Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Priming Agent
2.2. Plant Materials
2.3. Experimental Details
2.4. Crop Husbandry
2.5. Plant Survey
2.5.1. Stand Establishment Traits
2.5.2. Biochemical Traits
2.5.3. Yield and Yield Related Traits
2.6. Statistical analysis
3. Results
3.1. Stand Establishment Traits
3.2. Chlorophyll Contents in Flag Leaves
3.3. Antioxidant system in flag leaves
3.4. Grain Yield and Yield Related Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Basra, S.M.A.; Nawaz, M. Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). S. Afr. J. Bot. 2019. in Press. [Google Scholar] [CrossRef]
- Shan, Z.; Zu-liang, S.; Si-jun, Y.; Ke-jun, G.; Ting-bo, D.; Fei, W.; Xiang, L.; Ren-hua, S.; Xuebao, Y.S. Effects of nitrogen application rates and straw returning on nutrient balance and grain yield of late sowing wheat in rice-wheat rotation. Yingyong Shengtai Xuebao 2015, 26, 2714–2720. [Google Scholar]
- Khan, M.A. Wheat Crop Management for Yield Maximization; Annual Research Program, Arid Zone Research Institute: Bhakhar, Pakistan, 2004. [Google Scholar]
- Khan, M.B.; Ghurchani, M.; Hussain, M.; Mahmood, K. Wheat seed invigoration by pre-sowing chilling treatments. Pak. J. Bot 2010, 42, 1561–1566. [Google Scholar]
- Bedada, W.; Lemenih, M.; Karltun, E. Soil nutrient build-up, input interaction effects and plot level N and P balances under long-term addition of compost and NP fertilizer. Agric. Ecosyst. Environ. 2016, 218, 220–231. [Google Scholar] [CrossRef]
- Taylor, A.G.; Harman, G.E. Concepts and technologies of selected seed treatments. Ann. Rev. Phytopathol. 1990, 28, 321–339. [Google Scholar] [CrossRef]
- Yucel, N.C.; Heybet, E.H. Salicylicl acid and calcium treatments improves wheat vigor, lipids and phenolics under high salinity. Acta Chim. Slov. 2016, 2016 63, 738–746. [Google Scholar] [CrossRef]
- Mahboob, W.; Khan, M.A.; Shirazi, M.U.; Mumtaz, S.; Shereen, A. Using Growth and Ionic Contents of Wheat Seedlings as Rapid Screening Tool for Salt Tolerance. J. Crop Sci. Biotechnol. 2017, 21, 173–181. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment-A shotgun approach to improve germination, growth and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Barth, C.; De–Tullio, M.; Conklin, P.L. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 2006, 57, 1657–1665. [Google Scholar] [CrossRef]
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S.A. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 2013, 199, 12–22. [Google Scholar] [CrossRef]
- Conklin, P.L.; Barth, C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens and the onset of senescence. Plant Cell Environ. 2004, 27, 959–971. [Google Scholar] [CrossRef]
- Pinouchi, C.; Foyer, C.H. Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Curr. Opin. Plant Biol. 1990, 6, 379–389. [Google Scholar]
- Imran, S.; Afzal, I.; Basra, S.M.A.; Saqib, M. Integrated seed priming with growth promoting substances enhances germination and seedling vigor of spring maize at low temperature. Int. J. Agric. Biol. 2013, 15, 1251–1257. [Google Scholar]
- ISTA. International Rules for Seed Testing; ISTA: Secretariat, Switzerland, 2010. [Google Scholar]
- Ellis, R.A.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Hafeez, K.; Ahmad, N. Thermal hardening: A new seed vigor enhancement tool in rice. J. Integr. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Arnon, D.T. Copper enzyme in isolated chloroplasts polyphenols oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, M.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzym. 1955, 2, 764. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Deekey, D.A. Principles and procedures of statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co. Inc.: New York, NY, USA, 1997; pp. 400–428. [Google Scholar]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of Selenium Biofortification and Beneficial Microorganism Inoculation on Yield, Quality and Antioxidant Properties of Shallot Bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef]
- Jena, A.; Sing, R.K.; Sing, M.K. Mitigation measures for wheat production under heat stress condition. Int. J. Agric. Sci. Res. 2016, 7, 359–376. [Google Scholar]
- Basra, S.M.A.; Farooq, M.; Tabassum, R. Physiological and biochemical aspects of seed vigor enhancement treatments in fi ne rice (Oryza sativa L.). Seed Sci. Technol. 2005, 33, 623–628. [Google Scholar] [CrossRef]
- Zheng, M.; Tao, Y.; Hussain, S.; Jiang, Q.; Peng, S.; Huang, J.; Nie, L. Seed priming in dry direct-seeded rice: Consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul. 2016, 78, 167–178. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Modarressanavy, S.A.M. Effect of the ascorbic acid, pyridoxine and hydrogen peroxide treatments on germination, catalase activity, protein and malondialdehyde content of three oil seeds. Not. Bot. Horti Agrobot. Cluj-Napoca 2008, 36, 61–66. [Google Scholar]
- Ajouri, A.; Haben, A.; Baker, M. Seed priming enhances germination and seedling growth of barley under conditions of P and Zn deficiency. J. Plant Nutr. Soil Sci. 2004, 167, 630–636. [Google Scholar] [CrossRef]
- Rowse, H.R. Drum priming. A non-osmotic method of priming seeds. Seed Sci. Technol. 1995, 24, 281–294. [Google Scholar]
- Johkan, M.; Oda, M.; Maruo, T.; Shinohara, Y. Crop production and global warming. In Global Warming Impacts-Case Studies on the Economy, Human Health, and on Urban and Natural Environments; Casalegno, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 139–152. [Google Scholar]
- Feng, H.Q.; Li, X.; Duan, J.G.; Li, H.Y.; Liang, H.G. Chilling tolerance of wheat seedlings is related to an enhanced alternative respiratory pathway. Crop Sci. 2008, 48, 2381–2388. [Google Scholar] [CrossRef]
- Chivasa, W.; Harris, D.; Chiduza, C.; Nyamudeza, P.; Mashingaidze, A.B. Agronomic practices, major crops and farmers’ perceptions of the importance of good stand establishment in Musikavanhu Communal Area, Zimbabwe. J. Appl. Sci. S. Afr. 1998, 4, 9–25. [Google Scholar] [CrossRef]
- Joshi, A.K.; Rai, B.; Sing, M.P. Technology for late sowing wheat in eastern Uttar Perdesh. Indian Farm. 1992, 42, 15–16. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving the chilling tolerance. J. Agron. Crop Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 2nd ed.; Sinauer Associates Inc. Publishers: Sunderland, MA, USA, 2002. [Google Scholar]
- Yang, M.T.; Chen, S.L.; Lin, C.Y.; Chen, Y.M. Chilling stress suppresses chloroplast development and nuclear gene expression in leaves of mung bean seedlings. Planta 2005, 221, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Fayyaz-ul-Hassan. Response of spring wheat (Triticum aestivum L.) quality traits and yield to sowing date. PLoS ONE 2015, 10, 126097. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Perveen, M.; Gelani, S.; Basra, S.M.A. Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J. Plant Physiol. 2007, 164, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Pell, E.J.; Dann, M.S. Multiple stress-induced foliar senescence and implications for whole-plant longevity. In Response of Plants to Multiple Stresses; Mooney, H.A., Winner, W.E., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 189–204. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Khan, A.; Sajid, M.; Ahmad, A.; Athar, H.R.; Ashraf, M. Interactive effect of foliarly applied ascorbicacid and salt stress on wheat (Triticum aestivum L.) at the seedling stage. Pak. J. Bot. 2006, 38, 1407–1414. [Google Scholar]
- Appu, M.; Muthukrishnan, S. Foliar application of salicylic acid stimulates flowering and induce defense related proteins in finger millet plants. Univers. J. Plant Sci. 2014, 2, 14–18. [Google Scholar]
- Yu, H.; Wu, Q.L.; Hai, Z.; Ying, S.X.; Zhang, X.K. Changes of stomatal density, length, width and microstructure of maize leaves under water stress. J. Jilin Agri. Univ. 2003, 25, 239–242. [Google Scholar]
- Wang, H.L.; Gan, Y.T.; Wang, R.Y.; Niu, J.Y.; Zhao, H.; Yang, Q.G.; Li, G.C. Phenological trends in winter wheat and spring cotton in response to climate changes in Northwest China. Agric. For. Meteorol. 2008, 148, 1242–1251. [Google Scholar] [CrossRef]
- Iqbal, M.; Navabi, A.; Yang, R.C.; Salmon, D.F.; Spaner, D. The effect of vernalization genes on earliness and related agronomic traits of spring wheat in northern growing regions. Crop Sci. 2007, 47, 1031–1039. [Google Scholar] [CrossRef]
- Akhtar, M.; Cheema, M.S.; Jamil, M.; Ali, L. Effect of time of sowing on some important characters of wheat, Triticum aestivum genotypes. J. Agric. Res. 2006, 44, 255258. [Google Scholar]
- Liu, Z.; Guo, Y.; Bai, J. Exogenous H2O2 changes antioxidant enzyme activity and protects ultrastructure in leaves of two cucumber Ecotypes under osmotic stress. J. Plant Growth Regul. 2010, 29, 171–183. [Google Scholar] [CrossRef]
- Sattar, A.; Cheema, M.A.; Farooq, M.; Wahid, M.A.; Wahid, A.; Babar, B.H. Evaluating the performance of wheat varieties under late sown conditions. Int. J. Agric. Biol. 2010, 12, 561–565. [Google Scholar]
- Hung, S.H.; Yu, C.W.; Lin, C.H. Hydrogen peroxide functions as a stress signal in plants. Bot. Bull. Acad. Sin. 2005, 46, 1–10. [Google Scholar]
- Gapper, C.; Dolan, L. Control of plant development by ROS. Plant Physiol. 2006, 141, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Nguyen, V.; Schroeder, J.I. The role of ROS in hormonal responses. Plant Physiol. 2006, 141, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Hussain, S.; Yin, H.; Peng, S.; Khan, F.A.; Khan, F.; Sameeullah, M.; Hussain, H.A.; Huang, J.; Cui, K.; Nie, L. Comparative transcriptional profiling of primed and non-primed rice seedlings under submergence stress. Front. Plant Sci. 2016, 7, 1125. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Keskitalo, J.; Bergquist, G.; Gardeström, P.; Jansson, S. A Cellular timetable of autumn senescence. Plant Physiol. 2005, 139, 1635–1648. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.; Dangl, J.L. ROS signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, Y.K.; Lin, S.H.; Fang, Y.Y.; Bai, J.G. H2O2 pretreatment alters the activity of antioxidant enzymes and protects chloroplast ultrastructure in heat stressed cucumber leaves. Sci. Hortic. 2010, 126, 20–26. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Young, L.W.; Wilen, R.W.; Smith, P.C.B. High temperature stress of Brassica napus during flowering resuces micro and megagametophyte fertility, induces fruit abortion and disrupts seed production. J. Exp. Bot. 2004, 55, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Berzsenyi, Z.; Lap, D.Q. Responses of maize (Zea mays L.) hybrids to sowing date, N fertilizer and plant density in different years. Acta Agron. Hung. 2005, 53, 119–131. [Google Scholar] [CrossRef]
- Namakka, A.; Abubakar, I.U.; Sadik, I.A.; Sharifai, A.I.; Hassas, A.H. Effect of sowing date and nitrogen level on yield and yield components of extra early maize varieties (Zea mays L.) in Sudan Savana of Nigeria. ARPN. J. Agric. Biol. Sci. 2008, 3, 1–5. [Google Scholar]
- Yasmeen, H.A.; Basra, S.M.A.; Ahmad, R.; Wahid, A. Performance of late sown wheat in response to foliar application of Moringa oleifera lam. leaf extract. Chil. J. Agric. Res. 2012, 72, 92–97. [Google Scholar] [CrossRef]
- Ahmad, I.; Basra, S.M.A.; Wahid, A. Exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide improves the productivity of hybrid maize at low temperature stress. Int. J. Agric. Biol. 2014, 16, 825–830. [Google Scholar]
- Farooq, M.; Aziz, T.; Basra, S.M.A.; Cheema, M.A.; Rehman, H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 2008, 194, 161–168. [Google Scholar] [CrossRef]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol. Biochem. 2016, 118, 362–369. [Google Scholar] [CrossRef]
- Abid, M.; Hakeem, A.; Shao, Y.; Liu, Y.; Zahoor, R.; Fan, Y.; Suyu, J.; Ata-Ul-Karim, S.T.; Tian, Z.; Jiang, D.; et al. Seed osmopriming invokes stress memory against post-germinative drought stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2017, 145, 12–20. [Google Scholar] [CrossRef]










| Years | Mean Emergence Time (days) | Time to 50% Emergence (days) | Co-efficient of Uniformity Emergence (CUE) | Final Germination Count |
|---|---|---|---|---|
| 2016 | 10.77 | 6.16 | 0.78 | 208.9 |
| 2017 | 12.37 | 8.64 | 0.66 | 182.7 |
| LSD | 0.37 | 0.62 | 0.07 | 14.86 |
| Sowing Dates (SD) | ||||
| 15-November | 7.69 | 4.94 | 0.74 | 204.1 |
| 15-December | 16.10 | 11.34 | 0.57 | 161.4 |
| LSD | 1.44 | 0.95 | 0.26 | 9.16 |
| Priming Techniques (P) | ||||
| Control | 12.88 a | 9.51 a | 0.52 b | 152.4 c |
| Hydro-priming | 12.46 a | 8.57 b | 0.63 b | 171.4 b |
| AsA priming | 11.76 b | 7.83 c | 0.81 a | 224.4 a |
| LSD | 0.43 | 0.79 | 0.25 | 13.86 |
| Interaction | ||||
| SD × P (2016) | * (Figure 1) | * (Figure 2) | ns | * (Figure 3a) |
| SD × P (2017) | Ns | ns | ns | * (Figure 3b) |
| Years | Chlorophyll a (mg g−1) | Chlorophyll b (mg g−1) | Peroxidase (unit mg protein−1) | Catalase (unit mg protein−1) | Super Oxide Dismutase (unit mg protein−1) |
|---|---|---|---|---|---|
| 2016 | 2.09 | 0.718 | 8.88 | 14.13 | 137.2 |
| 2017 | 1.90 | 0.613 | 7.27 | 10.32 | 121.6 |
| LSD | 0.119 | 0.154 | 0.62 | 3.066 | 7.37 |
| Sowing Dates (SD) | |||||
| 15-November | 2.24 | 0.693 | 4.81 | 11.83 | 125.9 |
| 15-December | 2.13 | 0.533 | 9.73 | 8.80 | 148.4 |
| LSD | 0.22 | 0.255 | 1.35 | 2.51 | 3.09 |
| Priming Techniques (P) | |||||
| Control | 1.04 c | 0.551 c | 5.93 c | 8.79 b | 129.2 c |
| Hydropriming | 2.21 b | 0.621 b | 6.87 b | 9.97 b | 136.9 b |
| AsA priming | 2.23 a | 0.667 a | 8.03 a | 12.18 a | 145.5 a |
| LSD | 0.17 | 0.147 | 0.63 | 2.37 | 4.72 |
| Interaction | |||||
| SD × P (2016) | ns | ns | * (Figure 5a) | ns | ns |
| SD × P (2017) | * (Figure 4) | ns | * (Figure 5b) | ns | * (Figure 6) |
| Years | Tillers Per Unit Area (m−2) | Plant Height (cm) | Grain Number | 1000-Grain Weight (g) | Grain Yield (t ha−1) | Biological Yield (t ha−1) |
|---|---|---|---|---|---|---|
| 2016 | 345.7 | 94.8 | 43.5 | 35.7 | 4.0 | 14.2 |
| 2017 | 335.5 | 88.8 | 41.3 | 34.4 | 4.8 | 12.6 |
| LSD | 10.85 | 5.36 | 0.96 | 0.66 | 0.3 | 0.63 |
| Sowing Dates (SD) | ||||||
| 15-November | 376.7 | 97.1 | 45.7 | 38.0 | 5.0 | 16.7 |
| 15-December | 294.3 | 80.5 | 36.8 | 29.9 | 3.4 | 11.8 |
| LSD | 72.91 | 6.41 | 4.42 | 2.63 | 0.47 | 2.53 |
| Priming Techniques (P) | ||||||
| Control | 307.2 c | 81.0 b | 38.6 b | 31.7 c | 4.2 c | 13.2 c |
| Hydropriming | 337.7 b | 90.7 a | 41.3 ab | 34.6 b | 4.8 b | 14.3 b |
| AsA priming | 360.9 a | 93.8 a | 43.9 a | 36.9 a | 5.2 a | 15.1 a |
| LSD | 9.66 | 7.97 | 4.26 | 2.11 | 0.43 | 0.82 |
| Interaction | ||||||
| SD × P (2016) | * (Figure 7a) | ns | ns | * (Figure 8) | * (Figure 9) | ns |
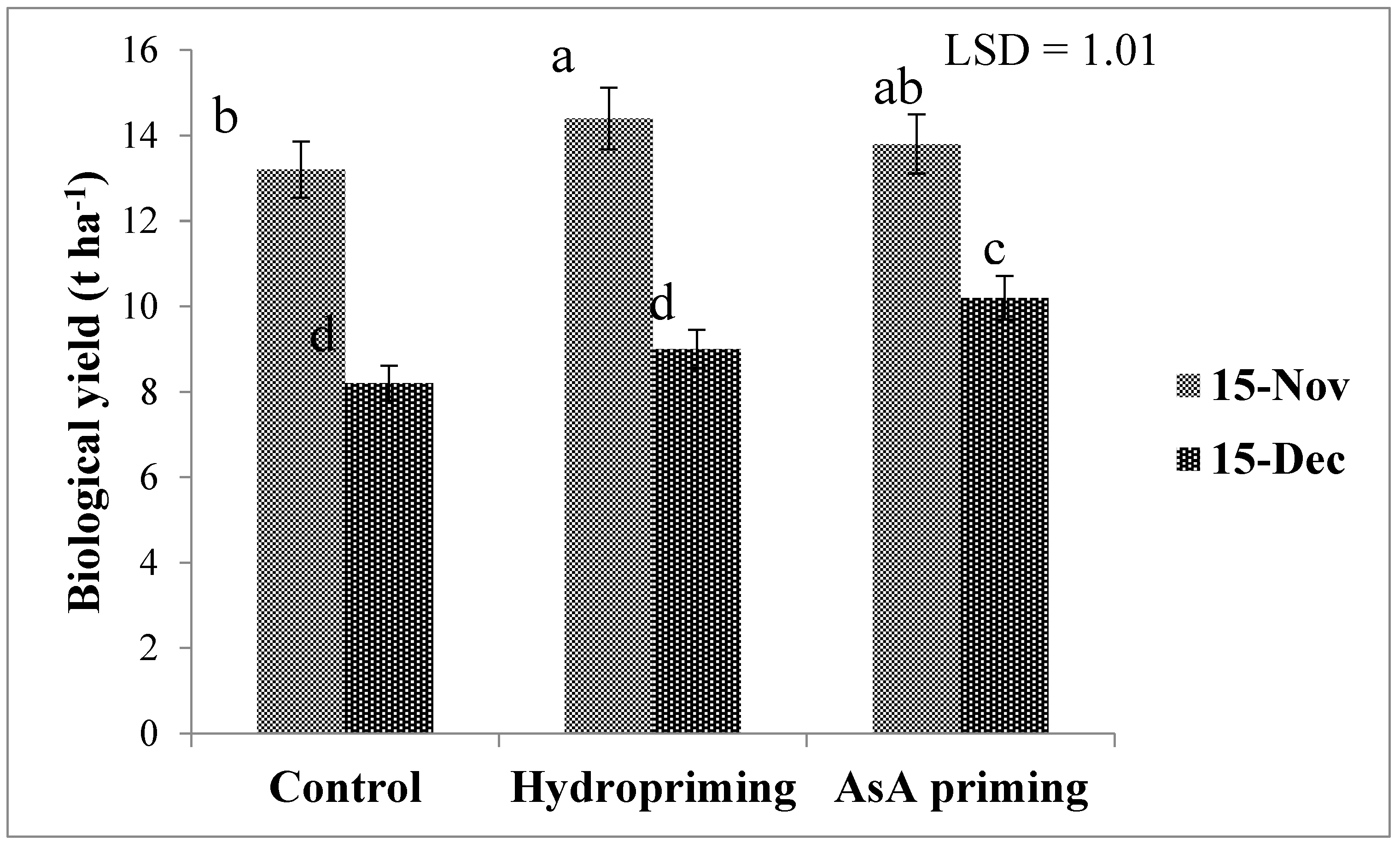
| SD × P (2017) | * (Figure 7b) | ns | ns | ns | ns | * (Figure 10) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L. Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan. Agronomy 2019, 9, 757. https://doi.org/10.3390/agronomy9110757
Shah T, Latif S, Khan H, Munsif F, Nie L. Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan. Agronomy. 2019; 9(11):757. https://doi.org/10.3390/agronomy9110757
Chicago/Turabian StyleShah, Tariq, Sadia Latif, Hamad Khan, Fazal Munsif, and Lixiao Nie. 2019. "Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan" Agronomy 9, no. 11: 757. https://doi.org/10.3390/agronomy9110757
APA StyleShah, T., Latif, S., Khan, H., Munsif, F., & Nie, L. (2019). Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan. Agronomy, 9(11), 757. https://doi.org/10.3390/agronomy9110757





