In Vitro Regeneration and ISSR-Based Genetic Fidelity Analysis of Orthosiphon stamineus Benth
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Growth Media and Plant Material
2.2. Induction of Direct Regeneration
2.3. Acclimatization
2.4. DNA Isolation and ISSR Analysis
3. Results
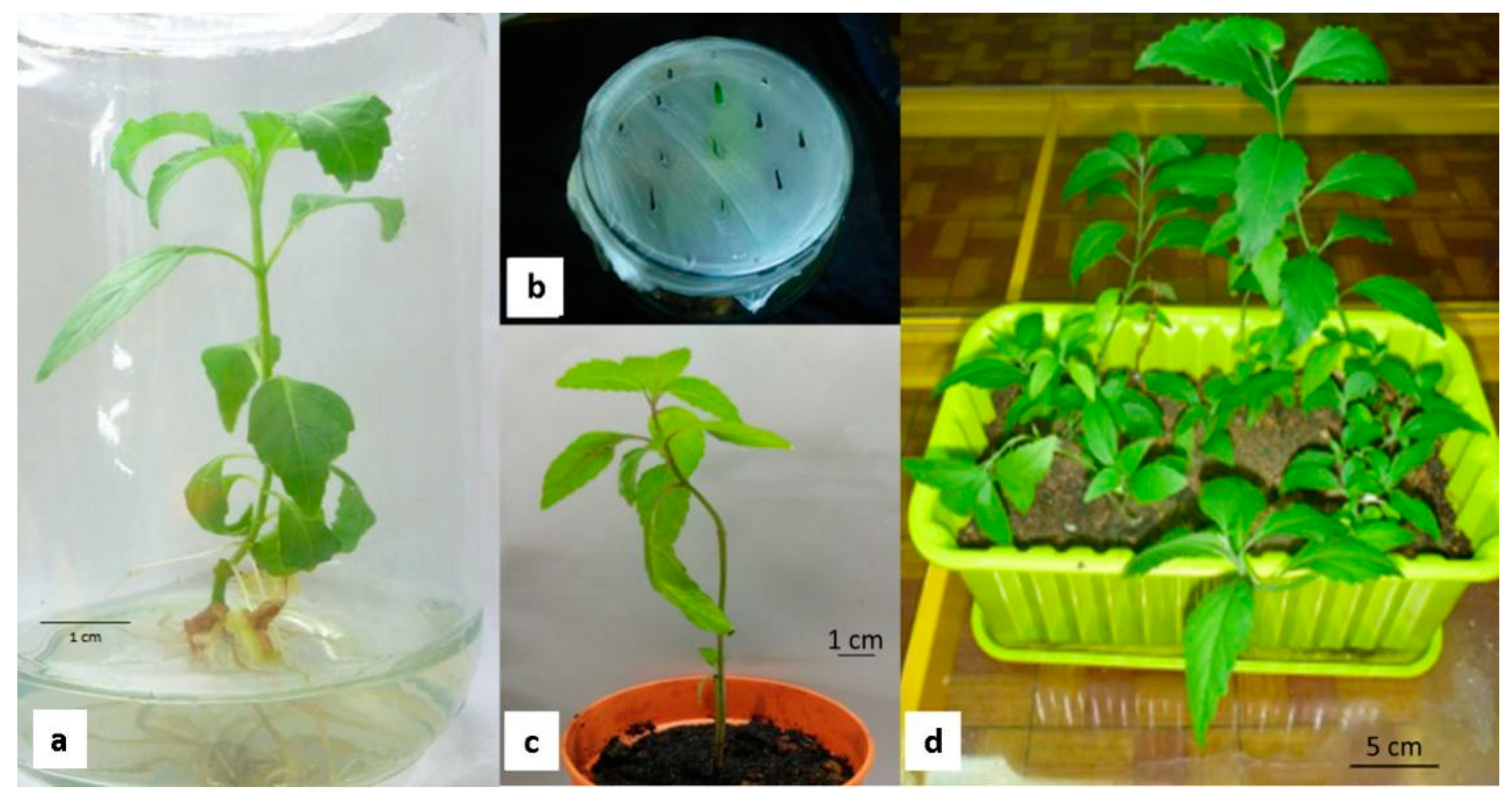
3.1. Direct Regeneration In Vitro
3.2. Production of Callus
3.3. Acclimatization
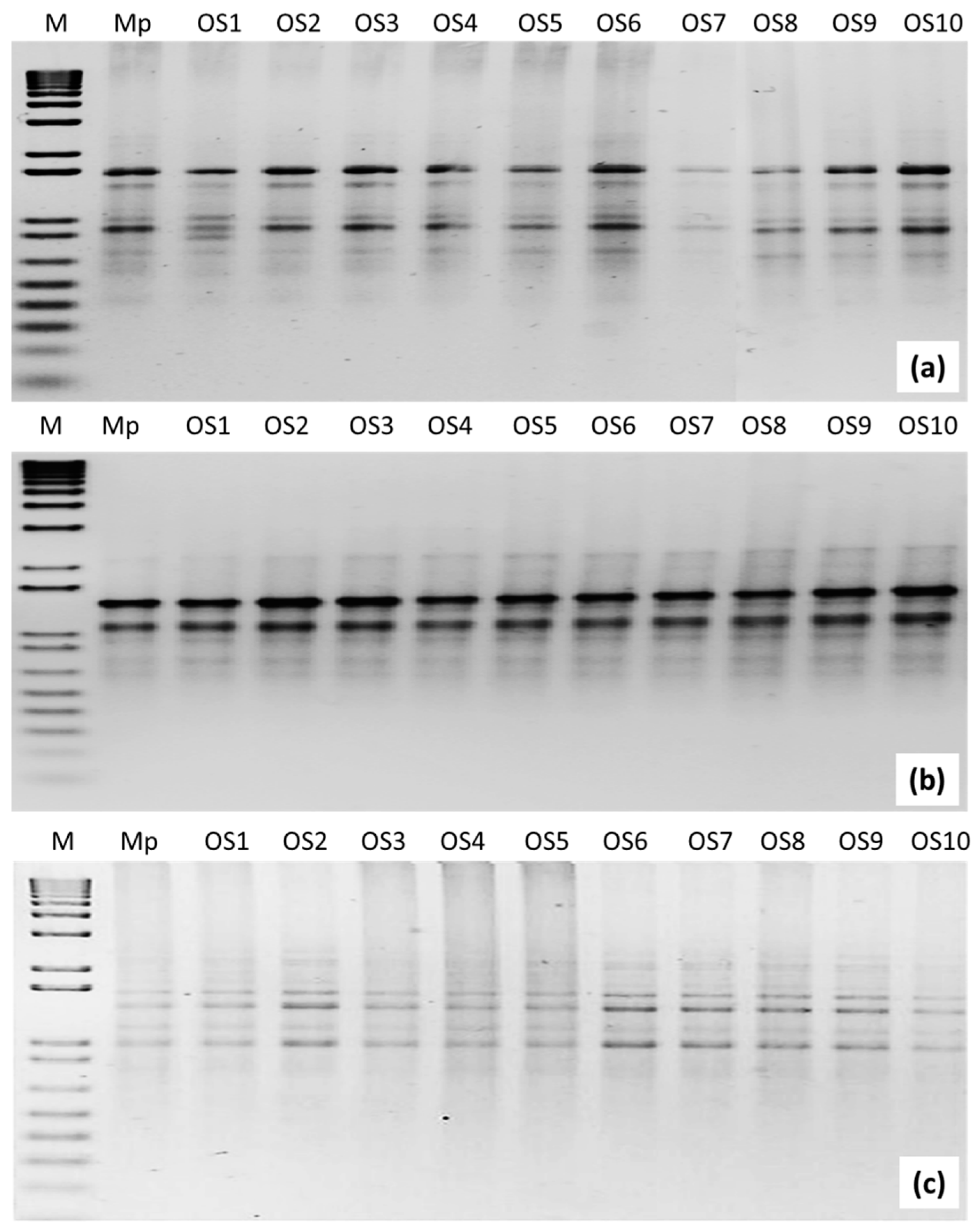
3.4. ISSR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almatar, M.; Rahmat, Z.; Salleh, F.M. Preliminary morphological and anatomical study of Orthosiphon stamineus. Asian J. Pharm. Biol. Res. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Akowuah, G.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Bever, B.O.; Zahnd, G.R. Plants with Oral Hypoglycaemic Action. Q. J. Crude Drug Res. 1979, 17, 139–196. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Tankhaeva, L.M. Physicochemical characteristics and antioxidant activity of melanoidin pigment from the fermented leaves of Orthosiphon stamineus. Rev. Bras. Farmacogn. 2012, 22, 284–290. [Google Scholar] [CrossRef]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Kadota, S. Siphonols A–E: Novel nitric oxide inhibitors from Orthosiphon stamineus of Indonesia. Bioorg. Med. Chem. Lett. 2003, 13, 31–35. [Google Scholar] [CrossRef]
- Adam, Y.; Somchit, M.N.; Sulaiman, M.R.; Nasaruddin, A.A.; Zuraini, A.; Bustamam, A.A.; Zakaria, Z.A. Diuretic properties of Orthosiphon stamineus Benth. J. Ethnopharmacol. 2009, 124, 154–158. [Google Scholar] [CrossRef]
- Olah, N.-K.; Radu, L.; Mogoşan, C.; Hanganu, D.; Gocan, S. Phytochemical and pharmacological studies on Orthosiphon stamineus Benth.(Lamiaceae) hydroalcoholic extracts. J. Pharm. Biomed. Anal. 2003, 33, 117–123. [Google Scholar] [CrossRef]
- Matsubara, T.; Bohgaki, T.; Watarai, M.; Suzuki, H.; Ohashi, K.; Shibuya, H. Antihypertensive actions of methylripariochromene A from Orthosiphon aristatus, an Indonesian traditional medicinal plant. Biol. Pharm. Bull. 1999, 22, 1083–1088. [Google Scholar] [CrossRef]
- Yehya, A.H.S.; Asif, M.; Kaur, G.; Hassan, L.E.A.; Al-Suede, F.S.R.; Abdul Majid, A.M.S.; Oon, C.E. Toxicological studies of Orthosiphon stamineus (Misai Kucing) standardized ethanol extract in combination with gemcitabine in athymic nude mice model. J. Adv. Res. 2019, 15, 59–68. [Google Scholar] [CrossRef]
- Robards, K.; Prernzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Maheswari, C.; Maryammal, R.; Venkatanarayanan, R. Hepatoprotective activity of Orthosiphon stamineus on liver damage caused by paracetamol in rats. Jordan J. Biol. Sci. 2008, 1, 105–108. [Google Scholar]
- Cai, X.; Xiao, C.; Xue, H.; Xiong, H.; Hang, Y.; Xu, J.; Lu, Y. A comparative study of the antioxidant and intestinal protective effects of extracts from different parts of Java tea (Orthosiphon stamineus). Food Sci. Nutr. 2018, 6, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Choo, B.K.M.; Kundap, U.P.; Kumari, Y.; Hue, S.M.; Othman, I.; Shaikh, M.F. Orthosiphon stamineus Leaf Extract Affects TNF-alpha and Seizures in a Zebrafish Model. Front. Pharmacol. 2018, 9, 139. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Mohamed, Z. Antiobesity and Lipid Lowering Effects of Orthosiphon stamineus in High-Fat Diet-Induced Obese Mice. Planta Med. 2017, 83, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Nawi, I.M.; Samad, A.A. Successful plant regeneration of Orthosiphon stamineus from petiole. J. Med. Plants Res. 2012, 6, 4276–4280. [Google Scholar]
- Affendy, H.; Aminuddin, M.; Azmy, M.; Amizi, M.; Assis, K.; Tamer, A. Effect of organic fertilizers application to the growth of Orthosiphon stamineus Benth. intercropped with Hevea brasiliensis Willd. and Durio zibethinus Murr. Int. J. Agric. Res. 2011, 6, 180–187. [Google Scholar]
- Dhoran, V.; Gudadhe, S. Effect of plant growth regulators on seed germination and seedling vigour in Asparagus sprengeri Regelin. Int. Res. J. Biol. Sci. 2012, 1, 6–10. [Google Scholar]
- Leng, L.W.; Lai-Keng, C. Plant regeneration from stem nodal segments of Orthosiphon stamineus Benth., a medicinal plant with diuretic activity. In Vitro Cell. Dev. Biol. Plant 2004, 40, 115–118. [Google Scholar] [CrossRef]
- Zainuddin, Z. In-Vitro Regeneration Of Orthosiphon stamineus (Misai Kucing) Using Axillary Bud. Sci. Herit. J. 2019, 3, 8–10. [Google Scholar] [CrossRef]
- Sheena, E.; Jeya Jothi, G. In vitro propagation of Orthosiphon stamineus Benth (Lamiaceae) an important medicinal plant using nodal and leaf explants. Pharma Innov. J. 2015, 4, 6–10. [Google Scholar]
- Zarnadze, N.; Diasamidze, I.; Varshanidze, N.; Dolidze, K.; Bolkvadze, T. In vitro reproduction of Kidney Tea (Orthosiphon stamineus Bents). J. Pharm. Pharmacol. 2018, 6, 695–699. [Google Scholar] [CrossRef][Green Version]
- Wai-Leng, L.; Lai-Keng, C. Establishment of Orthosiphon stamineus cell suspension culture for cell growth. Plant Cell Tissue Organ Cult. 2004, 78, 101–106. [Google Scholar] [CrossRef]
- Lim, F.L.; Yam, M.F.; Asmawi, M.Z.; Chan, L.-K. Elicitation of Orthosiphon stamineus cell suspension culture for enhancement of phenolic compounds biosynthesis and antioxidant activity. Ind. Crops Prod. 2013, 50, 436–442. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Yaacob, J.S.; Loh, H.-S.; Mat Taha, R. Protein Profiling and Histone Deacetylation Activities in Somaclonal Variants of Oil Palm (Elaeis guineensis Jacq.). Sci. World J. 2013, 2013, 613635. [Google Scholar] [CrossRef]
- Rani, V.; Raina, S. Genetic fidelity of organized meristem-derived micropropagated plants: A critical reappraisal. In Vitro Cell. Dev. Biol. Plant 2000, 36, 319–330. [Google Scholar] [CrossRef]
- Leva, A.; Rinaldi, L.; Petruccelli, R. Somaclonal Variation in Tissue Culture: A Case Study with Olive; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar]
- Yaacob, J.S.; Mat Taha, R. Genetic stability of in vitro propagated African blue lily (Agapanthus praecox ssp. minimus). Caryologia 2014, 67, 227–233. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vujović, T.; Ružić, D.; Cerović, R. In vitro shoot multiplication as influenced by repeated subculturing of shoots of contemporary fruit rootstocks. Hortic. Sci. 2012, 39, 101–107. [Google Scholar] [CrossRef]
- Viehmannova, I.; Bortlova, Z.; Vitamvas, J.; Cepkova, P.H.; Eliasova, K.; Svobodova, E.; Travnickova, M. Assessment of somaclonal variation in somatic embryo-derived plants of yacon [Smallanthus sonchifolius (Poepp. and Endl.) H. Robinson] using inter simple sequence repeat analysis and flow cytometry. Electron. J. Biotechnol. 2014, 17, 102–106. [Google Scholar] [CrossRef][Green Version]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. 2006. Available online: http://darwin.cirad.fr/ (accessed on 24 August 2019).
- Reshi, N.A.; Sudarshana, M. In vitro micropropagation of Orthosiphon aristatus (Blume) Miq. J. Med. Plants Res. 2015, 9, 962–967. [Google Scholar]
- Ignacimuthu, S.; Elangomathavan, R.; Prakash, S.; Kathiravan, K.; Seshadri, S. High frequency in vitro propagation of kidney tea plant. Plant Cell Tissue Organ Cult. 2003, 72, 83–86. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Bahar, E.; Lahham, J.; Schroeder, D.; Hussein, E. Regeneration and assessment of genetic fidelity of the endangered tree Moringa peregrina (Forsk.) Fiori using Inter Simple Sequence Repeat (ISSR). Physiol. Mol. Biol. Plants 2013, 19, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Veetil, S.E.; Gabriel, J.J. Comparative Phytochemical Screening and Antioxidant Properties of In Vitro and In Vivo Propagated Orthosiphon Stamineus Benth (Lamiaceae). Asian J. Pharm. Clin. Res. 2015, 8, 216–220. [Google Scholar]
- Sridhar, T.M.; Aswath, C.R. Influence of Additives on Enhanced in Vitro Shoot Multiplication of Stevia rebaudiana (Bert.)—An Important Anti Diabetic Medicinal Plant. Am. J. Plant Sci. 2014, 5, 192–199. [Google Scholar] [CrossRef][Green Version]
- Ling, A.P.K.; Kok, K.M.; Hussein, S.; Ong, S.L. Effects of plant growth regulators on adventitious roots induction from different explants of Orthosiphon stamineus. Am.-Eurasian J. Sustain. Agric. 2009, 3, 493–501. [Google Scholar]
- Hembrom, M.E.; Martin, K.; Patchathundikandi, S.K.; Madassery, J. Rapid in vitro production of true-to-type plants of Pogostemon heyneaus through dedieferentiated axillary buds. In Vitro Cell. Dev. Biol. Plant 2006, 42, 283–286. [Google Scholar] [CrossRef]
- Nordine, A.; Bousta, D.; El Khanchoufi, A.; El Meskaoui, A. An efficient and rapid in vitro propagation system of Thymus hyemalis Lange, a wild medicinal and aromatic plant of Mediterranean region. Int. J. Pharma Biosci. Technol. 2013, 1, 118–129. [Google Scholar]
- Saha, S.; Kader, A.; Sengupta, C.; Ghosh, P. In vitro propagation of Ocimum gratissimum L.(Lamiaceae) and its evaluation of genetic fidelity using RAPD marker. Am. J. Plant Sci. 2012, 3, 64. [Google Scholar] [CrossRef]
- Marin, J. High survival rates during acclimatization of micropropagated fruit tree rootstocks by increasing exposures to low relative humidity. In Proceedings of the I International Symposium on Acclimatization and Establishment of Micropropagated Plants, Sani-Halkidiki, Macedonia, Greece, 19 September 2001; pp. 139–142. [Google Scholar]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Hoque, M.; Ali, M.; Karim, N. Embryogenic callus induction and regeneration of elite Bangladeshi Indica rice cultivars. Plant Tissue Cult. Biotechnol. 2007, 17, 65–70. [Google Scholar] [CrossRef]
- Mishra, R.; Wang, H.-Y.; Yadav, N.R.; Wilkins, T.A. Development of a highly regenerable elite Acala cotton (Gossypium hirsutum cv. Maxxa)—A step towards genotype-independent regeneration. Plant Cell Tissue Organ Cult. 2003, 73, 21–35. [Google Scholar] [CrossRef]
- Rashid, H.; Khan, S.A.; Zia, M.; Chaudhary, M.F.; Hanif, Z.; Chaudary, Z. Callus induction and regeneration in elite sugarcane cultivar HSF-240. Pak. J. Bot. 2009, 41, 1645–1649. [Google Scholar]
- Dang, J.C.; Kumaria, S.; Kumar, S.; Tandon, P. Micropropagation of Ilex khasiana, a critically endangered and endemic holly of Northeast India. AoB Plants 2011, 2011. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci. Rep. 2019, 9, 9634. [Google Scholar]
- Markovic, M.; Grbic, M.; Djukic, M. Micropropagation of Endangered and Decorative Species Dianthus pinifolius Sibth. et Sm. Braz. Arch. Biol. Technol. 2016, 59, e16150320. [Google Scholar] [CrossRef][Green Version]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Manimekalai, R.; Nagarajan, P.; Kumaran, P. Comparison of effectiveness of RAPD, ISSR and SSR markers for analysis of coconut (Cocos nucifera L.) germplasm accessions. Trop. Agric. Res. 2006, 18, 217. [Google Scholar]
- Sornakili, A.; Rathinam, P.K.; Thiruvengadum, R.; Kuppusamy, P. Comparative assessment of RAPD and ISSR markers to study genetic polymorphism in Colletotrichum gloeosporioides isolates of mango. Asian J. Plant Pathol. 2017, 11, 130–138. [Google Scholar] [CrossRef]
- Rayar, J.K.; Arif, M.; Singh, U.S. Relative efficiency of RAPD and ISSR markers in assessment of DNA polymorphism and genetic diversity among Pseudomonas strains. Afr. J. Biotechnol. 2015, 14, 1097–1106. [Google Scholar]
- Fernandez, M.; Figueiras, A.; Benito, C. The use of ISSR and RAPD markers for detecting DNA polymorphism, genotype identification and genetic diversity among barley cultivars with known origin. Theor. Appl. Genet. 2002, 104, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Saharan, M.; Verma, A.; Sharma, I. Comparative analysis of RAPD and ISSR marker assays for detecting genetic polymorphism in Tilletia indica. Eur. J. Exp. Biol. 2013, 3, 380–387. [Google Scholar]
- Pathak, H.; Dhawan, V. ISSR assay for ascertaining genetic fidelity of micropropagated plants of apple rootstock Merton 793. In Vitro Cell. Dev. Biol. Plant 2012, 48, 137–143. [Google Scholar] [CrossRef]
- Muthusamy, S.; Kanagarajan, S.; Ponnusamy, S. Efficiency of RAPD and ISSR markers system in accessing genetic variation of rice bean (Vigna umbellata) landraces. Electron. J. Biotechnol. 2008, 11, 32–41. [Google Scholar] [CrossRef]
- Seth, S.; Panigrahi, J. In vitro organogenesis of Abutilon indicum (L.) Sweet from leaf derived callus and assessment of genetic fidelity using ISSR markers. J. Hortic. Sci. Biotechnol. 2019, 94, 70–79. [Google Scholar] [CrossRef]
- Dinara, S.M.; Elena, V.K.; Tatyana, I.N. Assessment of Genetic Fidelity of Fritillaria dagana (Liliaceae) Regenerated Plants Using ISSR Markers. In Proceedings of the BIO Web of Conferences, Novosibirsk, Russia, 8–12 October 2018; p. 00029. [Google Scholar]
- Babu, G.A.; Vinoth, A.; Ravindhran, R. Direct shoot regeneration and genetic fidelity analysis in finger millet using ISSR markers. Plant Cell Tissue Organ Cult. 2018, 132, 157–164. [Google Scholar] [CrossRef]
- Rohela, G.K.; Jogam, P.; Shabnam, A.A.; Shukla, P.; Abbagani, S.; Ghosh, M.K. In vitro regeneration and assessment of genetic fidelity of acclimated plantlets by using ISSR markers in PPR-1 (Morus sp.): An economically important plant. Sci. Hortic. 2018, 241, 313–321. [Google Scholar] [CrossRef]
- Mudoi, K.D.; Saikia, S.P.; Goswami, A.; Gogoi, A.; Bora, D.; Borthakur, M. Micropropagation of important bamboos: A review. Afr. J. Biotechnol. 2013, 12, 2770–2785. [Google Scholar]
- Zakaria, M.H. Review of Policies and Issues in the Malaysian Herbal Industry; FFTC Agricultural Policy Articles; Food and Fertilizer Technology Centre (FTTC): Taiper, Taiwan, 2015. [Google Scholar]
- Gonçalves, S.; Romano, A. Production of plant secondary metabolites by using biotechnological tools. In Secondary Metabolites: Sources and Applications; INTECH Open Access Publisher: London, UK, 2018; pp. 81–99. [Google Scholar]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
| MS + Hormone (mg L−1) | Number of Shoots per Explant | Multiplication Index | Number of Leaves per Explant | Number of Roots per Explant | Percentage (%) of Explant Producing Callus |
|---|---|---|---|---|---|
| Control | 2.67 ± 0.09 a | 1.67 ± 0.09 a | 4.21 ± 0.32 a | NR | NR |
| 0.5 Kin | 2.90 ± 0.11 a | 1.89 ± 0.11 b | 8.93 ± 0.47 b | 0.31 ± 0.10 a | 93.33 ± 0.05 b |
| 1.0 Kin | 3.77 ± 0.16 b | 2.77 ± 0.16 b | 11.70 ± 0.70 c | 1.03 ± 0.30 a | 40.00 ± 0.09 a |
| 1.5 Kin | 4.00 ± 0.32 b | 3.00 ± 0.32 b | 15.10 ± 0.15 d | 0.34 ± 0.12 a | 90.00 ± 0.06 b |
| 2.0 Kin | 3.90 ± 0.23 b | 2.90 ± 0.23 b | 13.00 ± 1.09 c | 0.23 ± 0.10 a | 96.67 ± 0.03 b |
| 0.5 IAA | 3.63 ± 0.09 b | 2.63 ± 0.09 b | 9.23 ± 0.49 b | 2.93 ± 0.64 b | 50.00 ± 0.09 a |
| 1.0 IAA | 2.62 ± 0.13 a | 1.62 ± 0.13 a | 8.69 ± 0.55 b | 4.00 ± 0.74 bc | 80.00 ± 0.07 b |
| 1.5 IAA | 2.55 ± 0.11 a | 1.55 ± 0.11 a | 9.28 ± 0.65 b | 3.31 ± 0.61 bc | 96.67 ± 0.03 b |
| 2.0 IAA | 2.63 ± 0.12 a | 1.63 ± 0.12 a | 7.77 ± 0.67 b | 4.60 ± 0.65 c | 96.67 ± 0.03 b |
| MS + Hormone (mg L−1) | Number of Shoots Per Explant | Multiplication Index | Number of Leaves per Explant | Number of Roots per Explant | Percentage (%) of Explant Producing Callus |
|---|---|---|---|---|---|
| Control | 2.67 ± 0.09 a | 1.67 ± 0.09 a | 4.21 ± 0.32 a | NR | NR |
| 0.5 Kin + 0.5 IAA | 2.93 ± 0.14 ab | 1.93 ± 0.14 ab | 10.20 ± 0.65 b | 0.73 ± 0.21 abcd | 100.00 ± 0.00 b |
| 0.5 Kin + 1.0 IAA | 3.23 ± 0.11 abcd | 2.23 ± 0.11 abcd | 12.70 ± 0.51 bcde | 1.60 ± 0.28 de | 93.33 ± 0.05 b |
| 0.5 Kin + 1.5 IAA | 3.13 ± 0.20 abc | 2.13 ± 0.19 abc | 11.20 ± 0.58 b | 1.07 ± 0.42 bcde | 80.00 ± 0.07 a |
| 0.5 Kin + 2.0 IAA | 2.87 ± 0.14 ab | 1.87 ± 0.14 ab | 12.11 ± 0.84 bcd | 1.93 ± 0.59 e | 96.67 ± 0.03 b |
| 1.0 Kin + 0.5 IAA | 3.77 ± 0.16 ab | 2.77 ± 0.16 cdefg | 11.30 ± 1.01 bc | 1.80 ± 0.60 cde | 93.33 ± 0.05 b |
| 1.0 Kin + 1.0 IAA | 4.53 ± 0.25 cdefg | 3.53 ± 0.25 g | 12.03 ± 0.86 bcd | 1.47 ± 0.48 de | 96.67± 0.03 b |
| 1.0 Kin + 1.5 IAA | 4.10 ± 0.16 efg | 3.10 ± 0.16 efg | 10.52 ± 0.60 b | 0.10 ± 0.07 a | 100.00 ± 0.00 b |
| 1.0 Kin + 2.0 IAA | 4.43 ± 0.17 g | 3.43 ± 0.18 g | 11.60 ± 0.91 b | 0.43 ± 0.18 abc | 96.67 ± 0.03 b |
| 1.5 Kin + 0.5 IAA | 4.23 ± 0.28 fg | 3.23 ± 0.28 fg | 15.80 ± 0.89 ef | 0.07 ± 0.05 a | 70.00 ± 0.09 a |
| 1.5 Kin + 1.0 IAA | 3.93 ± 0.29 defg | 2.93 ± 0.29 defg | 15.53 ± 1.29 ef | 0.27 ± 0.14 abc | 100.00 ± 0.00 b |
| 1.5 Kin + 1.5 IAA | 3.40 ± 0.17 abcde | 2.40 ± 0.17 abcde | 12.11 ± 0.87 bcd | 0.15 ± 0.08 ab | 100.00 ± 0.00 b |
| 1.5 Kin + 2.0 IAA | 4.20 ± 0.26 fg | 3.20 ± 0.26 fg | 15.23 ± 1.12 def | 0.70 ± 0.23 abcd | 93.33 ± 0.05 b |
| 2.0 Kin + 0.5 IAA | 5.57 ± 0.42 h | 4.57 ± 0.42 h | 20.53 ± 1.91 g | 0.73 ± 0.27 abcd | 93.33 ± 0.05 b |
| 2.0 Kin + 1.0 IAA | 3.60 ± 0.24 bcdef | 2.60 ± 0.24 bcdef | 16.83 ± 1.21 f | 1.69 ± 0.29 de | 100.00 ± 0.00 b |
| 2.0 Kin + 1.5 IAA | 4.20 ± 0.36 fg | 3.20 ± 0.36 fg | 14.50 ± 1.42 cdef | 0.27 ± 0.15 abc | 96.67 ± 0.03 b |
| 2.0 Kin + 2.0 IAA | 4.40 ± 0.27 g | 3.40 ± 0.27 g | 15.07 ± 1.08 def | 0.83 ± 0.30 abcd | 96.67 ± 0.03 b |
| Sample ID | Potting Mixture | Survival % after 30 Days | Survival % after 60 Days | Survival % after 90 Days |
|---|---|---|---|---|
| PA | Black soil | 66.67 ± 12.60 ab | 40.00 ± 13.09 a | 26.67 ± 11.82 a |
| PB | Red soil | 57.14 ± 12.78 a | 57.14 ± 12.78 ab | 57.14 ± 12.78 b |
| PC | Black soil + red soil | 78.57 ± 10.60 abc | 64.33 ± 12.37 abc | 64.33 ± 12.37 bc |
| PD | Black soil + compost | 100.00 ± 0.00 c | 92.31 ± 6.63 cd | 92.31 ± 6.63 cd |
| PE | Red soil + compost | 86.67 ± 9.09 bc | 86.67 ± 9.09 bcd | 80.00 ± 10.69 bcd |
| PF | Black soil + red soil + compost | 100.00 ± 0.00 c | 100.00 ± 0.00 d | 100.00 ± 0.00 d |
| Primer Code(UBC) | Sequence 5′–3′ | Annealing Temperature (°C) | Total Number of Bands Amplified | Number of Scorable Bands per Primer | No. and Frequency of Polymorphic Bands per Primer | Range of Amplification (bp) |
|---|---|---|---|---|---|---|
| UBC807 | (AG)8T | 46.5 | 68 | 8 | 0 | 500–2000 |
| UBC829 | (TG)8C | 52.5 | 80 | 8 | 0 | 600–1300 |
| UBC835 | (AG)8YC | 50.0 | 59 | 7 | 2 (28.57%) | 550–2500 |
| UBC836 | (AG)8YA | 48.0 | 68 | 11 | 1 (9.90%) | 400–2300 |
| UBC840 | (GA)8YT | 46.5 | 49 | 6 | 0 | 300–1300 |
| UBC841 | (GA)8YC | 52.0 | 36 | 5 | 2 (40.00%) | 400–2500 |
| UBC845 | (CT)8RG | 47.5 | 71 | 13 | 1 (7.69%) | 400–1800 |
| UBC854 | (TC)8RG | 50.0 | 40 | 10 | 0 | 500–1800 |
| UBC855 | (AC)8YT | 53.0 | 100 | 10 | 0 | 400–2000 |
| UBC856 | (AC)8YA | 54.0 | 39 | 4 | 0 | 700–1500 |
| Total | 610 | 82 | 6 (7.32%) |
| OS1 | OS2 | OS3 | OS4 | OS5 | OS6 | OS7 | OS8 | OS9 | OS10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| OS2 | 0.110625 | |||||||||
| OS3 | 0.077377 | 0.110625 | ||||||||
| OS4 | 0.021594 | 0.110625 | 0.077377 | |||||||
| OS5 | 0.021594 | 0.110625 | 0.077377 | 0.021594 | ||||||
| OS6 | 0.077377 | 0.110625 | 0.077377 | 0.077377 | 0.077377 | |||||
| OS7 | 0.077377 | 0.110625 | 0.077377 | 0.077377 | 0.077377 | 0.077377 | ||||
| OS8 | 0.077377 | 0.110625 | 0.077377 | 0.077377 | 0.077377 | 0.077377 | 0.000000 | |||
| OS9 | 0.077377 | 0.110625 | 0.077377 | 0.077377 | 0.077377 | 0.077377 | 0.077377 | 0.077377 | ||
| OS10 | 0.077377 | 0.110625 | 0.077377 | 0.077377 | 0.077377 | 0.077377 | 0.000000 | 0.000000 | 0.077377 | |
| Mp | 0.124336 | 0.124336 | 0.124336 | 0.124336 | 0.124336 | 0.124336 | 0.124336 | 0.124336 | 0.124336 | 0.124336 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.; Musa, I.F.; Abu Bakar, N.A.; Karsani, S.A.; Yaacob, J.S. In Vitro Regeneration and ISSR-Based Genetic Fidelity Analysis of Orthosiphon stamineus Benth. Agronomy 2019, 9, 778. https://doi.org/10.3390/agronomy9120778
Ali H, Musa IF, Abu Bakar NA, Karsani SA, Yaacob JS. In Vitro Regeneration and ISSR-Based Genetic Fidelity Analysis of Orthosiphon stamineus Benth. Agronomy. 2019; 9(12):778. https://doi.org/10.3390/agronomy9120778
Chicago/Turabian StyleAli, Hanisah, Izzah Farhanah Musa, Nurul Atikhah Abu Bakar, Saiful Anuar Karsani, and Jamilah Syafawati Yaacob. 2019. "In Vitro Regeneration and ISSR-Based Genetic Fidelity Analysis of Orthosiphon stamineus Benth" Agronomy 9, no. 12: 778. https://doi.org/10.3390/agronomy9120778
APA StyleAli, H., Musa, I. F., Abu Bakar, N. A., Karsani, S. A., & Yaacob, J. S. (2019). In Vitro Regeneration and ISSR-Based Genetic Fidelity Analysis of Orthosiphon stamineus Benth. Agronomy, 9(12), 778. https://doi.org/10.3390/agronomy9120778





