; Elferjani, H.S.; Haroun, F.A.; Abdelnabi, F.F. Determination of available nitrate, phosphate and sulphate in soil samples. Int. J. PharmTech Res. 2009, 1, 598–604. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total organic carbon and available phosphorus in soils. Soil Sci. 1945, 59, 39–48. [Google Scholar] [CrossRef]
- Bremner, J.M.; Jenkinson, D.S. Determination of organic carbon in soil. I. oxidation by dichromate of organic matter in soil and plant materials. J. Soil Sci. 1960, 11, 394–402. [Google Scholar] [CrossRef]
- Islam, M.S.; Halim, M.A.; Safiullah, S.; Islam, M.S.; Islam, M.M. Analysis of organic matter, ion and manganese in soil of arsenic affected Singair Area, Bangladesh. Res. J. Environ. Toxicol. 2009, 3, 31–35. [Google Scholar]
- Harrigan, W.F.; McCance, M.E. Laboratory Methods in Foods and Dairy Microbiology, 8th ed.; Academic Press: London, UK, 1990. [Google Scholar]
- Holt, J.G.; Sneath, P.H.; Krieg, N.R. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Lippincott, Williams and Wilkins Publishers: Baltimore, MD, USA, 2002; p. 787. [Google Scholar]
- Zhang, J.; Kirkham, M.B. Enzymatic Responses of the Ascorbate-Gluta-thione Cycle to Drought in Sorghum and Sunflower Plants. Plant Sci. 1996, 113, 139–147. [Google Scholar] [CrossRef]
- Zou, X.; Hu, C.; Zeng, L.; Xu, M.; Zhang, X. A comparison of screening methods to identify waterlogging tolerance in the field in Brassica napus (L.) during plant ontogeny. PLoS ONE 2014, 9, e89731. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Samarajeewa, P.K.; Barrero, R.A.; Nishigushi, M.; Uchimiya, H. Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation of rice roots. Planta 1998, 204, 277–287. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.-J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL 1 and SNORKEL 2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef]
- Mensah, J.K.; Obadoni, B. Effects of sodium azide on yield parameters of groundnut (Arachis hypogaea L.). Afr. J. Biotechnol. 2007, 6, 668–671. [Google Scholar]
- Nakweti, R.K.; Franche, C.; Ndiku, S.L. Effects of sodium azide (NaN3) on seeds germination, plantlets growth and in vitro antimalarial activities of Phyllantus odontadenius Mull. Arg. Amer. J. Exp. Agric. 2015, 5, 226–238. [Google Scholar]
- Unger, I.M.; Kennedy, A.C.; Muzika, R.-M. Flooding effects on soil microbial communities. Appl. Soil Ecol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Suzuki, C.; Kunito, T.; Aono, T.; Liu, C.-T.; Oyaizu, H. Microbial indices of soil fertility. J. Appl. Microbiol. 2005, 98, 1062–1074. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–378. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Vo, D.; Graham, K.J.; Scow, K.M. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 2004, 48, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Mentzer, J.L.; Goodman, R.M.; Balser, T.C. Microbial responses over time to hydrologic and fertilization treatments in a simulated wet prairie. Plant Soil 2006, 284, 85–100. [Google Scholar] [CrossRef]
- Stover, R.H. Flooding of soil for disease control. In Soil Disinfection; Mulder, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Labuda, S.Z.; Vetchinnikov, A.A. Soil susceptibility on reduction as an index of soil properties applied in the investigation upon soil devastation. Ecol. Chem. Eng. 2011, 18, 333–344. [Google Scholar]
- Vepraskas, M.J.; Faulkner, S.P. Redox chemistry of hydric soils. In Wetlands Soils: Genesis, Hydrology, Landscapes and Classification; Richardson, J.L., Vepraskas, M.J., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Jeng, T.L.; Tseng, T.H.; Lai, C.C.; Wu, M.T.; Sung, J.M. Antioxidative charactrisation of NaN3- induced common bean mutants. Food Chemistry. 2010, 119, 1006–1011. [Google Scholar] [CrossRef]
- Elfeky, S.; Abo-Hamad, S.; Saad-Allah, K.M. Physiological impact of sodium azide on Helianthus annus seedlings. Int. J. Agron. Agric. Res. 2014, 4, 102–109. [Google Scholar]

| NaN3 Treatment | Waterlogging Conditions | 2 WAP | 4 WAP | 6 WAP | 8 WAP | 10 WAP |
|---|---|---|---|---|---|---|
| 0% | Non-waterlogging | 8.6 b ± 0.45 | 14.7 e ± 0.78 | 18.8 a ± 0.28 | 23.7 a ± 1.19 | 31.5 a ± 1.28 |
| One-week waterlogging | 7.7 c ± 0.47 | 17.2 ab ± 0.68 | 17.9 abc ± 0.43 | 18.3 c ± 0.25 | 15.2 c ± 0.62 | |
| Two weeks waterlogging | 8.3 b ± 0.30 | 15.8 cd ± 0.24 | 16.8 d ± 0.94 | 18.9 bc ± 1.05 | 19.3 b ± 0.29 | |
| 0.02% | Non-waterlogging | 9.5 a ± 0.62 | 12.0 f ± 0.30 | 18.0 abc ± 0.60 | 24.5 a ± 1.31 | 29.5 ab ± 0.68 |
| One-week waterlogging | 8.3 bc ± 0.45 | 17.9 ab ± 0.66 | 18.4 ab ± 0.42 | 18.8 bc ± 0.47 | 21.8 d ± 0.35 | |
| Two weeks waterlogging | 7.9 bc ± 0.09 | 17.7 ab ± 0.91 | 18.5 ab ± 1.23 | 19.7 b ± 1.30 | 22.4 bc ± 0.45 | |
| 0.05% | Non-waterlogging | 8.1 bc ± 0.78 | 11.9 f ± 0.83 | 17.6 bcd ± 0.49 | 23.5 a ± 0.88 | 31.1 a ± 0.98 |
| One-week waterlogging | 7.9 bc ± 0.21 | 17.2 ab ± 0.48 | 17.7 bcd ± 0.63 | 18.0 c ± 0.72 | 19.4 c ± 1.60 | |
| Two weeks waterlogging | 7.8 bc ± 0.22 | 16.1 bc ± 0.28 | 17.1 cd ± 0.20 | 18.1 c ± 0.71 | 19.9 c ± 0.34 |
| NaN3 Treatment | Waterlogging Conditions | 2 WAP | 4 WAP | 6 WAP | 8 WAP | 10 WAP |
|---|---|---|---|---|---|---|
| 0% | Non-waterlogging | 0.81 a ± 0.02 | 0.95 c ± 0.05 | 1.10 b ± 0.08 | 1.25 a ± 0.05 | 1.35 a ± 0.05 |
| One-week waterlogging | 0.80 a ± 0.01 | 1.02 b ± 0.09 | 1.07 b ± 0.09 | 1.15 b ± 0.05 | 1.27 b ± 0.05 | |
| Two weeks waterlogging | 0.85 a ± 0.05 | 1.17 a ± 0.05 | 1.27 a ± 0.05 | 1.27 a ± 0.05 | 1.27 b ± 0.05 | |
| 0.02% | Non-waterlogging | 0.76 b ± 0.05 | 1.02 b ± 0.09 | 1.10 c ± 0.08 | 1.25 a ± 0.05 | 1.37 a ± 0.05 |
| One-week waterlogging | 0.89 a ± 0.09 | 1.12 a ± 0.05 | 1.17 b ± 0.05 | 1.25 a ± 0.05 | 1.37 a ± 0.05 | |
| Two weeks waterlogging | 0.80 b ± 0.08 | 1.15 a ± 0.05 | 1.27 a ± 0.05 | 1.27 a ± 0.05 | 1.30 b ± 0.00 | |
| 0.05% | Non-waterlogging | 0.75 b ± 0.05 | 1.05 b ± 0.05 | 1.17 b ± 0.05 | 1.30 a ± 0.08 | 1.45 a ± 0.05 |
| One-week waterlogging | 0.82 a ± 0.07 | 1.07 b ± 0.05 | 1.20 b ± 0.08 | 1.20 b ± 0.08 | 1.32 b ± 0.09 | |
| Two weeks waterlogging | 0.85 a ± 0.05 | 1.17 a ± 0.09 | 1.27 a ± 0.05 | 1.27 a ± 0.05 | 1.32 b ± 0.05 |
| NaN3 Treatment | Waterlogging Conditions | No. Leaves per Plant | No. Adventitious Roots per Plant | Survival Percentage |
|---|---|---|---|---|
| 0% | Non-waterlogging | 16.0 a ± 2.30 | 0 c | 100.0 a ± 0.00 |
| One-week waterlogging | 13.0 b ± 1.10 | 10.7 b ± 7.18 | 33.3 b ± 27.22 | |
| Two weeks waterlogging | 12.0 b ± 0.00 | 13.0 a ± 8.67 | 25.0 b ± 16.67 | |
| 0.02% | Non- waterlogging | 16.5 a ± 1.00 | 0 c | 100.0 a ± 0.00 |
| One-week waterlogging | 14.0 b ± 1.60 | 15.5 b ± 1.29 | 50.0 b ± 19.25 | |
| Two weeks waterlogging | 13.0 b ± 1.15 | 21.0 a ± 0.81 | 33.3 c ± 0.00 | |
| 0.05% | Non- waterlogging | 16.5 a ± 1.00 | 0 c | 100.0 a ± 0.00 |
| One-week waterlogging | 15.0 b ± 1.15 | 18.0 b ± 1.63 | 50.0 b ± 19.25 | |
| Two weeks waterlogging | 13.0 c ± 1.15 | 22.2 a ± 1.25 | 50.0 b ± 19.25 |
| NaN3 Treatment | Waterlogging Conditions | Number of Flower Buds | Number of Flowers | Number of Fruits |
|---|---|---|---|---|
| 0% | Non-waterlogging | 5.5 a ± 0.57 | 5.0 a ± 0.81 | 4.5 a ± 0.57 |
| One-week waterlogging | 2.7 b ± 1.25 | 2.0 b ± 1.41 | 1.2 b ± 0.95 | |
| Two weeks waterlogging | 1.7 b ± 1.25 | 1.0 b ± 0.81 | 0.5 b ± 0.57 | |
| 0.02% | Non-waterlogging | 5.0 a ± 0.81 | 5.0 a ± 0.81 | 3.5 a ± 1.29 |
| One-week waterlogging | 2.5 b ± 0.57 | 1.7 b ± 0.50 | 1.5 b ± 1.00 | |
| Two weeks waterlogging | 1.7 b ± 0.5 | 1.2 b ± 0.95 | 1.2 b ± 0.95 | |
| 0.05% | Non-waterlogging | 5.0 a ± 0.81 | 4.5 a ± 1.29 | 3.7 a ± 1.89 |
| One-week waterlogging | 3.2 b ± 0.95 | 2.5 b ± 0.57 | 2.2 b ± 0.91 | |
| Two weeks waterlogging | 2.2 b ± 0.95 | 1.5 b ± 0.57 | 1.5 b ± 0.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vwioko, E.D.; El-Esawi, M.A.; Imoni, M.E.; Al-Ghamdi, A.A.; Ali, H.M.; El-Sheekh, M.M.; Abdeldaym, E.A.; Al-Dosary, M.A. Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.). Agronomy 2019, 9, 679. https://doi.org/10.3390/agronomy9110679
Vwioko ED, El-Esawi MA, Imoni ME, Al-Ghamdi AA, Ali HM, El-Sheekh MM, Abdeldaym EA, Al-Dosary MA. Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.). Agronomy. 2019; 9(11):679. https://doi.org/10.3390/agronomy9110679
Chicago/Turabian StyleVwioko, Emuejevoke D., Mohamed A. El-Esawi, Marcus E. Imoni, Abdullah A. Al-Ghamdi, Hayssam M. Ali, Mostafa M. El-Sheekh, Emad A. Abdeldaym, and Monerah A. Al-Dosary. 2019. "Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.)" Agronomy 9, no. 11: 679. https://doi.org/10.3390/agronomy9110679
APA StyleVwioko, E. D., El-Esawi, M. A., Imoni, M. E., Al-Ghamdi, A. A., Ali, H. M., El-Sheekh, M. M., Abdeldaym, E. A., & Al-Dosary, M. A. (2019). Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.). Agronomy, 9(11), 679. https://doi.org/10.3390/agronomy9110679








