Factors Underlying Seed Yield in Red Clover: Review of Current Knowledge and Perspectives
Abstract
:1. Introduction
2. Reproductive Characteristics of Red Clover and Implications for Breeding
3. Steps Undertaken to Increase Seed Yield in Red Clover
3.1. Improvement of Agricultural Practices
3.2. Breeding for Higher Seed Yield
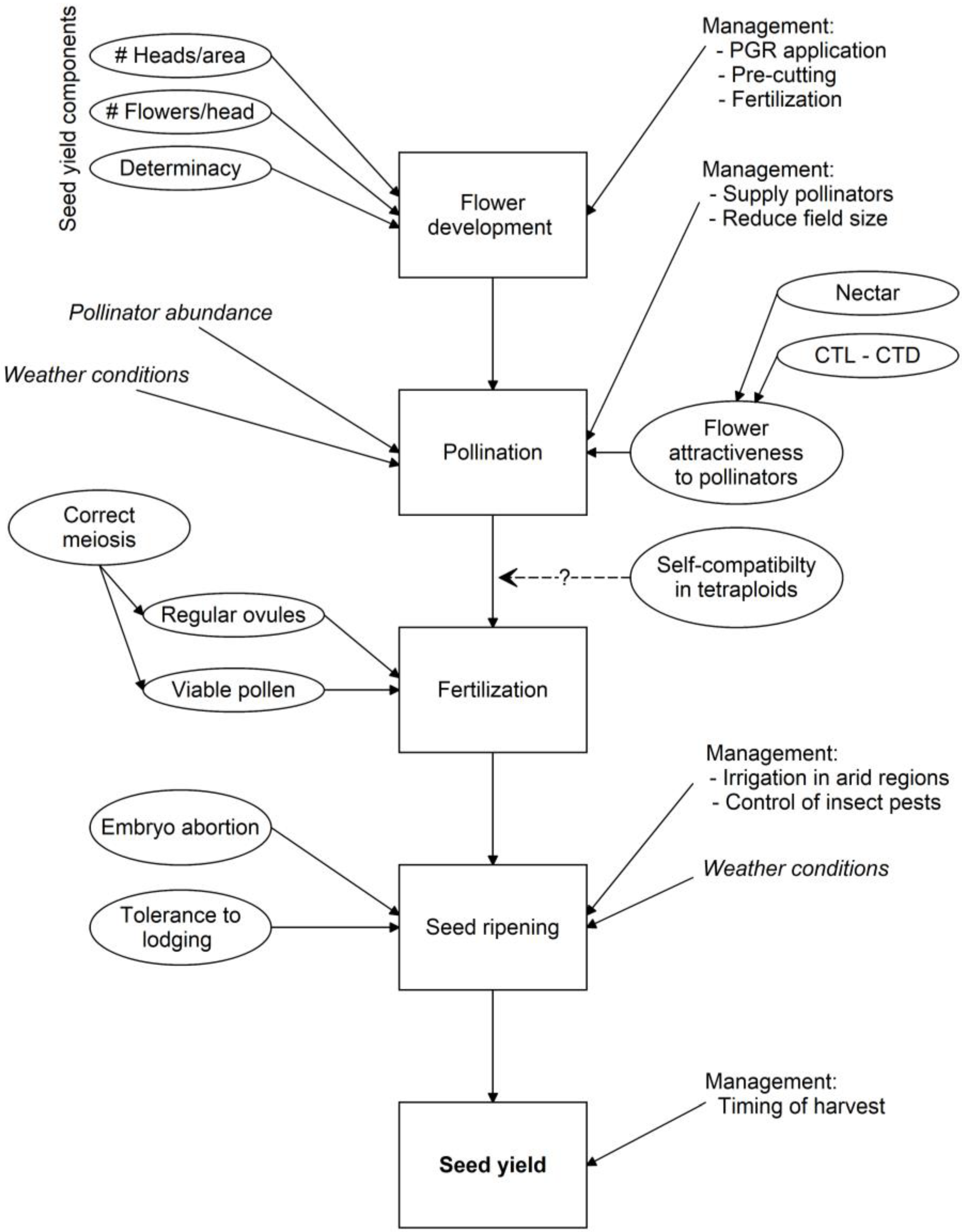
4. Factors Explaining Seed Yield in Red Clover
4.1. Seed Yield Components
4.2. Inadequate Pollination
4.3. Fertility Problems
4.4. Genomic Regions Associated with Seed Development and Seed Yield
4.5. Implications for Breeding
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CTL | corolla tube length |
| CTD | corolla tube diameter |
| HN | head number per plant at seed harvest |
| SN/H | seed number per head at seed harvest |
| PGR | plant growth regulator |
| QTL | Quantitative trait locus |
References
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and challenges in improving temperate perennial forage legumes. Crit. Rev. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Stockdale, E.A.; Lampkin, N.H.; Hovi, M.; Keatinge, R.; Lennartsson, E.K.M.; Macdonald, D.W.; Padel, S.; Tattersall, F.H.; Wolfe, M.S.; Watson, C.A. Agronomic and environmental implications of organic farming systems. Adv. Agron. 2003, 70, 261–327. [Google Scholar]
- Taylor, N.L.; Quesenberry, K.H. Red Clover Science; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- DeWhurst, R.J.; Delaby, L.; Moloney, A.L.; Boland, T.; Lewis, E. Nutritive value of forage legumes used for grazing and silage. Ir. J. Agric. Food Res. 2009, 48, 167–187. [Google Scholar]
- Huss-Danell, K.; Chaia, E.; Carlsson, G. N2 fixation and nitrogen allocation to above and below ground plant parts in red clover-grasslands. Plant Soil 2007, 299, 215–226. [Google Scholar] [CrossRef]
- Boller, B.; Schubiger, F.X.; Kölliker, R. Red clover. In Handbook of Plant Breeding; Boller, B., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 439–455. [Google Scholar]
- Sjödin, J.; Ellerström, S. Autopolyploid forage crops. In Research and Results in Plant Breeding; Olsson, G., Ed.; Svalöf: Stockholm, Sweden, 1986; pp. 102–113. [Google Scholar]
- Meglic, V.; Smith, R.R. Self-incompatibility and seed set in colchicine-, nitrous oxide-, and sexually derived tetraploid red clover. Crop Sci. 1992, 32, 1133–1137. [Google Scholar] [CrossRef]
- Amdahl, H.; Aamlid, T.S.; Ergon, A.; Kovi, M.R.; Marum, P.; Alsheikh, M.; Rognli, O.A. Seed yield of Norwegian and Swedish tetraploid red clover (Trifolium pratense L.) populations. Crop Sci. 2016, 56, 603–612. [Google Scholar] [CrossRef]
- Hejduk, S.; Knot, P. Effect of provenance and ploidity of red clover varieties on productivity, persistence and growth pattern in mixture with grasses. Plant Soil Environ. 2010, 56, 111–119. [Google Scholar] [CrossRef]
- Boelt, B.; Julier, B.; Karagic, D.; Hampton, J. Legume seed production meeting market requirements and economic impacts. Crit. Rev. Plant Sci. 2015, 34, 412–427. [Google Scholar] [CrossRef]
- Jing, S. Pollination and Seed Setting of Diploid and Tetraploid Red Clover (Trifolium pratense L.). Master’s Thesis, Aarhus University, Aarhus, Denmark, 2017. [Google Scholar]
- Karagic, D.; Jevtic, G.; Terzic, D. Forage legumes seed production in Serbia. Biotechnol. Anim. Husb. 2010, 26, 133–148. [Google Scholar]
- Anderson, N.P.; Garbaçik, C.J.; Chastain, T.G.; Elias, S. Boron effects on red clover seed production and quality. Seed Prod. Res. 2019, 9, 9–11. [Google Scholar]
- Petrauskas, G.; Mikaliuniene, J.; Norkeviciene, E.; Statkeviciute, G.; Kemesyte, V. Breeding for improved seed yield of red clover. In Breeding Grasses and Protein Crops in the Era of Genomics, Proceedings of the Joint Meeting of EUCARPIA Fodder Crops and Amenity Grasses Section and Protein Crops Working Group of Oil and Protein Crops Section, Vilnius, Lithuania, 11–14 September 2017; Brazauskas, G., Statkeviciute, G., Jonaviciene, G., Eds.; Springer: Cham, Switzerland, 2018; pp. 96–100. [Google Scholar]
- Brodsgaard, C.J.; Hansen, H. Pollination of red clover in Denmark. DIAS Rep. 2002, 71, 1–50. [Google Scholar]
- Eckhart, T.; (Saatzucht Steinach, Steinach, Germany); Hartmann, S.; (Bavarian State Research Center for Agriculture, Freising, Germany). Personal communication, 2019.
- Hejduk, S.; (Mendel University, Brno, Czech Republic). Personal communication, 2019.
- Wermuth, K.H.; Dupont, Y.L. Effects of field characteristics on abundance of bumblebees (Bombus spp.) and seed yield in red clover fields. Apidologie 2010, 41, 657–666. [Google Scholar] [CrossRef]
- Bommarco, R.; Lundin, O.; Smith, H.G.; Rundlöf, M. Drastic historic shifts in bumble-bee community composition in Sweden. Proc. R. Soc. B 2012, 279, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Julén, G. Rotklee, Trifolium pratense L. In Handbuch der Pflanzenzüchtung; Julén, G., Ed.; Paul Parey Publishing: Berlin, Germany, 1959; pp. 239–305. [Google Scholar]
- Lawrence, M.J. Number of incompatibility alleles in clover and other species. Heredity 1996, 76, 610–615. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Boller, B.; Brummer, E.C.; Reheul, D. Improving the focus of forage breeding research. In Breeding in a World of Scarcity; Roldán-Ruiz, I., Baert, J., Reheul, D., Eds.; Springer: Cham, Switzerland, 2016; pp. 251–270. [Google Scholar]
- Free, J.B. Leguminosae: Trifolium. In Insect Pollination of Crops, 2nd ed.; Academic Press: London, UK, 1993; pp. 271–297. [Google Scholar]
- McGregor, S.E. Clover and some relatives. In Insect Pollination of Cultivated Crop Plants; McGregor, S.E., Ed.; USDA-ARS: Washington, DC, USA, 1976; pp. 162–235. [Google Scholar]
- Vleugels, T.; Ceuppens, B.; Cnops, G.; Lootens, P.; van Parijs, F.R.D.; Smagghe, G.; Roldán-Ruiz, I. Models with only two predictor variables can accurately predict seed yield in diploid and tetraploid red clover. Euphytica 2016, 209, 507–523. [Google Scholar] [CrossRef]
- Dunning, J.W. The importation of humble bees into New Zealand. Trans. R. Entomol. Soc. Lond. 1886, 6, 32–34. [Google Scholar]
- Hawkins, R.P. Observations on the pollination of red clover by bees. Ann. Appl. Biol. 1961, 49, 55–65. [Google Scholar] [CrossRef]
- Gurr, L. The role of bumblebees as pollinators of red clover and Lucerne in New Zealand: A review and prospect. Proc. N. Z. Grassl. Assoc. 1974, 36, 111–122. [Google Scholar]
- Hawkins, R.P. A preliminary survey of red clover seed production. Ann. Appl. Biol. 1956, 44, 657–664. [Google Scholar] [CrossRef]
- Hawkins, R.P. A survey of late-flowering and single cut red clover seed crops. J. Natin. Inst. Agric. Bot. 1958, 8, 450–461. [Google Scholar]
- Goetze, G. Versuche zur Ausnutzung des Rotklees durch die Honigbienen. Beitr. Agrarwiss. 1948, 2, 35–47. [Google Scholar]
- Rao, S.; Stephen, W.P. Bumble bee pollinators in red clover seed production. Crop Sci. 2009, 49, 2207–2214. [Google Scholar] [CrossRef]
- Ruszkowski, A.; Sowa, S.; Biliński, M.; Kochańska, Z.; Galas, J.; Mostowska, I. The tongue length of the honeybee and the manner in which it forages on red clover. Pszczel. Zesz. Nauk. 1980, 24, 37–41. [Google Scholar]
- Vanommeslaeghe, A.; Meeus, I.; Cnops, G.; Vleugels, T.; Merchiers, M.; Duquenne, B.; Roldán-Ruiz, I.; Smagghe, G. Influence of pollinator abundance and flower visitation on seed yield in red clover. Arthropod Plant Interact. 2018, 12, 339–349. [Google Scholar] [CrossRef]
- Rundlöf, M.; Lundin, O.; Bommarco, R. Annual flower strips support pollinators and potentially enhance red clover seed yield. Ecol. Evol. 2018, 8, 7974–7985. [Google Scholar] [CrossRef]
- Richards, K.W. Effectiveness of the alfalfa leafcutter bee Megachile rotundata Fab. to pollinate perennial clovers. J. Apic. Res. 2016, 55, 259–267. [Google Scholar] [CrossRef]
- Stoltz, E.; Wallenhammer, A.C. Influence of boron in organic red clover (Trifolium pratense L.) seed production. Grass Forage Sci. 2014, 69, 285–293. [Google Scholar] [CrossRef]
- Anderson, N.P.; Garbacik, C.J. Irrigation and trinexapac-ethyl effects on seed yield in first-and second-year red clover stands. Agron. J. 2016, 108, 1116–1123. [Google Scholar] [CrossRef]
- Oliva, R.N.; Steiner, J.J.; Young, W.C. Red clover seed production: II. Plant water status on yield and yield components. Crop Sci. 1994, 34, 184–192. [Google Scholar] [CrossRef]
- Kolařík, P.; Rotrekl, J. Regulation of the abundance of clover seed weevils, Apion spp. (Coleoptera: Curculionidae) in a seed stand of red clover (Trifolium pratense L.). J. Entomol. Acarol. Res. 2013, 45, e19. [Google Scholar] [CrossRef]
- Lundin, O.; Rundlöf, M.; Smith, H.G.; Bommarco, R. Towards integrated pest management in red clover seed production. J. Econ. Entomol. 2012, 105, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Mihovsky, T.; Naydenova, G. Phenotypic analysis and heritability of seed production components in red clover (Trifolium pratense L.). Bulg. J. Agric. Sci. 2018, 24, 46–49. [Google Scholar]
- Bender, A. An impact of polyploidization on the dimensions of red clover flowers, species’ composition and number of insect pollinators. Agraarteadus 1999, 4, 9–23. [Google Scholar]
- Chastain, T.G.; Anderson, N.P.; Garbacik, C.J.; Angsumalee, D.; Elias, S.G. Irrigation and trinexapac-ethyl effects on seed yield in a second-year red clover stand. In 2013 Seed Production Research Report; Young, W.C., Ed.; Oregon State University: Salem, MA, USA, 2015; pp. 1–3. [Google Scholar]
- Øverland, J.I.; Aamlid, T.S. Plant growth regulators and insect control in seed production of red clover (Trifolium pratense). In Seed Production in the Northern Light, Proceedings of the 6th International Herbage Seeds Conference, Gjennestad, Norway, 18–20 June 2007; Aamlid, T.S., Havstad, L.T., Boelt, B., Eds.; Bioforsk: Grimstad, Norway, 2007; pp. 226–230. [Google Scholar]
- Rao, S.; Anderson, N.P. Comparison of bee pollinator abundance in red clover seed production fields with and without honey bee hives in the Willamette valley. In 2009 Seed Production Research Report; Oregon State University: Corvallis, OR, USA, 2009; pp. 45–49. [Google Scholar]
- Dupont, Y.L.; Damgaard, C.; Simonsen, V. Quantitative historical change in bumblebee (Bombus spp.) assemblages of red clover fields. PLoS ONE 2011, 6, e25172. [Google Scholar] [CrossRef]
- Rincker, C.M.; Rampton, H.H. Seed production. In Clover Science and Technology; Taylor, N.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1985; pp. 417–443. [Google Scholar]
- Buchholz, L. Increasing red clover seed production by saturation of pollinators. In Greenbook 2001; Minnesota Department of Agriculture: Minnesota, WI, USA, 2001; pp. 13–14. [Google Scholar]
- Jevtic, G.; Andelkovic, B.; Lugic, Z.; Mladenovic, M.; Nedic, N. The influence of the hive distance and the use of corn syrup on pollinator visits and red clover seed yield. Biotechnol. Anim. Husb. 2010, 26, 167–172. [Google Scholar]
- Kovi, M.K.; Amdahl, H.; Alsheikh, M.; Rognli, O.A. De novo and reference transcriptome assembly of transcripts expressed during flowering provide insight into seed setting in tetraploid red clover. Sci. Rep. 2017, 7, 44383. [Google Scholar] [CrossRef]
- Herrmann, D.; Boller, B.; Studer, B.; Widmer, F.; Kölliker, R. Improving persistence in red clover: Insights from QTL analysis and comparative phenotypic evaluation. Crop Sci. 2008, 48, 269–277. [Google Scholar] [CrossRef]
- Steiner, J.J.; Smith, R.R.; Alderman, S.C. Red clover seed production: 4. Root rot resistance under forage and seed production systems. Crop Sci. 1997, 37, 1278–1282. [Google Scholar] [CrossRef]
- Vleugels, T.; Cnops, G.; Roldán-Ruiz, I. Improving seed yield in red clover through marker assisted parentage analysis. Euphytica 2014, 200, 305–320. [Google Scholar] [CrossRef]
- Vleugels, T.; Roldán-Ruiz, I.; Cnops, G. Marker-assisted parentage analysis reveals high individual selfing rates in tetraploid red clover genotypes selected for seed yield. Plant Breed. 2019, 138, 947–957. [Google Scholar] [CrossRef]
- Montardo, D.P.; Dall’Agnol, M.; Crusius, A.F.; Paim, N.R. Path analysis for seed production in red clover (Trifolium pratense L.). Rev. Bras. Zootec. 2003, 32, 1076–1082. [Google Scholar] [CrossRef]
- Herrmann, D.; Boller, B.; Studer, B.; Widmer, F.; Kölliker, R. QTL analysis of seed yield components in red clover (Trifolium pratense L.). Theor. Appl. Genet. 2006, 112, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J. The importance of two-seeded pods in red clover (Trifolium pratense L.). Euphytica 1969, 18, 340–351. [Google Scholar] [CrossRef]
- Amdahl, H.; Aamlid, T.S.; Marum, P.; Ergon, A.; Alsheikh, M.; Rognli, O.A. Seed yield components in single plants of diverse Scandinavian tetraploid red clover populations (Trifolium pratense L.). Crop Sci. 2017, 57, 108–117. [Google Scholar] [CrossRef]
- Vleugels, T.; Roldán-Ruiz, I.; Cnops, G. Influence of flower and flowering characteristics on seed yield in diploid and tetraploid red clover. Plant Breed. 2015, 134, 56–61. [Google Scholar] [CrossRef]
- Clifford, P.T.P.; Scott, D. Inflorescence, bumble bee and climate interactions in seed crops of a tetraploid red clover (Trifolium pratense L.). J. Appl. Seed Prod. 1989, 7, 38–45. [Google Scholar]
- Tasei, J.N. Légumineuses fourragères et protéagineuses. In Pollinisation et Productions Végétales; Pesson, P., Louveaux, J., Eds.; INRA: Paris, France, 1984; pp. 261–308. [Google Scholar]
- Pammel, L.H.; King, C.M. Pollination of clover. Proc. Iowa Acad. Sci. 1911, 18, 35–45. [Google Scholar]
- Plath, O.E. The role of bumblebees in the pollination of certain cultivated plants. Am. Nat. 1925, 59, 441–451. [Google Scholar] [CrossRef]
- Palmer-Jones, T.; Forster, I.W.; Clinch, P.G. Observations on the pollination of Montgomery red clover (Trifolium pratense L.). N. Z. J. Agric. Res. 1966, 9, 738–747. [Google Scholar] [CrossRef]
- Hawkins, R.P. Factors affecting the yield of seed produced by different varieties of red clover. J. Agric. Sci. 1965, 65, 245–253. [Google Scholar] [CrossRef]
- Bond, D.A. Variation between tetraploid red clover plants in corolla tube length and height of nectar. J. Agric. Sci. 1968, 71, 113–116. [Google Scholar] [CrossRef]
- Skirde, W. Studies of morphology and secretion in diploid and tetraploid clovers. Ann. Agric. Fenn. 1963, 2, 90. [Google Scholar]
- Bingefors, S.; Ellerstrom, S. Polyploidy breeding in red clover: The tetraploid variety Svalof’s Ulva compared with some diploid and tetraploid varieties. Z. Pflanz. 1964, 51, 315–334. [Google Scholar]
- Starling, T.M.; Wilsie, C.P.; Gilbert, N.W. Corolla tube length studies in red clover. Agron. J. 1950, 42, 25–32. [Google Scholar] [CrossRef]
- Hawkins, R.P. Selection for height of nectar in the corolla tube of English singlecut Red Clover. J. Argic. Sci. Camb. 1971, 77, 347–350. [Google Scholar] [CrossRef]
- Julén, G. Aspects on the breeding of tetraploid red clover with special reference to the seed setting problem. In Proceedings of the European Grassland Conference, Paris, France, 21–24 June 1954; European Productivity Agency; Organisation for European Economic Co-operation: Paris, France, 1954; pp. 69–72. [Google Scholar]
- Vleugels, T.; Roldán-Ruiz, I.; Ceuppens, B.; Smagghe, G.; Cnops, G. Are corolla tube dimensions the reason for low seed yield in tetraploid red clover? In Breeding in a World of Scarcity, Proceedings of the 2015 Meeting of the Section “Forage Crops and Turfgrasses” of Eucarpia, Ghent, Belgium, 13–17 September 2015; Roldán-Ruiz, I., Baert, J., Reheul, D., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 293–297. [Google Scholar]
- Jablonski, B. A preliminary appreciation of agricultural and beekeeping value of a red clover population with a shortened flower tube. Acta Hortic. 2001, 561, 215–217. [Google Scholar] [CrossRef]
- Han, F.; Wallberg, A.; Webster, M. From where did the Western honeybee (Apis mellifera) originate? Ecol. Evol. 2012, 2, 1949–1957. [Google Scholar] [CrossRef]
- Grebrenisan, M.; Savatti, M. The comparative effects of the micro- and macrosporogenesis of the red clover with different levels of ploidy, in relation with its fertility. Bull. Univ. Agric. Sci. Vet. Med. Agric. 2011, 68, 138–143. [Google Scholar]
- Mackiewicz, T. Low seed setting in tetraploid red clover (Trifolium pratense L.) in the light of cytoembryological analyses. Genet. Pol. 1965, 6, 5–39. [Google Scholar]
- Büyükkartal, H.N. The reasons of sterility during pollen grain formation in the natural tetraploid Trifolium pratense L. Int. J. Bot. 2007, 3, 188–195. [Google Scholar]
- Vleugels, T.; van Laere, K.; Roldán-Ruiz, I.; Cnops, G. Seed yield in red clover is associated with meiotic abnormalities and in tetraploid genotypes also with self-compatibility. Euphytica 2019, 215, 79. [Google Scholar] [CrossRef]
- Büyükkartal, H.N. In vitro pollen germination and pollen tube characteristics in tetraploid red clover (Trifolium pratense L.). Turk. J. Bot. 2003, 27, 57–61. [Google Scholar]
- Akhalkatsi, M.; Pfauth, M.; Calvin, C.L. Structural aspects of ovule and seed development and nonrandom abortion in Melilotus officinalis (Fabaceae). Protoplasma 1999, 208, 211–223. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, J.; Feng, G.; Zhang, S.; Huang, L.; Zhuo, R.; Jin, L. Characterization of nine alfalfa cultivars for differences in ovule numbers and ovule sterility. Aust. J. Crop Sci. 2011, 5, 447–452. [Google Scholar]
- Povilaitis, B.; Boyes, J.W. Ovule development in diploid red clover. Can. J. Bot. 1960, 38, 507–523. [Google Scholar] [CrossRef]
- Büyükkartal, H.N. Causes of low seed set in the natural tetraploid Trifolium pratense L. (Fabaceae). Afr. J. Biotechnol. 2008, 7, 1240–1249. [Google Scholar]
- Marble, B.K. Polyploidy and self-compatibility: Is there an association? New Phytol. 2004, 162, 803–811. [Google Scholar] [CrossRef]
- Drach, N.P.; Rogovchenko, V.M.; Skorobagat’ko, T.N.; Efimenko, G.M. Significance of self pollination in the reproduction of early red clover. Sb. Nauchnykh Tr. Prikl. Bot. Genet. Sel. 1986, 103, 31–34. [Google Scholar]
- Elgersma, A. Effects of aneuploidy on seed production in autopolyploid forage crops. In Ploidy and Chromosome Manipulation in Forage Breeding, Proceedings of the 17th Meeting of the Fodder Crops Section of EUCARPIA, Alghero, Italy, 14–18 October 1991; Caredda, S., Veronesi, F., Bullitta, S., Eds.; Consiglio Nazionale delle Ricerche: Rome, Italy, 1991; pp. 124–131. [Google Scholar]
- Laczynska-Hulewicz, T. Self-fertility and inbreeding in tetraploid red clover. Genet. Pol. 1963, 4, 97–119. [Google Scholar]
- Vleugels, T.; Roldán-Ruiz, I.; Cnops, G. Comparing mitotic and meiotic tetraploid red clover plants for seed yield. In Breeding Grasses and Protein Crops in the Era of Genomics, Joint Meeting of EUCARPIA Fodder Crops and Amenity Grasses Section and Protein Crops Working Group of Oil and Protein Crops Section, Vilnius, Lithuania, 11–14 September 2017; Brazauskas, G., Statkeviciute, G., Jonaviciene, G., Eds.; Springer: Cham, Switzerland, 2018; pp. 39–45. [Google Scholar]
- Williams, R.D.; Williams, W. Genetics of red clover (Trifolium pratense L.) compatibility III. The frequency of incompatibility S alleles in two non-pedigree populations of red clover. J. Genet. 1947, 48, 69–79. [Google Scholar] [CrossRef]
- Riday, H.; Reisen, P.; Raasch, J.A.; Santa-Martinez, E.; Brunet, J. Selfing rate in an alfalfa seed production field pollinated with leafcutter bees. Crop Sci. 2015, 55, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- De Vega, J.J.; Ayling, S.A.; Hegarty, M.; Kudrna, D.; Goicoechea, J.L.; Ergon, A.; Rognli, O.A.; Jones, C.; Swain, M.; Geurts, R.; et al. Red clover (Trifolium pratense L.) draft genome provides a platform for trait improvement. Sci. Rep. 2015, 5, e17394. [Google Scholar] [CrossRef] [PubMed]
- Picard, J.; Berthaut, J. Fertility problems in autotetraploid red clover, an insight into the efficiency of selection at the diploid level. In Proceedings of the 10th International Grassland Congress, Helsinki, Finland, 7–16 July 1966; pp. 664–666. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vleugels, T.; Amdahl, H.; Roldán-Ruiz, I.; Cnops, G. Factors Underlying Seed Yield in Red Clover: Review of Current Knowledge and Perspectives. Agronomy 2019, 9, 829. https://doi.org/10.3390/agronomy9120829
Vleugels T, Amdahl H, Roldán-Ruiz I, Cnops G. Factors Underlying Seed Yield in Red Clover: Review of Current Knowledge and Perspectives. Agronomy. 2019; 9(12):829. https://doi.org/10.3390/agronomy9120829
Chicago/Turabian StyleVleugels, Tim, Helga Amdahl, Isabel Roldán-Ruiz, and Gerda Cnops. 2019. "Factors Underlying Seed Yield in Red Clover: Review of Current Knowledge and Perspectives" Agronomy 9, no. 12: 829. https://doi.org/10.3390/agronomy9120829
APA StyleVleugels, T., Amdahl, H., Roldán-Ruiz, I., & Cnops, G. (2019). Factors Underlying Seed Yield in Red Clover: Review of Current Knowledge and Perspectives. Agronomy, 9(12), 829. https://doi.org/10.3390/agronomy9120829




