Carbon Dioxide Enrichment Combined with Supplemental Light Improve Growth and Quality of Plug Seedlings of Astragalus membranaceus Bunge and Codonopsis lanceolata Benth. et Hook. f.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
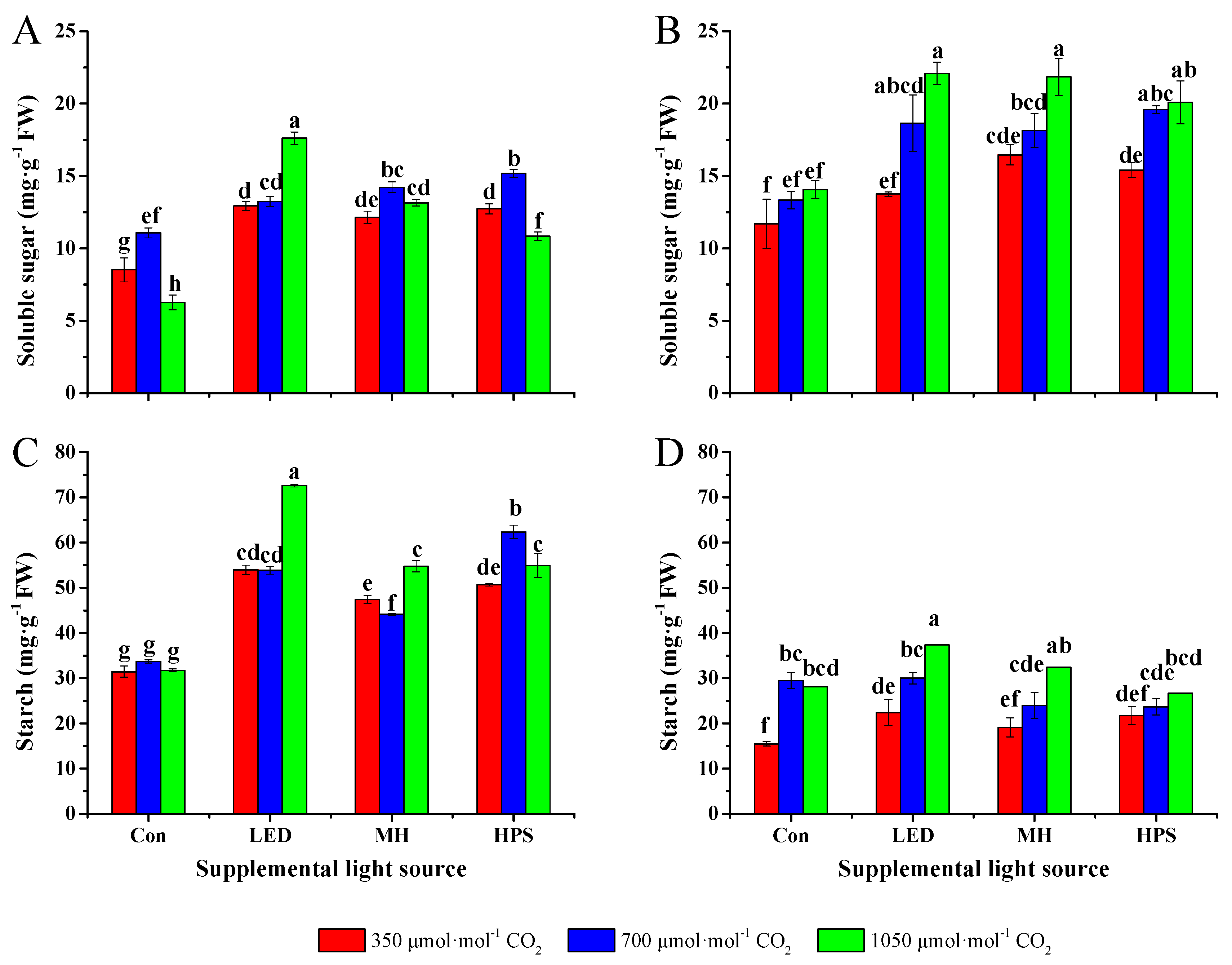
2.2. Contents of Soluble Sugar and Starch
2.3. Contents of Total Phenols and Flavonoids
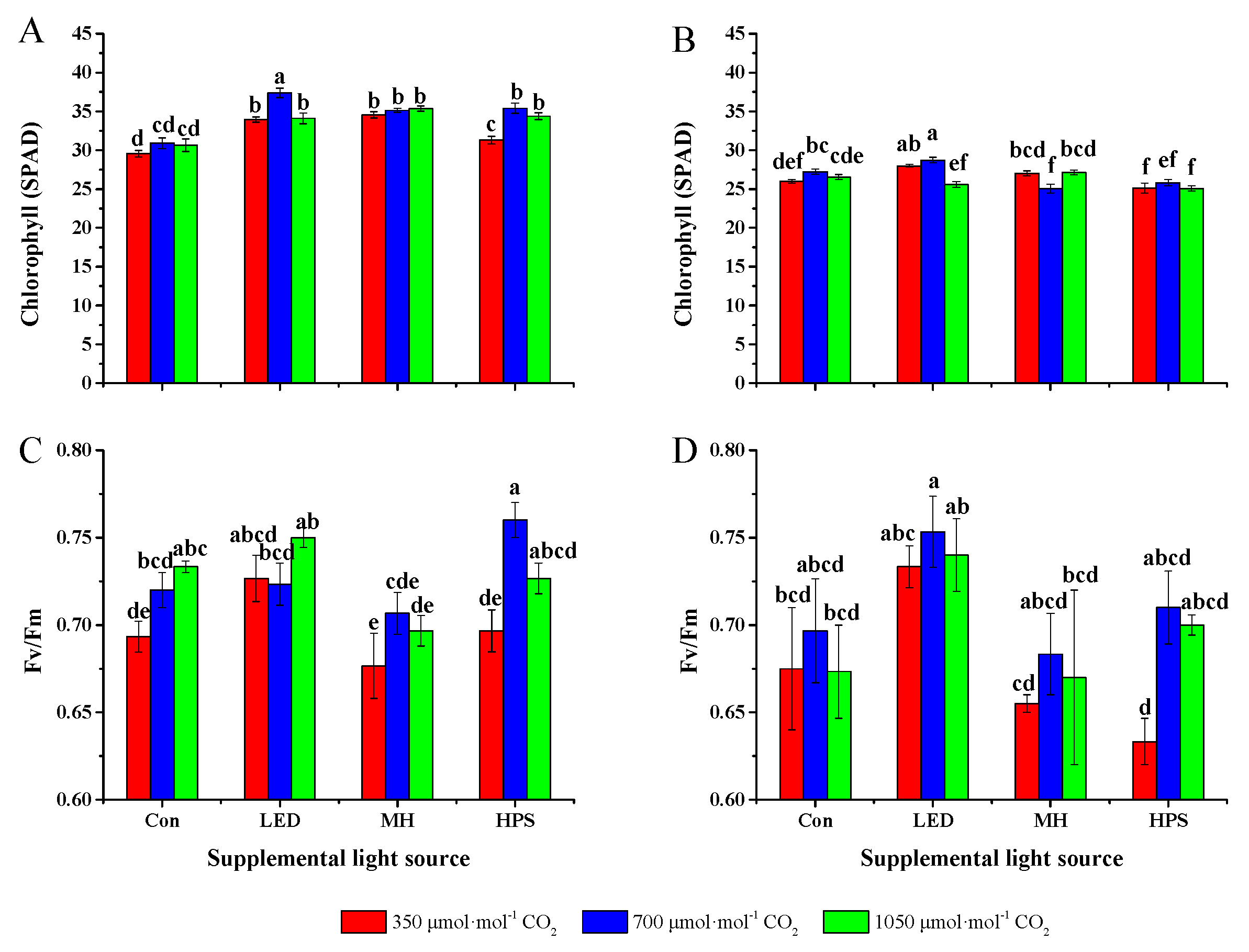
2.4. Assessment of the Chlorophyll Content, Quantum Yield, and Stomatal Conductance
2.5. Data Collection and Analysis
3. Results and Discussion
3.1. Growth, Development, and Morphology
3.2. Contents of Soluble Sugar and Starch
3.3. Contents of Total Phenols and Flavonoids
3.4. Chlorophyll Content, Quantum Yield, and Stomatal Conductance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tuan, P.A.; Chung, E.; Thwe, A.A.; Li, X.; Kim, Y.B.; Mariadhas, V.A.; Al-Dhabi, N.A.; Lee, J.H.; Park, S.U. Transcriptional profiling and molecular characterization of astragalosides, calycosin, and calycosin-7-o-beta-d-glucoside biosynthesis in the hairy roots of Astragalus membranaceus in response to methyl jasmonate. J. Agric. Food Chem. 2015, 63, 6231–6240. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Gong, L.; Ding, Z.F.; Li, Y.D.; Li, F.X.; Zhao, S.P.; Liu, B. Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolata Benth. et Hook. F., as revealed by ISSR and rapd markers. Plant Cell Rep. 2006, 25, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jia, M.M.; Zhang, X.J.; Wang, P.L. Calycosin attenuates MPTP-induced Parkinson’s disease by suppressing the activation of TLR/NF-kappa B and MAPK pathways. Phytother. Res. 2019, 33, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Han, A.Y.; Lee, Y.S.; Kwon, S.; Lee, H.S.; Lee, K.W.; Seol, G.H. Codonopsis lanceolata extract prevents hypertension in rats. Phytomedicine 2018, 39, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.O.; Kim, H.H.; Barasch, D.; Nemirovski, A.; Lee, M.S.; Gorinstein, S.; Ku, Y.G. Codonopsis lanceolata and Nelumbo nucifera Gaertn. Root extracts for functional food: Metabolic profiling by MS, FTIR and fluorescence and evaluation of cytotoxicity and anti-obesity properties on 3T3-L1 cell line. Eur. Food Res. Technol. 2017, 243, 689–700. [Google Scholar] [CrossRef]
- Moon, K.G.; Um, I.S.; Jeon, S.H.; Cho, Y.S.; Kim, Y.G.; Rho, I.R. Effect of organic fertilizer application on growth characteristics and saponin content in Codonopsis lanceolata. Hortic. Environ. Biotechnol. 2018, 59, 125–130. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Seong, E.S.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Evaluation of phenolic compounds and antimicrobial activities in transgenic Codonopsis lanceolata plants via overexpression of the gamma-tocopherol methyltransferase (gamma-tmt) gene. S. Afr. J. Bot. 2017, 109, 25–33. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.W.; Wang, H.Q.; Yu, J.Q.; Duan, J.A. Flowers of Astragalus membranaceus var. mongholicus as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Molecules 2019, 24, 434. [Google Scholar] [CrossRef]
- Mamatha, H.; Rao, N.K.S.; Laxman, R.H.; Shivashankara, K.S.; Bhatt, R.M.; Pavithra, K.C. Impact of elevated CO2 on growth, physiology, yield, and quality of tomato (Lycopersicon esculentum Mill) cv. Arka Ashish. Photosynthetica 2014, 52, 519–528. [Google Scholar] [CrossRef]
- Pan, T.H.; Ding, J.J.; Qin, G.G.; Wang, Y.L.; Xi, L.J.; Yang, J.W.; Li, J.M.; Zhang, J.; Zou, Z.R. Interaction of supplementary light and CO2 enrichment improves growth, photosynthesis, yield, and quality of tomato in autumn through spring greenhouse production. HortScience 2019, 54, 246–252. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, H.; Dong, B.; Shi, C.; Li, Y.; Zhai, H.; Liu, M. Effects of elevated CO2 concentration on growth and water use efficiency of winter wheat under two soil water regimes. Agric. Water Manag. 2010, 97, 1742–1748. [Google Scholar] [CrossRef]
- Cheng, W.; Sakai, H.; Yagi, K.; Hasegawa, T. Interactions of elevated CO2 and night temperature on rice growth and yield. Agric. For. Meteorol. 2009, 149, 51–58. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Schjoerring, J.K. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of current hypotheses. Agric. Ecosyst. Environ. 2013, 178, 57–63. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, J.; Yao, F.; Hao, C. Interactive effects of elevated CO2 concentration and irrigation on photosynthetic parameters and yield of maize in northeast China. PLoS ONE 2014, 9, e98318. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brestic, M.; Tan, D.-X.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin alleviates low PSⅠ-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Erice, G.; Sanz-Sáez, A.; Abadie, C.; Gilard, F.; Gil-Quintana, E.; Avice, J.C.; Staudinger, C.; Wienkoop, S.; Araus, J.L.; et al. Differential CO2 effect on primary carbon metabolism of flag leaves in durum wheat (Triticum durum Desf.). Plant Cell Environ. 2015, 38, 2780–2794. [Google Scholar] [CrossRef]
- Bunce, J.A. Acclimation to temperature of the response of photosynthesis to increased carbon dioxide concentration in Taraxacum officinale. Photosynth. Res. 2000, 64, 89–94. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef]
- Jiang, C.Y.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Photosynthesis, plant growth, and fruit production of single-truss tomato improves with supplemental lighting provided from underneath or within the inner canopy. Sci. Hortic. 2017, 222, 221–229. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Whitman, C.M.; Runkle, E.S. Manipulating growth, color, and taste attributes of fresh cut lettuce by greenhouse supplemental lighting. Sci. Hortic. 2019, 252, 274–282. [Google Scholar] [CrossRef]
- Bergstrand, K.J.; Schussler, H.K. Growth, development and photosynthesis of some horticultural plants as affected by different supplementary lighting technologies. Eur. J. Hortic. Sci. 2013, 78, 119–125. [Google Scholar]
- Chen, X.L.; Wang, L.C.; Li, T.; Yang, Q.C.; Guo, W.Z. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue led light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef] [PubMed]
- Tewolde, F.T.; Lu, N.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Nighttime supplemental led inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Front. Plant Sci. 2016, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Cui, L.R.; Ye, L.; Zhou, X.T.; Bao, E.C.; Zhao, H.L.; Zou, Z.R. Effects of red light night break treatment on growth and flowering of tomato plants. Front. Plant Sci. 2016, 7, 527. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of light intensity and photoperiod on improving steviol glycosides content in stevia rebaudiana (bertoni) bertoni while conserving light energy consumption. J. Appl. Res. Med. Aroma. 2017, 7, 64–73. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Soundararajan, P.; Manivnnan, A.; Jeong, B.R. Light quality during night interruption affects morphogenesis and flowering in petunia hybrida, a qualitative long-day plant. Hortic. Environ. Biotechnol. 2016, 57, 371–377. [Google Scholar] [CrossRef]
- Ouzounis, T.; Frette, X.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. J. Plant Physiol. 2014, 171, 1491–1499. [Google Scholar] [CrossRef]
- Paradiso, R.; Meinen, E.; Snel, J.F.H.; De Visser, P.; Van Ieperen, W.; Hogewoning, S.W.; Marcelis, L.F.M. Spectral dependence of photosynthesis and light absorptance in single leaves and canopy in rose. Sci. Hortic. 2011, 127, 548–554. [Google Scholar] [CrossRef]
- Shukla, M.; Tabassum, R.; Singh, R.; Dhar, D.W. Influence of light intensity, temperature and CO2 concentration on growth and lipids in green algae and cyanobacteria. Indian J. Exp. Biol. 2016, 54, 482–487. [Google Scholar]
- Correll, M.J.; Weathers, P.J. Effects of light, CO2 and humidity on carnation growth, hyperhydration and cuticular wax development in a mist reactor. In Vitr. Cell. Dev. Biol.-Plant 2001, 37, 405–413. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Zhang, X.; Korpelainen, H.; Berninger, F.; Li, C. Effects of elevated CO2 and temperature on photosynthesis and leaf traits of an understory dwarf bamboo in subalpine forest zone, China. Physiol. Plant 2013, 148, 261–272. [Google Scholar] [CrossRef]
- Hwang, C.H.; Park, Y.G.; Jeong, B.R. Changes in content of total polyphenol and activities of antioxidizing enzymes in Perilla frutescens var. Acuta kudo and Salvia plebeia R. Br. as affected by light intensity. Hortic. Environ. Biotechnol. 2014, 55, 489–497. [Google Scholar] [CrossRef]
- Matsuda, R.; Ozawa, N.; Fujiwara, K. Leaf photosynthesis, plant growth, and carbohydrate accumulation of tomato under different photoperiods and diurnal temperature differences. Sci. Hortic. 2014, 170, 150–158. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Jeong, R.B. Night temperature affects the growth, metabolism, and photosynthetic gene expression in astragalus membranaceus and codonopsis lanceolata plug seedlings. Plants 2019, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Guo, S.S.; Xin, X.L.; Chen, L. Changes in volatile constituents and phenols from Gynura bicolor DC grown in elevated CO2 and LED lighting. Sci. Hortic. 2014, 175, 243–250. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. In vitro propagation, phytochemical analysis, and evaluation of free radical scavenging property of Scrophularia kakudensis Franch tissue extracts. Biomed Res. Int. 2015, 2015, 480564. [Google Scholar] [CrossRef]
- Kitaya, Y.; Niu, G.; Kozai, T.; Ohashi, M. Photosynthetic photon flux, photoperiod, and CO2 concentration affect growth and morphology of lettuce plug transplants. HortScience 1998, 33, 58–62. [Google Scholar] [CrossRef]
- Kim, H.M.; Hwang, S.J. The growth and development of ‘mini chal’ tomato plug seedlings grown under various wavelengths using light emitting diodes. Agronomy 2019, 9, 157. [Google Scholar] [CrossRef]
- Yücedağ, C.; Bilir, N.; Özel, H.B. Phytohormone effect on seedling quality in Hungarian oak. For. Syst. 2019, 28, 5. [Google Scholar] [CrossRef]
- Elfadl, A.M. Growth performance and physiological characteristics of seedlings of six tropical dry land forest tree species in the sudan. J. Nat. Resour. Environ. Stud. 2013, 1, 25–33. [Google Scholar]
- Park, Y.G.; Park, J.E.; Hwang, S.J.; Jeong, B.R. Light source and CO2 concentration affect growth and anthocyanin content of lettuce under controlled environment. Hortic. Environ. Biotechnol. 2012, 53, 460–466. [Google Scholar] [CrossRef]
- Hoddinott, J.; Scott, R. The influence of light quality and carbon dioxide enrichment on the growth and physiology of seedlings of three conifer species. II. Physiological responses. Can. J. Bot. 1996, 74, 391–402. [Google Scholar] [CrossRef]
- Yilmaz, O.; Kahraman, K.; Ozturk, L. Elevated carbon dioxide exacerbates adverse effects of mg deficiency in durum wheat. Plant Soil 2017, 410, 41–50. [Google Scholar] [CrossRef]
- Park, Y.G.; Oh, H.J.; Jeong, B.R. Growth and anthocyanin concentration of Perilla frutescens var. acuta Kudo as affected by light source and DIF under controlled environment. Hortic. Environ. Biotechnol. 2013, 54, 103–108. [Google Scholar] [CrossRef]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Jeong, H.K.; Wei, H.; Jeong, B.R. Growth and physiological responses of Adenophora triphylla (Thunb.) A.DC. plug seedlings to day and night temperature regimes. Agronomy 2018, 8, 173. [Google Scholar] [CrossRef]
- Dreywood, R. Qualitative test for carbohydrate material. Ind. Eng. Chem. Anal. Ed. 1946, 18, 499. [Google Scholar] [CrossRef]
- Santos-Moura, S.D.; Alves, E.U.; Ursulino, M.M.; Bruno, R.D.A.; dos Anjos Neto, A.P. Effect of shading on Dimorphandra gardneriana Tul. seedling production. Biosci. J. 2018, 34, 1147–1157. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Ma, X.; Grodzinski, B. The effect of spectral quality on daily patterns of gas exchange, biomass gain, and water-use-efficiency in tomatoes and lisianthus: An assessment of whole plant measurements. Front. Plant Sci. 2017, 8, 1076. [Google Scholar] [CrossRef]
- Randall, W.C.; Lopez, R.G. Comparison of supplemental lighting from high-pressure sodium lamps and light-emitting diodes during bedding plant seedling production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of different led sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Wohlfahrt, Y.; Smith, J.P.; Tittmann, S.; Honermeier, B.; Stoll, M. Primary productivity and physiological responses of Vitis vinifera L. cvs. under free air carbon dioxide enrichment (FACE). Eur. J. Agron. 2018, 101, 149–162. [Google Scholar] [CrossRef]
- Terfa, M.T.; Solhaug, K.A.; Gislerod, H.R.; Olsen, J.E.; Torre, S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa x hybrida but does not affect time to flower opening. Physiol. Plant 2013, 148, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.L.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z.Q. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Lee, J.H.; Oh, Y.; Kim, D.; Oh, M.M.; In, B.C. Growth and bioactive compound synthesis in cultivated lettuce subject to light-quality changes. HortScience 2017, 52, 584–591. [Google Scholar] [CrossRef]
- Ebisawa, M.; Shoji, K.; Kato, M.; Shimomura, K.; Goto, F.; Yoshihara, T. Supplementary ultraviolet radiation b together with blue light at night increased quercetin content and flavonol synthase gene expression in leaf lettuce (Lactuca sativa L.). Environ. Control Biol. 2008, 46, 1–11. [Google Scholar] [CrossRef]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Fan, X.; Zang, J.; Xu, Z.; Guo, S.; Jiao, X.; Liu, X.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. 2009, 96, 30–37. [Google Scholar] [CrossRef]
- Sezgin, A.; Altuntaş, C.; Demiralay, M.; Cinemre, S.; Terzi, R. Exogenous alpha lipoic acid can stimulate photosystem Ⅱ activity and the gene expressions of carbon fixation and chlorophyll metabolism enzymes in maize seedlings under drought. J. Plant Physiol. 2019, 232, 65–73. [Google Scholar] [CrossRef]
- Ksiksi, T.S.; Ppoyil, S.B.T.; Palakkott, A.R. Co2 enrichment affects eco-physiological growth of maize and alfalfa under different water stress regimes in the uae. Physiol. Mol. Biol. Plants 2018, 24, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.S.; Ibrahim, R.; Damalas, C.A.; Noorhosseini, S.A. Effects of gamma stress and carbon dioxide on eight bioactive flavonoids and photosynthetic efficiency in Centella asiatica. J. Plant Growth Regul. 2017, 36, 957–969. [Google Scholar] [CrossRef]
- Ruhil, K.; Ahmad, A.; Iqbal, M.; Tripathy, B.C. Photosynthesis and growth responses of mustard (Brassica juncea L. cv pusa Bold) plants to free air carbon dioxide enrichment (FACE). Protoplasma 2015, 252, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Samphumphung, T.; Theerawitaya, C.; Prommee, W.; Cha-um, S. In vitro photoautotrophic acclimatization, direct transplantation and ex vitro adaptation of rubber tree (Hevea brasiliensis). Plant Cell Tissue Organ Cult. 2018, 133, 215–223. [Google Scholar] [CrossRef]
- Bunce, J.A.; Ziska, L.H. Impact of measurement irradiance on acclimation of photosynthesis to elevated CO2 concentration in several plant species. Photosynthetica 1999, 37, 509–517. [Google Scholar] [CrossRef]
- Li, X.; Lu, W.; Hu, G.; Wang, X.C.; Zhang, Y.; Sun, G.X.; Fang, Z. Effects of light-emitting diode supplementary lighting on the winter growth of greenhouse plants in the Yangtze River Delta of China. Bot. Stud. 2016, 57, 2. [Google Scholar] [CrossRef]
- Bergstrand, K.J.; Mortensen, L.M.; Suthaparan, A.; Gislerod, H.R. Acclimatisation of greenhouse crops to differing light quality. Sci. Hortic. 2016, 204, 1–7. [Google Scholar] [CrossRef]
- Prince, C.M.; MacDonald, G.E.; Erickson, J.E. Effects of elevated temperature and carbon dioxide concentrations on the response of two common reed (Phragmites australis) haplotypes to glyphosate. Invasive Plant Sci. Manag. 2018, 11, 181–190. [Google Scholar] [CrossRef]
- Tom-Dery, D.; Eller, F.; Jensen, K.; Reisdorff, C. Effects of elevated carbon dioxide and climate change on biomass and nutritive value of kyasuwa (Cenchrus pedicellatus Trin.). J. Appl. Bot. Food Qual. 2018, 91, 88–95. [Google Scholar]
- Song, J.X.; Meng, Q.W.; Du, W.F.; He, D.X. Effects of light quality on growth and development of cucumber seedlings in controlled environment. Int. J. Agric. Biol. Eng. 2017, 10, 312–318. [Google Scholar]
- Borowski, E.; Michalek, S.; Rubinowska, K.; Hawrylak-Nowak, B.; Grudzinski, W. The effects of light quality on photosynthetic parameters and yield of lettuce plants. Acta Sci. Pol. 2015, 14, 177–188. [Google Scholar]
- Liu, Y.; Ren, X.; Jeong, B.R. Supplementary light source affects growth, metabolism, and physiology of Adenophora triphylla (Thunb.) A.DC. seedlings. Biomed. Res. Int. 2019, 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomata development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [PubMed]



| Light (L) | Length (cm) | Dry Weight (mg) | Leaf Area (cm−2) | Stem Diameter (mm) | Dickson’s Quality Index (×10−4) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Leaf | |||||
| 350 | Con | 8.4 ± 0.5 e z | 5.0 ± 0.4 b | 39.1 ± 2.5 e | 6.4 ± 0.8 e | 8.2 ± 0.6 g | 4.8 ± 0.3 e z | 0.99 ± 0.02 d | 5.1 ± 0.3 d |
| LED | 14.9 ± 0.6 a | 4.9 ± 0.4 b | 105.5 ± 9.6 bc | 15.8 ± 1.6 ad | 17.4 ± 1.6 be | 7.9 ± 0.5 bd | 1.37 ± 0.06 bc | 10.5 ± 1.0 bc | |
| MH | 14.7 ± 0.5 ab | 4.7 ± 0.3 b | 103.8 ± 11.3 bc | 15.8 ± 2.6 ad | 16.9 ± 1.6 bf | 8.0 ± 0.5 bd | 1.32 ± 0.09 bc | 10.4 ± 1.8 bc | |
| HPS | 13.1 ± 0.5 c | 4.7 ± 0.3 b | 84.6 ± 8.0 cd | 11.4 ± 1.4 ce | 14.1 ± 1.8 df | 7.6 ± 0.6 bd | 1.25 ± 0.07 c | 8.7 ± 1.0 cd | |
| 700 | Con | 10.7 ± 0.3 d | 4.5 ± 0.3 b | 73.2 ± 7.2 d | 9.5 ± 1.8 de | 11.8 ± 1.2 fg | 6.7 ± 0.4 cd | 1.24 ± 0.04 c | 8.8 ± 1.1 cd |
| LED | 13.3 ± 0.3 bc | 4.1 ± 0.2 b | 134.8 ± 9.0 a | 17.0 ± 3.7 ac | 25.1 ± 2.0 a | 10.2 ± 0.6 a | 1.62 ± 0.06 a | 16.0 ± 1.7 a | |
| MH | 13.3 ± 0.5 bc | 5.9 ± 0.4 a | 108.2 ± 7.4 bc | 16.4 ± 1.4 ac | 19.3 ± 2.2 bd | 9.3 ± 0.9 ab | 1.38 ± 0.04 bc | 12.1 ± 0.8 a-c | |
| HPS | 13.4 ± 0.2 bc | 4.1 ± 0.2 b | 98.3 ± 10.2 bd | 15.0 ± 2.2 ad | 16.9 ± 2.5 bf | 6.5 ± 0.4 d | 1.28 ± 0.06 c | 10.4 ± 1.4 bc | |
| 1050 | Con | 12.2 ± 0.6 c | 4.9 ± 0.2 b | 76.8 ± 6.2 d | 12.8 ± 1.2 be | 13.5 ± 0.9 ef | 7.3 ± 0.4 cd | 1.23 ± 0.06 c | 8.7 ± 1.2 cd |
| LED | 12.7 ± 0.7 c | 4.5 ± 0.2 b | 111.2 ± 10.0 ac | 20.4 ± 3.1 a | 20.4 ± 1.9 ac | 8.2 ± 0.5 b-d | 1.48 ± 0.06 ab | 15.5 ± 1.5 a | |
| MH | 13.6 ± 0.3 ac | 4.0 ± 0.2 b | 121.4 ± 11.3 ab | 18.9 ± 2.2 ab | 21.4 ± 1.4 ab | 8.4 ± 0.6 bc | 1.48 ± 0.10 ab | 14.6 ± 2.3 ab | |
| HPS | 12.7 ± 0.7 c | 4.1 ± 0.4 b | 96.6 ± 5.6 bd | 14.1 ± 1.1 ad | 16.1 ± 0.9 cf | 6.8 ± 0.3 cd | 1.30 ± 0.03 bc | 10.8 ± 0.8 bc | |
| F-test | C | NS | NS | ** | ** | ** | * | ** | *** |
| L | *** | NS | *** | *** | *** | *** | *** | *** | |
| C×L | *** | *** | NS | NS | NS | ** | ** | NS | |
| CO2 (C) | Light (L) | Length(cm) | Dry Weight (mg) | Leaf Area (cm−2) | Stem Diameter (mm) | Dickson’s Quality Index (×10−4) | |||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | Leaf | |||||
| 350 | Con | 10.50.4 bdz | 4.4 ± 0.3 a | 30.6 ± 4.7 f | 6.1 ± 1.2 e | 5.6 ± 0.4 d | 4.6 ± 0.2 f | 1.56 ± 0.09 f | 5.1 ± 0.9 e |
| LED | 11.3 ± 0.3 ac | 3.8 ± 0.4 ab | 52.9 ± 4.3 bd | 12.0 ± 2.8 cd | 8.9 ± 0.8 bc | 7.2 ± 0.6 ac | 2.01 ± 0.08 bc | 10.8 ± 1.9 bc | |
| MH | 10.0 ± 0.6 d | 3.0 ± 0.3 b | 49.1 ± 4.9 ce | 12.5 ± 2.5 bd | 8.2 ± 0.7 bc | 5.6 ± 0.4 df | 1.69 ± 0.06 ef | 9.7 ± 1.1 bd | |
| HPS | 10.5 ± 0.3 bd | 3.7 ± 0.2 ab | 46.5 ± 2.2 de | 12.8 ± 1.4 bd | 8.3 ± 0.6 bc | 7.0 ± 0.7 ad | 1.99 ± 0.07 bc | 9.9 ± 0.8 bd | |
| 700 | Con | 10.2 ± 0.3 cd | 4.4 ± 0.4 a | 37.2 ± 2.2 ef | 9.1 ± 0.7 de | 6.5 ± 0.5 cd | 5.4 ± 0.5 ef | 1.73 ± 0.04 df | 7.3 ± 0.5 ce |
| LED | 12.0 ± 0.2 a | 4.2 ± 0.2 a | 61.9 ± 6.7 bc | 16.2 ± 1.9 ac | 9.7 ± 1.2 b | 7.3 ± 0.5 ab | 2.04 ± 0.04 b | 12.5 ± 1.4 b | |
| MH | 11.0 ± 0.5 ad | 4.0 ± 0.2 a | 63.4 ± 3.8 b | 15.6 ± 1.8 ac | 9.8 ± 0.4 b | 7.1 ± 0.4 ad | 1.81 ± 0.06 ce | 12.3 ± 1.2 b | |
| HPS | 11.6 ± 0.5 ab | 4.5 ± 0.4 a | 53.7 ± 4.5 bd | 13.8 ± 2.3 bd | 7.5 ± 0.6 bd | 5.7 ± 0.4 bf | 1.89 ± 0.05 be | 10.2 ± 0.7 bd | |
| 1050 | Con | 11.0 ± 0.5 ad | 4.5 ± 0.3 a | 36.5 ± 3.2 ef | 10.4 ± 1.2 ce | 6.6 ± 0.6 cd | 5.7 ± 0.4 bf | 1.70 ± 0.03 ef | 6.8 ± 0.6 de |
| LED | 12.2 ± 0.2 a | 4.4 ± 0.3 a | 80.3 ± 6.8 a | 20.5 ± 1.4 a | 12.1 ± 1.4 a | 7.7 ± 0.9 a | 2.30 ± 0.10 a | 17.9 ± 2.1 a | |
| MH | 11.5 ± 0.2 ab | 4.1 ± 0.4 a | 61.7 ± 4.6 bc | 18.3 ± 2.5 ab | 9.5 ± 0.7 b | 6.7 ± 0.3 ae | 1.96 ± 0.04 bc | 12.8 ± 1.1 b | |
| HPS | 10.6 ± 0.5 bd | 4.5 ± 0.1 a | 45.1 ± 2.8 de | 15.0 ± 1.3 ad | 6.7 ± 0.5 cd | 5.6 ± 0.4 cf | 1.90 ± 0.03 bd | 10.2 ± 0.6 bd | |
| F-test | C | * | ** | ** | *** | NS | NS | ** | ** |
| L | ** | * | *** | *** | *** | *** | *** | *** | |
| C×L | NS | NS | * | NS | NS | NS | NS | NS | |
| Species | Factor | Soluble Sugar | Starch | Total Phenols | Total Flavonoids |
|---|---|---|---|---|---|
| A. membranaceus | CO2 (C) | *** | *** | *** | *** |
| Light(L) | *** | *** | NS | *** | |
| C × L | *** | *** | *** | *** | |
| C. lanceolata | CO2 (C) | *** | *** | *** | *** |
| Light(L) | *** | ** | ** | *** | |
| C × L | NS | NS | *** | *** |
| Species | Factor | Chlorophyll | Quantum Yield | Stomatal Conductance | |
|---|---|---|---|---|---|
| Day | Night | ||||
| A. membranaceus | CO2 (C) | *** | ** | NS | * |
| Light(L) | *** | ** | *** | *** | |
| C×L | ** | NS | ** | ** | |
| C. lanceolata | CO2 (C) | NS | NS | ** | ** |
| Light(L) | *** | ** | NS | *** | |
| C×L | *** | NS | NS | NS | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ren, X.; Jeong, B.R. Carbon Dioxide Enrichment Combined with Supplemental Light Improve Growth and Quality of Plug Seedlings of Astragalus membranaceus Bunge and Codonopsis lanceolata Benth. et Hook. f. Agronomy 2019, 9, 715. https://doi.org/10.3390/agronomy9110715
Liu Y, Ren X, Jeong BR. Carbon Dioxide Enrichment Combined with Supplemental Light Improve Growth and Quality of Plug Seedlings of Astragalus membranaceus Bunge and Codonopsis lanceolata Benth. et Hook. f. Agronomy. 2019; 9(11):715. https://doi.org/10.3390/agronomy9110715
Chicago/Turabian StyleLiu, Ya, Xiuxia Ren, and Byoung Ryong Jeong. 2019. "Carbon Dioxide Enrichment Combined with Supplemental Light Improve Growth and Quality of Plug Seedlings of Astragalus membranaceus Bunge and Codonopsis lanceolata Benth. et Hook. f." Agronomy 9, no. 11: 715. https://doi.org/10.3390/agronomy9110715






