Growth, Evapotranspiration, and Ion Uptake Characteristics of Alfalfa and Triticale Irrigated with Brackish Groundwater and Desalination Concentrate
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
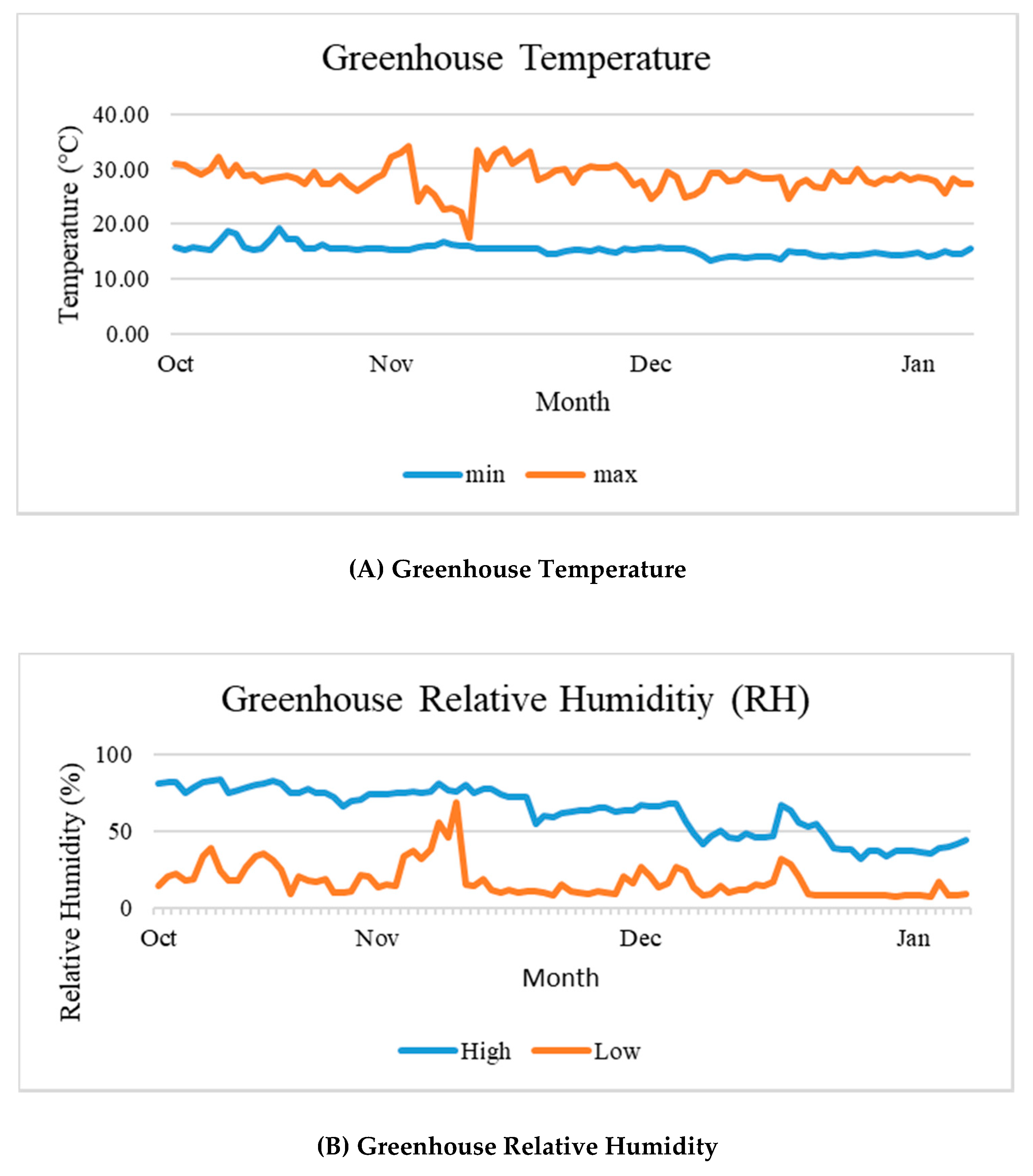
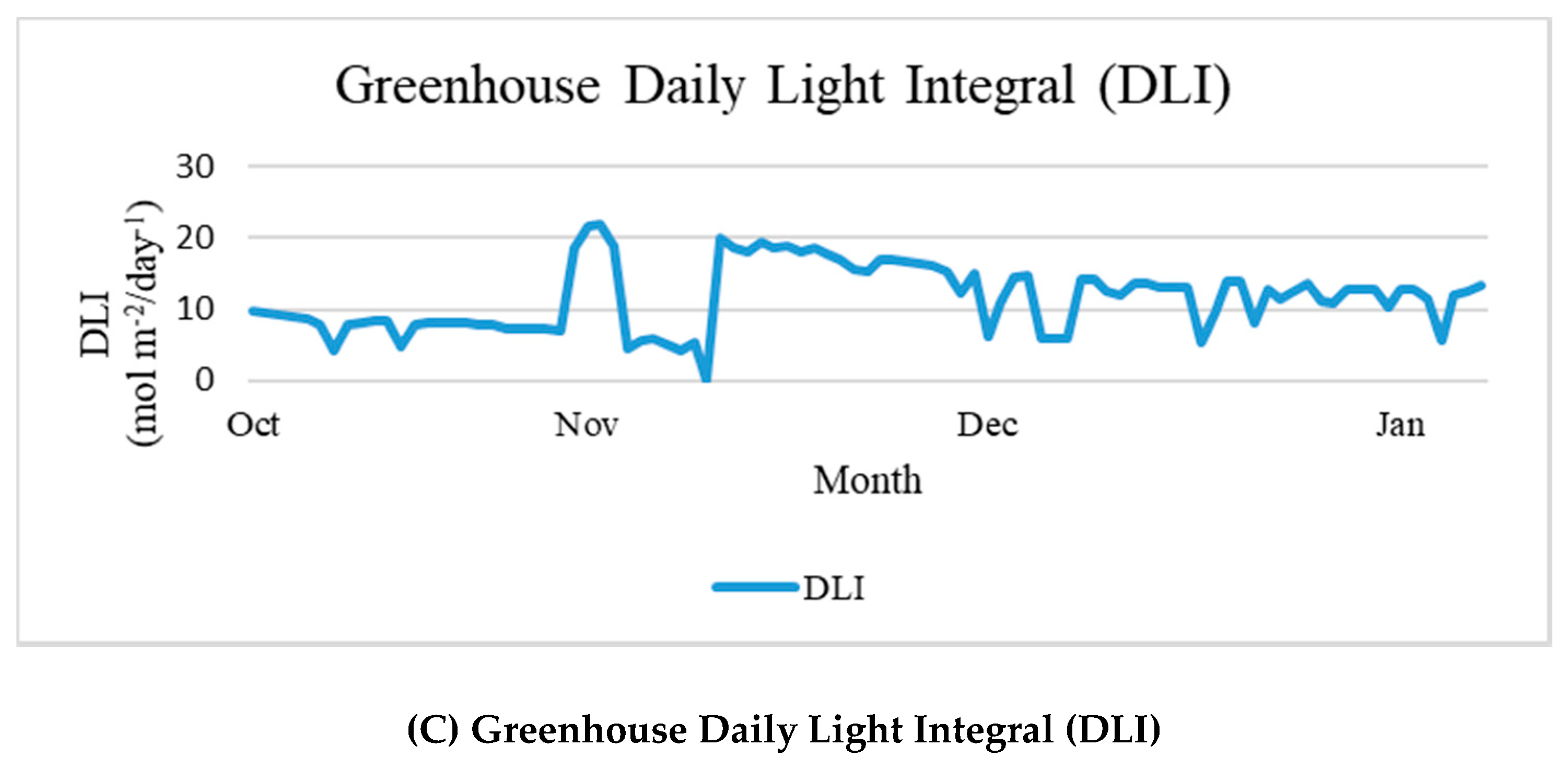
3.1. Greenhouse Meteorological Data
3.2. Evapotranspiration and Deep Percolation
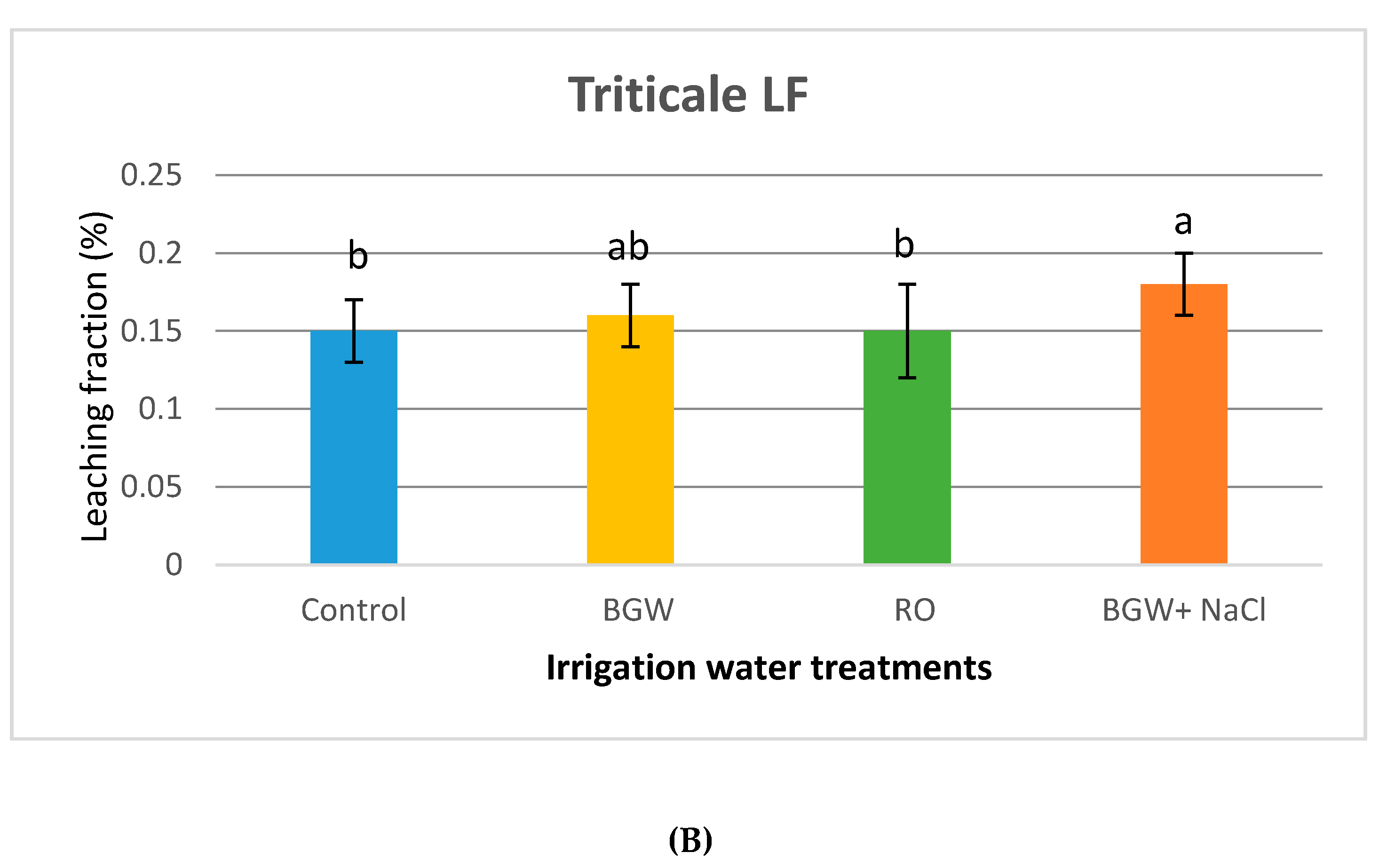
3.3. Volumetric Leaching Fractions
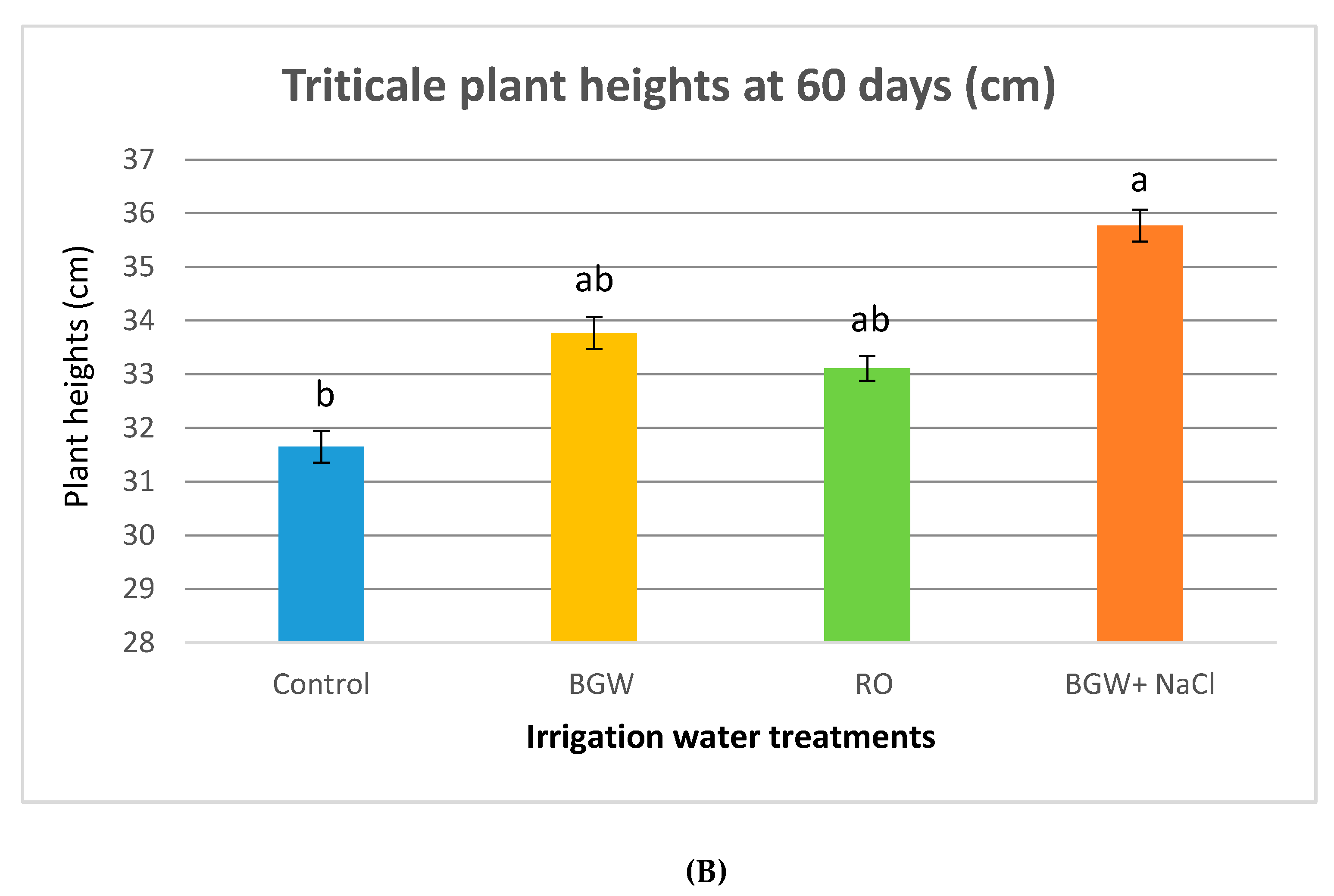
3.4. Plant Heights
3.5. Fresh and Dry Aboveground Biomass
3.6. Shoot Ion Concentration
3.7. Soil Ion Concentrations
3.8. Leachate Ion Concentrations
4. Discussion
4.1. Water Balance Parameters
4.2. Plant Heights and Biomass
4.3. Shoot Ion Content
4.4. Soil and Leachate Ion Content
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwabe, K.; Albiac, J.; Connor, J.D.; Hassan, R.; Meza-Gonzalez, L. Introduction. In Drought in Arid and Semi-Arid Regions; Schwabe, K., Albiac, J., Connor, J.D., Hassan, R., Meza-Gonzalez, L., Eds.; Springer: New York, NY, USA, 2013; pp. 1–21. [Google Scholar]
- Szabolcs, I. Soils and salinization. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 3–11. [Google Scholar]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Ann. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Lansford, R.; Hernandez, J.; Enis, P.; Truby, D.; Mapel, C. Evaluation of Available Saline Water Resources In New Mexico for the Production of Microalgae; Solar Energy Research Institute: Golden, CO, USA, 1990. [Google Scholar]
- Water Resources Research Institute (WRRI). Transboundary Aquifers of the El/Ciudad Juarez/Las Cruces Region, VI; Texas Water Development Board and New Mexico Water Resources Research Institute; U.S. Environmental Protection Agency: Region, CA, USA, 1997.
- Stanton, J.S.; Anning, D.W.; Brown, C.J.; Moore, R.B.; McGuire, V.L.; Qi, S.L.; Böhlke, J.K. Brackish Groundwater in the United States; US Geological Survey: Reston, VA, USA, 2017; p. 1833. [Google Scholar] [CrossRef]
- New Mexico Bureau of Geology and Mineral Resources. Brackish and Saline Groundwater in New Mexico. Earth Matters 2015, 15, 1–6. [Google Scholar]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Babcock, M.; Shukla, M.K.; Picchioni, G.; Mexal, J.; Daniel, D. Chemical and physical properties of Chihuahuan desert soils irrigated with industrial effluent. Arid Land Res. Manag. 2009, 23, 47–66. [Google Scholar] [CrossRef]
- Flores, A.M.; Shukla, M.K.; Daniel, D.; Ulery, A.L.; Schutte, B.J.; Picchioni, G.A.; Fernald, S. Evapotranspiration changes with irrigation using saline groundwater and RO concentrate. J. Arid Environ. 2016, 131, 35–45. [Google Scholar] [CrossRef]
- Ries, R.E.; Hofmann, L. Effect of sodium and magnesium sulfate on forage seed germination. J. Range Manag. 1983, 36, 658–662. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Flowers, T.J.; Läuchli, A. Sodium versus potassium: Substitution and compartmentation. In Encyclopedia of Plant Physiology. Inorganic Plant Nutrition; Läuchli, A., Bieleski, R.C., Eds.; New Series; Springer: New York, NY, USA, 1983; Volume 15B, pp. 651–681. [Google Scholar]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A functional plant nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Shukla, M.K. Soil Physics, an Introduction; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Khan, M.J.; Edward, P. Glenn Yield and evapotranspiration of two barley varieties as affected by sodium chloride salinity and leaching fraction in lysimeter tanks. Commun. Soil Sci. Plant Anal. 1996, 27, 157–177. [Google Scholar] [CrossRef]
- Li, C.; Lei, J.; Zhao, Y.; Xu, X.; Li, S. Effect of saline water irrigation on soil development and plant growth in the Taklimakan Desert Highway shelterbelt. Soil Tillage Res. 2015, 146, 99–107. [Google Scholar] [CrossRef]
- Malek, E.; Bingham, G.E.; McCurdy, G.D.; Hanks, R.J. Determination of evapotranspiration from an alfalfa crop irrigated with saline waste water from an electrical power plant. Irrig. Sci. 1992, 13, 73–80. [Google Scholar] [CrossRef]
- Ozturk, O.F.; Shukla, M.K.; Stringam, B.; Picchioni, G.A.; Gard, C. Irrigation with brackish water changes evapotranspiration, growth and ion uptake of halophytes. Agric. Water Manag. 2018, 195, 142–153. [Google Scholar] [CrossRef]
- Salehi, M.; Arzani, A. Evaluation of triticale genotypes for salt tolerance using physiological traits. Emir. J. Food Agric. 2014, 26, 277–283. [Google Scholar] [CrossRef]
- Cachorro, P.; Ortiz, A.; Cerda, A. Implications of calcium nutrition on the response of Phaseolus vulgaris L. to salinity. Plant Soil 1994, 159, 205–221. [Google Scholar] [CrossRef]
- USDA-ARS. Roadmap for Alfalfa Research. 2014. Available online: http://ars.usda.gov/SP2UserFiles/Place/54281000/alfalfaroadmap2.pdf (accessed on 18 November 2018).
- Hanks, R.J.; Mace, W.R.; Schick, J.H. Manual for Using Model PLSAPIST–A Simple Model for Estimation Water and Salt Flow Assuming Piston Flow of Water and Salt; Dept. of Plants, Soils and Biometeorology, Utah State University: Logan, UT, USA, 1990. [Google Scholar]
- Ferreira, J.F.S.; Cornacchione, M.V.; Liu, X.; Suarez, D.L. Nutrient Composition, Forage Parameters, and Antioxidant Capacity of Alfalfa (Medicago sativa L.) in Response to Saline Irrigation Water. Agriculture 2015, 5, 577–597. [Google Scholar] [CrossRef]
- Mergoum, M.; Singh, P.; Pena, R.; Lozano-del Río, A.; Cooper, K.; Salmon, D.; Macpherson, H.G. Triticale: A “new” crop with old challenges. In Cereals; Springer: New York, NY, USA, 2009. [Google Scholar]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Pfeiffer, W.H. Triticale: Potential and Research Status of a Manmade Cereal Crop. In Background Material for the Germplasm Improvement Subprogram External Review; Wheat Program; CIMMYT: Ciudad Obregón, Sonora, Mexico, 1994; pp. 82–92. [Google Scholar]
- Hinojosa, M.B.; Hede, A.; Rajaram, S.; del Río, J.L.; Gonzalez, A.V. Triticale: An Alternative Forage Crop under Rainfed Conditions in Chihuahua, México. In Proceedings of the 5th International Triticale Symp, Radzikow, Poland, 30 June–5 July 2002; Plant Breeding and Acclimatization Institute: Radzikow, Poland, 2002; pp. 22–29. [Google Scholar]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Chapter 13 Plant salt tolerance. Agric. Ultural Salin. Assess. Manag. 2012, 71, 405–459. [Google Scholar]
- Fisher, R.A.; Maurer, R. Drought resistance in spring wheat cultivars. I. Grain yield responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Fisher, R.A.; Sanchez, M. Drought resistance in spring wheat cultivars. 2. Effects on plant water relations. Aust. J. Agric. Res. 1979, 30, 801–814. [Google Scholar]
- Sutton, B.G.; Dubblede, E.A. Effects of water deficits on yield of wheat and triticale. Aust. J. Exp. Agric. 1980, 20, 594–598. [Google Scholar] [CrossRef]
- Masters, D.G.; Benes, S.E.; Norman, H.C. Biosaline agriculture for forage and livestock production. Agric. Ecosyst. Environ. 2007, 119, 234–248. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Experiment Stn. Circ. 1950, 347, 1–32. [Google Scholar] [CrossRef]
- Ayers, R.S.; Wescot, D.W. Water Quality for Agriculture. 1985. Available online: http://www.fao.org/docrep/003/t0234e/T0234E02.htm (accessed on 11 August 2019).
- Katerji, N.; Mastrorilli, M.; van Hoorn, J.W.; Lahmer, F.Z.; Hamdy, A.; Oweis, T. Durum wheat and barley productivity in saline drought environments. Eur. J. Agron. 2009, 31, 1–9. [Google Scholar] [CrossRef]
- Beltran, J.M. Irrigation with saline water: Benefits and environmental impact. Agric. Water Manag. 1999, 40, 183–194. [Google Scholar] [CrossRef]
- Shabala, S.; Hariadi, Y.; Jacobsen, S. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na + loading and stomatal density Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na + loading and stomatal density. J. Plant Physiol. 2013, 170, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Shalaby, E.E.; Epstein, E.; Qualset, C.O. Variation in salt tolerance among some wheat and triticale genotypes. J. Agron. Crop Sci. 1993, 171, 298–304. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, S.; Zheng, H.; Chi, L.; He, X. Abnormal relationship between rust particles size and rust layer compactness of weathering steels. Acta Metall. Sin. Engl. Lett. 2010, 23, 57–62. [Google Scholar] [CrossRef]
- Slama, F. Effect of ammonium nitrate on the degree of tolerance to a strong NaCl amount of ten varieties of wheat. In Regional NaCl Seminar of Ten Varieties of Wheat. Regional Seminar on Market Gardenings; Staples, R.C., Toenniessen, G.H., Eds.; Tunisia, 1987; pp. 206–207. [Google Scholar]
- Hasegawa, P.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Ann. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance (June). Ann. Rev. Plant Biol. 2008, 59, 651–668. [Google Scholar] [CrossRef]
- Davenport, R.J.; Tester, M. A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol. 2000, 122, 823–834. [Google Scholar] [CrossRef]
- Flores, A.M.; Shukla, M.K.; Schutte, B.J.; Picchioni, G.; Daniel, D. Physiologic response of six plant species grown in two contrasting soils and irrigated with 399 brackish groundwater and RO concentrate. J. Arid Land Res. Manag. 2017, 31, 182–203. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives 414, 2nd ed.; Sinanuer Associates: Sunderland, MA, USA, 2005. [Google Scholar]
- Flowers, T.J.; Yeo, A.R. Ion relations of salt tolerance. In Solute 441 Transport in Plant Cells and Tissues; Baker, D., Halls, J., Eds.; Longman: Harlow, UK, 1988; pp. 392–416. [Google Scholar]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the 598 plant: A review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef] [Green Version]
- McNeal, B.L.; Layfield, D.A.; Norvell, W.A.; Rhoades, J.D. Factors influencing 508 hydraulic conductivity of soils in the presence of mixed salt solutions. Soil Sci. Soc. Am. J. 1968, 32, 187–190. [Google Scholar] [CrossRef]






| Ion Concentration | ||||||||
|---|---|---|---|---|---|---|---|---|
| meq L−1 | mg L−1 | |||||||
| Treatment | Na+ | Ca+ | Mg2+ | K+ | Cl− | SAR | EC (dSm−1) | pH |
| Control (tap water) | 2.53 | 2.59 | 0.79 | 5.33 | 57.2 | 1.95 | 0.7 | 7.3 |
| BGW | 15.87 | 20.4 | 16.54 | 6.74 | 697.7 | 3.69 | 4 | 7.4 |
| RO (Ca+ dominant) | 30.09 | 34.88 | 30.12 | 14.0 | 892.7 | 5.28 | 8 | 7.4 |
| BGW + NaCl (Na+ dominant) | 50.11 | 18.78 | 15.98 | - | - | 12.02 | 8 | 7.4 |
| Shoot Ion Concentrations (% of Dry Wt) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alfalfa (%) | Triticale (%) | |||||||
| Salinity | Na+ | Ca2+ | Mg2+ | Cl− | Na+ | Ca2+ | Mg2+ | Cl− |
| Control | 0.08 ± 0.009(d) | 0.53 ± 0.06(c) | 0.20 ± 0.05(c) | 0.42 ± 0.03(c) | 0.1 ± 0.01(c) | 0.58 ± 0.01(c) | 0.23 ± 0.00(c) | 1.75 ± 0.03(c) |
| BGW | 0.58 ± 0.003(c) | 0.81 ± 0.03(b) | 0.60 ± 0.04(b) | 0.17 ± 0.10(d) | 0.36 ± 0.03(c) | 0.67 ± 0.009(b) | 0.49 ± 0.01(b) | 2.16 ± 0.05(cb) |
| RO | 0.66 ± 0.02(b) | 1.85 ± 0.04(a) | 0.86 ± 0.04(a) | 1.39 ± 0.05(b) | 0.62 ± 0.10 (b) | 0.57 ± 0.02(c) | 0.59 ± 0.14(a) | 2.32 ± 0.19(b) |
| BGW + NaCl | 1.06 ± 0.03(a) | 1.81 ± 0.05(a) | 0.64 ± 0.02(b) | 2.60 ± 0.10(a) | 1.22 ± 0.03(a) | 0.82 ± 0.03(a) | 0.55 ± 0.03(ab) | 3.66 ± 0.19(a) |
| Soil Ion Concentrations (meq L−1) | Soil Ion Concentrations (meq L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alfalfa | Triticale | |||||||
| Salinity | Na+ | Ca2+ | Mg2+ | Cl− (%) | Na+ | Ca2+ | Mg2+ | Cl− (%) |
| Control | 1.17 ± 0.03(d) | 90.80 ± 0.72(a) | 5.57 ± 0.58(c) | 0.02 ± 0.00(a) | 1.67 ± 0.15(d) | 89.43 ± 0.72(a) | 6.33 ± 0.43(b) | 0.02 ± 0.00(c) |
| BGW | 3.53 ± 0.19(c) | 82.90 ± 0.78(b) | 11.83 ± 0.59(b) | 0.02 ± 0.02(a) | 3.90 ± 0.20(c) | 81.2 ± 0.61(b) | 12.97 ± 0.46(a) | 0.03 ± 0.03(b) |
| RO | 6.57 ± 0.30(b) | 76.47 ± 1.07(c) | 15.23 ± 0.80(b) | 0.02 ± 0.02(a) | 5.67 ± 0.32(b) | 80.50 ± 0.60(b) | 12.57 ± 0.26(a) | 0.04 ± 0.02(b) |
| BGW + NaCl | 9.43 ± 0.49(a) | 75.87 ± 0.62(c) | 12.90 ± 0.12(a) | 0.05 ± 0.02(a) | 11.43 ± 0.96(a) | 73.90 ± 1.99(c) | 12.83 ± 0.90(b) | 0.11 ± 0.05(a) |
| Leachate Ion Concentrations (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alfalfa | Triticale | |||||||
| Salinity | Na+ | Ca2+ | Mg2+ | Cl− | Na+ | Ca2+ | Mg2+ | Cl− |
| Control | 299.61 ± 61.18(d) | 100.01 ± 43.30(c) | 26.45 ± 5.39(c) | 249.67 ± 84.77(c) | 507.29 ± 83.29(d) | 243.80 ± 27.32(c) | 39.86 ± 5.75 (c) | 511.33 ± 101.58(c) |
| BGW | 2726.09 ± 457.40(c) | 1033.37 ± 107.68(b) | 891.18 ± 79.67(b) | 4745.00 ± 505.00(b) | 2337.67 ± 219.59(c) | 967.92 ± 10.66(b) | 953.32 ± 96.7(b) | 4030.00 ± 290.00(b) |
| RO | 4737.43 ± 265.93(b) | 974.50 ± 19.58(b) | 1683.1 ± 132.94(a) | 5470 ± 166.43(b) | -- | -- | -- | -- |
| BGW + NaCl | 6251.06 ± 592.806(a) | 1264.76 ± 119.78(a) | 963.937 ± 86.57(b) | 7696.67 ± 522.632(a) | 10248.03 ± 1047.79(a) | 1054.14 ± 38.56(a) | 2115.44 ± 273.13(b) | 10000.00 ± 0.00(a) |
| Total Salt Applied (mg) | Total Salt in Leachate (mg) | Total Salt in Shoots (mg) | Total Salt in Soil (mg) | |||||
|---|---|---|---|---|---|---|---|---|
| Salinity | Na+ | Ca2+ | Na+ | Ca2+ | Na | Ca2+ | Na+ | Ca2+ |
| Alfalfa | ||||||||
| Control | 465.5 | 414.4 | 299.6 | 100.0 | 11.5 | 7.6 | 85.7 | 5785.8 |
| BGW | 2920.1 | 3264.0 | 2726.1 | 1033.4 | 90.3 | 12.6 | 258.7 | 5282.4 |
| RO | 5536.6 | 5580.8 | 4737.4 | 974.5 | 98.6 | 276.4 | 481.4 | 4872.7 |
| BGW + NaCl | 9220.2 | 3004.8 | 6251.1 | 1264.8 | 154.8 | 264.3 | 691.0 | 4834.4 |
| Triticale | ||||||||
| Control | 605.18 | 538.72 | 507.29 | 243.80 | 18.36 | 107.77 | 122.37 | 5698.48 |
| BGW | 3796.10 | 4243.20 | 2337.67 | 967.92 | 68.76 | 27.97 | 285.78 | 5174.06 |
| RO | 7197.53 | 7255.04 | 0.00 | 0.00 | 134.91 | 124.03 | 415.49 | 5129.46 |
| BGW + NaCl | 11986.31 | 3906.24 | 10248.03 | 1054.14 | 268.28 | 180.32 | 837.57 | 4708.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kankarla, V.; Shukla, M.K.; VanLeeuwen, D.; Schutte, B.J.; Picchioni, G.A. Growth, Evapotranspiration, and Ion Uptake Characteristics of Alfalfa and Triticale Irrigated with Brackish Groundwater and Desalination Concentrate. Agronomy 2019, 9, 789. https://doi.org/10.3390/agronomy9120789
Kankarla V, Shukla MK, VanLeeuwen D, Schutte BJ, Picchioni GA. Growth, Evapotranspiration, and Ion Uptake Characteristics of Alfalfa and Triticale Irrigated with Brackish Groundwater and Desalination Concentrate. Agronomy. 2019; 9(12):789. https://doi.org/10.3390/agronomy9120789
Chicago/Turabian StyleKankarla, Vanaja, Manoj K. Shukla, Dawn VanLeeuwen, Brian J. Schutte, and Geno A. Picchioni. 2019. "Growth, Evapotranspiration, and Ion Uptake Characteristics of Alfalfa and Triticale Irrigated with Brackish Groundwater and Desalination Concentrate" Agronomy 9, no. 12: 789. https://doi.org/10.3390/agronomy9120789





