Water Deficit Effects on Soybean Root Morphology and Early-Season Vigor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Condition and Plant Culture
2.2. Treatments
2.3. Plant Growth, Developmental, and Physiological Measurements
2.3.1. Phenology and Growth
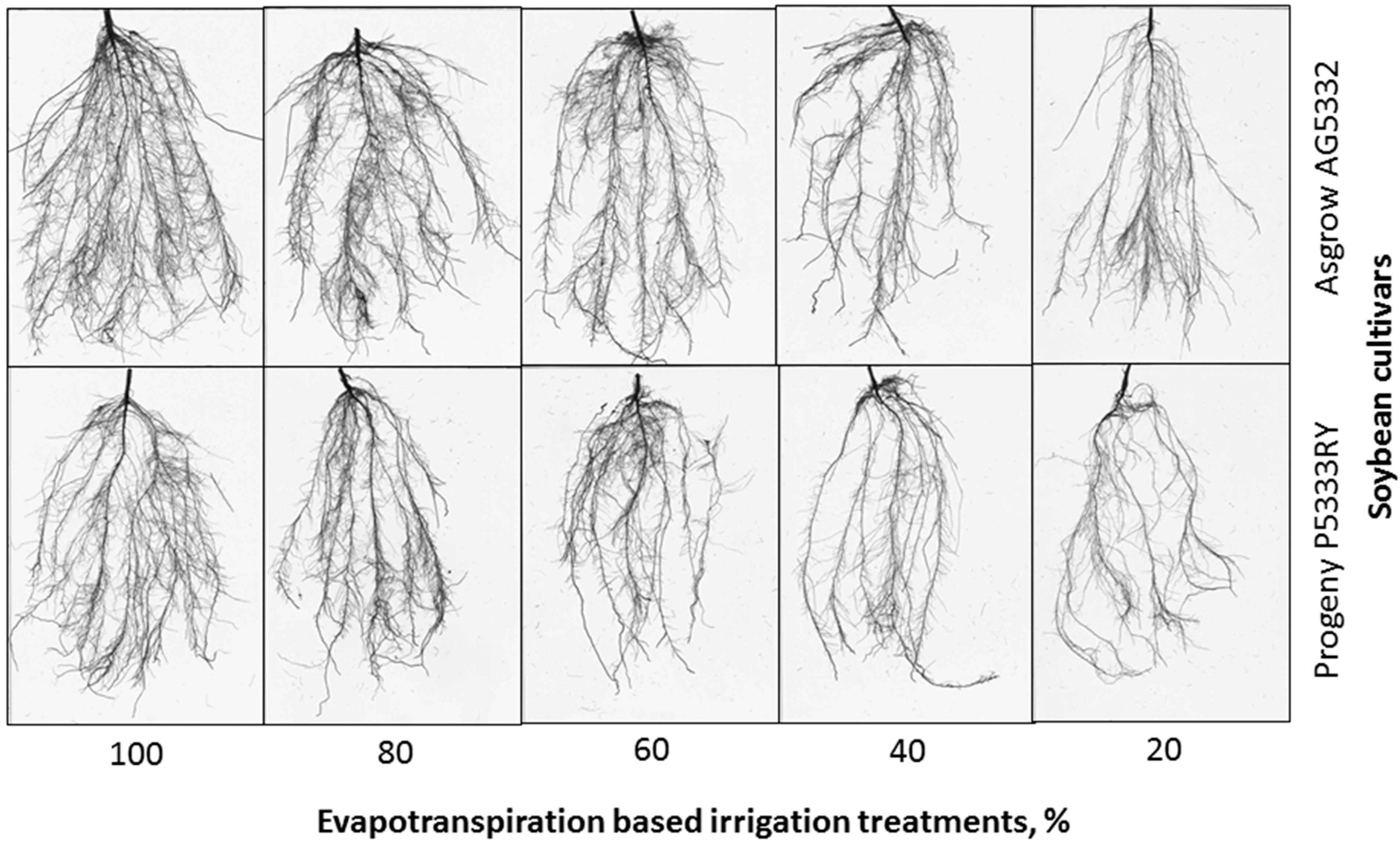
2.3.2. Root Morphology
2.3.3. Physiological and Gas-exchange Measurements
2.4. Data Analysis
Environment Productivity Index Concept and Critical Soil Moisture Limits
3. Results
3.1. Management of Soil Water Deficit Treatments
3.2. Growth and Shoot Pparameters
3.3. Root Parameters
3.4. Photosynthesis and Fluorescence Parameters
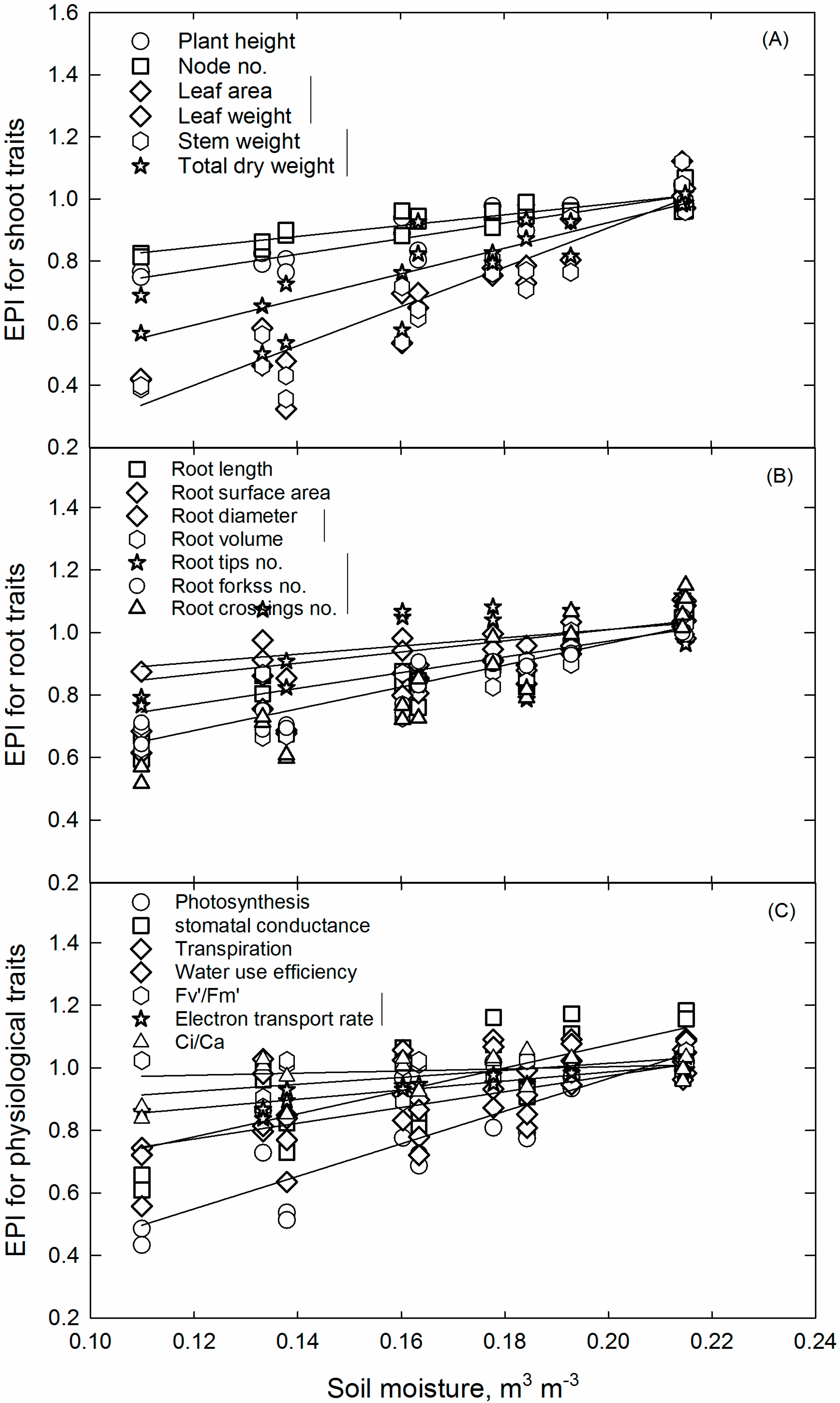
3.5. Soil Water Deficit Response for Soybean Growth, Developmental, and Physiological Pprocesses and CriticalLlimits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DAS | days after seeding |
| ET | evapotranspiration |
| SPAR | soil-plant-atmosphere-research |
| gs | stomatal conductance |
| Ci/Ca | internal to external CO2 ratio |
| E | transpiration |
| WUE | water use efficiency |
| ETR | electron transport rate |
| Fv′/Fm′ | chlorophyll fluorescence. |
References
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, J.; Liao, H.; Nian, H.; Hu, Y.; Qiu, L.; Dong, Y.; Yan, X. Characterization of root architecture in an applied core collection for phosphorus efficiency of soybean germplasm. Chin. Sci. Bull. 2017, 49, 1611–1620. [Google Scholar] [CrossRef]
- Kosturkova, G.; Todorova, R.; Tasheva, K.; Dimitrova, M. Screening of soybean against water stress mediated through polyethylene glycol. Turk. J. Agric. Nat. Sci. 2014, 1, 895–899. [Google Scholar]
- Frederick, J.R.; Camp, C.R.; Bauer, P.J. Drought stress effects on branch and mainstem seed yield and yield components of determinate soybean. Crop Sci. 2001, 41, 759–763. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Karim, M.A.; Haque, M.M. Genotypic differences in growth and ions accumulation in soybean under NaCl salinity and water stress conditions. Bangladesh Agron. J. 2014, 18, 267–288. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms, and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Brevedan, R.; Egli, D.B. Short periods of water stress during seed filling, leaf senescence, and yield of soybean. Crop Sci. 2003, 43, 2083–2088. [Google Scholar] [CrossRef]
- Board, J.E. Yield components related to seed yield in determinate soybean. Crop Sci. 1987, 27, 1296–1297. [Google Scholar] [CrossRef]
- Dornbos, D.L.; Mullen, R.E.; Shibles, R.E. Drought stress effects during seed fill on soybean seed germination and vigor. Crop Sci. 1989, 29, 476–480. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Alsajri, F.A.; Irby, T.; Krutz, J.; Golden, B. Quantifying soil moisture deficit effects on soybean yield and yield component distribution patterns. Irrig. Sci. 2018, 36, 241–255. [Google Scholar] [CrossRef]
- Bellaloui, N.; Mengistu, A.; Fisher, D.K.; Abel, C.A. Soybean seed composition constituents as affected by drought and phomopsisin phomopsis susceptible and resistant genotypes. J. Crop Improv. 2012, 26, 428–453. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Bellaloui, N. Soybean seed physiology, quality, and chemical composition under soil moisture stress. J. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Pantalone, V.R.; Rebetzke, G.L.; Burton, J.W.; Carter, T.E. Phenotypic evaluation of root traits in soybean and applicability to plant breeding. Crop Sci. 1996, 36, 456–459. [Google Scholar] [CrossRef]
- Fenta, B.A.; Beebe, S.E.; Kunert, K.J.; Burridge, J.D.; Barlow, K.M.; Lynch, P.J.; Foyer, C. Field phenotyping of soybean roots for drought stress tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.R.; Brand, D.; Wijewardana, C.; Gao, W. Temperature effects on cotton seedling emergence, growth, and development. Agron. J. 2017, 109, 1379–1387. [Google Scholar] [CrossRef]
- Brand, D.; Wijewardana, C.; Gao, W.; Reddy, K.R. Interactive effects of carbon dioxide, low temperature, and ultraviolet-B radiation on cotton seedling root and shoot morphology and growth. Front. Earth Sci. 2016, 10, 607–620. [Google Scholar] [CrossRef]
- Singh, K.; Wijewardana, C.; Gajanayake, B.; Lokhande, S.; Wallace, T.; Jones, D. Genotypic variability among cotton cultivars for heat and drought tolerance using reproductive and physiological traits. Euphytica 2018, 214, 57. [Google Scholar] [CrossRef]
- Bengough, A.G.; Mckenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, J.G.; Nielsen, D.C. Water deficit effects on root distribution of soybean, field pea, and chickpea. Field Crops Res. 2006, 97, 248–253. [Google Scholar] [CrossRef]
- Manavalan, L.P.; Guttikonda, S.K.; Nguyen, V.T.; Shannon, J.G.; Nguyen, H.T. Evaluation of diverse soybean germplasm for root growth and architecture. Plant Soil 2010, 330, 503–514. [Google Scholar] [CrossRef]
- Ao, J.; Fu, J.; Tian, J.; Yan, X.; Liao, H. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean. Funct. Plant Biol. 2010, 37, 304–312. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, J.; Liao, H.; He, Y.; Nian, H.; Hu, Y.; Qui, L.; Dong, Y.; Yan, X. Comparative proteomic analysis of drought response in roots of two soybean genotypes. Crop Pasture Sci. 2004, 68, 609–619. [Google Scholar]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byne, P.F.; Dierig, D.A. Root traits are contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serraj, R.; Bona, S.; Purcell, L.C.; Sinclair, T.R. Nitrogen accumulation and nodule activity of field grown ‘Jackson’ soybean in response to water deficits. Field Crops Res. 1997, 52, 109–116. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Manavalan, L.P.; Guttikonda, S.K.; Phan, T.L.S.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [Green Version]
- Wijewardana, C.; Henry, W.B.; Reddy, K.R. Evaluation of drought tolerant maize germplasm to induced drought stress. Miss. Acad. Sci. 2017, 62, 316–329. [Google Scholar]
- Keating, B.A.; Carberry, P.S.; Hammer, G.L.; Probert, M.E.; Robertson, M.J.; Holzworth, D.; Huth, N.I.; Hargreaves, J.N.G.; Meinke, H.; Hochman, Z.; et al. An overview of APSIM, a model designed for farming systems simulation. Eur. J. Agron. 2003, 17, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Boote, K.J.; Jones, J.W.; Batchelor, W.D.; Nafziger, E.D.; Myers, O. Genetic coefficients in the CROPGRO-soybean model: Links to field performance and genomics. Agron. J. 2003, 95, 32–51. [Google Scholar] [CrossRef]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Cassman, K.G.; Specht, J.E.; Dobermann, A.; Weiss, A.; Yang, H.; Conley, S.P.; Robinson, A.P.; Pedersen, P.; De Bruin, J.L. Simulation of soybean growth and yield in near-optimal growth conditions. Field Crops Res. 2010, 119, 161–174. [Google Scholar] [CrossRef]
- Acock, B.; Reddy, V.R.; Whisler, E.D.; Baker, D.N.; Hodges, H.F.; Boote, K.J. The Soybean Crop Simulator GLYCIM. Model Documentation; PB85171163/AS; U.S. Department of Agriculture: Washington, DC, USA, 1982.
- Nendel, C.; Berg, M.; Kersebaum, K.C.; Mirschel, W.; Specka, X.; Wegehenkel, M.; Wenkel, K.O.; Wieland, R. The MONICA model: Testing predictability for crop growth, soil moisture and nitrogen dynamics. Ecol. Model. 2011, 222, 1614–1625. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hodges, H.F.; Read, J.J.; McKinion, J.M.; Baker, J.T.; Tarpley, L.; Reddy, V.R. Soil-Plant-Atmosphere-Research (SPAR) facility: A tool for plant research and modeling. Biotronics 2001, 30, 27–50. [Google Scholar]
- Murray, F.W. On the computation of saturation vapor pressure. J. Appl. Meteorol. 1967, 6, 203–204. [Google Scholar] [CrossRef]
- McKinion, J.M.; Hodges, H.F. Automated system for measurement of evapotranspiration from closed environmental growth chambers. Trans. Am. Soc. Agric. Eng. 1985, 28, 1825–1828. [Google Scholar] [CrossRef]
- Timlin, D.; Fleisher, D.; Kim, S.H.; Reddy, V.; Baker, J. Evapotranspiration measurements in controlled environment chambers. Agron. J. 2007, 99, 166–173. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Shankle, M.W.; Meyers, S.; Gao, W. Low and high temperature effects on sweetpotato storage root initiation and early transplant establishment. Sci. Hortic. 2018, 240, 38–48. [Google Scholar] [CrossRef]
- Nobel, P.S. Environmental productivity indices and productivity for Opuntia ficus indica under current and elevated atmospheric CO2 levels. Plant Cell Environ. 1991, 14, 637–646. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hodges, H.F.; McKinion, J.M. Crop modeling and application: A cotton example. Adv. Agron. 1997, 59, 225–290. [Google Scholar]
- Reddy, K.R.; Kakani, V.G.; Hodges, H.F. Exploring the Use of Environmental Productivity Index Concept for Crop Production and Modeling. In Response of Crops to Limited Water: Understanding and Modeling of Water Stress Effects on Plant Growth Processes; Ahuja, L.R., Reddy, V., Saseendran, S.A., Yu, Q., Eds.; ASA, CSSA, and SSSA: Madison, WI, USA, 2008; pp. 387–410. [Google Scholar]
- Guimarães-Dias, F.; Neves-Borges, A.C.; Viana, A.A.; Mesquita, R.O.; Romano, E.; de Fátima Grossi-de-Sá, M.F.; Nepomuceno, A.L.; Loureiro, M.E.; Alves-Ferreira, M. Expression analysis in response to drought stress in soybean: Shedding light on the regulation of metabolic pathway genes. Genet. Mol. Biol. 2012, 35, 222–232. [Google Scholar] [CrossRef]
- Desclaux, D.; Huynh, T.T.; Roumet, P. Identification of soybean plant characteristics that indicate the timing of drought stress. Crop Sci. 2000, 40, 716–727. [Google Scholar] [CrossRef]
- Ku, Y.; Wan-Kin, A.; Yung, Y.; Li, M.; Wen, C.; Liu, X.; Lam, H. Drought stress and tolerance in soybean. InTech 2013. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, M.M.; Muchow, R.C. A critical evaluation of traits for improved crop yields in water limited environments. Adv. Agron. 1990, 43, 107–153. [Google Scholar]
- Arnon, I. Crop Production in Dry Regions, Background, and Principles; Polunin, N., Ed.; Leonard Hill Book: London, UK, 1972; pp. 203–211. [Google Scholar]
- Lynch, J.P.; Nielsen, K.L. Simulation of root system architecture. In Plant Roots: The Hidden Half, 2nd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 247–257. [Google Scholar]
- Yamauchi, A.; Pardales, J.R.; Kono, Y. Root system structure and its relation to stress tolerance. In Dyanamics of Roots and Nitrogen in Cropping Systems of the Semi-Arid Tropics; Ito, O., Johansen, C., Adu-Gyamfi, J.J., Katayama, K., Kumar, J.V.D.K., Rego, T.J., Eds.; Japan International Research Center for Ag Sciences: Tsukuba, Japan, 1996; pp. 211–233. [Google Scholar]
- Thu, N.B.; Nguyen, Q.T.; Hoang, X.L.; Thao, N.P.; Trans, L.S. Evaluation of drought tolerance of the Vietnamese soybean cultivars provides potential resources for soybean production and engineering. BioMed Res. Int. 2014, 2014, 809736. [Google Scholar] [CrossRef] [Green Version]
- Kunert, K.J.; Vorster, B.; Fenta, B.A.; Kibido, T.; Dionisio, G.; Foyer, C.H. Drought stress responses in soybean roots and nodules. Front. Plant Sci. 2016, 7, 1015. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.S.; Araus, J.L.; van Heerden, P.D.R.; Foyer, C.H. Enhancing drought tolerance in C4 crops. J. Exp. Bot. 2011, 62, 3135–3153. [Google Scholar] [CrossRef]
- Taylor, H.M. Modifying root systems of cotton and soybean. In Adaptation of Plants to Water and High Temperature Stress; Turner, N.C., Kramer, P.J., Eds.; Wiley-Interscience: New York, NY, USA, 1980; pp. 75–84. [Google Scholar]
- Taylor, H.M.; Klepper, B. Rooting density and water extraction patterns for com (Zea mays L.). Agron. J. 1973, 65, 965–968. [Google Scholar] [CrossRef]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root development and nutrient uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Stolf-Moreira, R.; Medri, M.E.; Neumaier, N.; Lemos, N.G.; Pimenta, J.A.; Tobita, S.; Brogin, R.L.; Marcelino-Guimarães, F.C.; Oliveira, M.C.; Farias, J.R.; et al. Soybean physiology and gene expression during drought. Genet. Mol. Res. 2010, 9, 1946–1956. [Google Scholar] [CrossRef]
- Lokhande, S.; Reddy, K.R. Reproductive and fiber quality responses of upland cotton to moisture deficiency. Agron. J. 2014, 106, 1060–1069. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought stress effects on photosynthetic rate and leaf gas exchange of wheat. Bot. Bull. Acad. Sin. 1999, 40, 141–145. [Google Scholar]
- Genty, B.; Briantais, J.M.; Silva, J.B.V. Effects of drought on primary photosynthetic processes of cotton leaves. Plant Physiol. 1987, 83, 360–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatments | Temperature | CO2 | VPD | ET | |
|---|---|---|---|---|---|
| Soil moisture | Day/night | Day/night | Day/night | Day/night | |
| m3 m−3 | °C | ppm | kPa | L m−2d−1 | |
| 18 days of the experiment (treatment period 4–18 days) | |||||
| 100 | 0.214a | 25.01a | 432.50a | 5.28a | 2.36a |
| 80 | 0.184b | 25.13a | 431.33a | 5.34a | 2.22a |
| 60 | 0.163c | 24.99a | 431.42a | 5.28a | 1.98b |
| 40 | 0.139d | 25.18a | 428.47a | 5.32a | 1.62c |
| 20 | 0.109e | 25.13a | 430.30a | 5.32a | 0.85d |
| 30 days of the experiment (treatment period 10–30 days) | |||||
| 100 | 0.212a | 24.33a | 429.37a | 5.75a | 2.79a |
| 80 | 0.192b | 24.53a | 428.17a | 5.65a | 1.97b |
| 60 | 0.177c | 24.49a | 428.86a | 5.55a | 1.90b |
| 40 | 0.160d | 24.58a | 428.03a | 5.13a | 1.75c |
| 20 | 0.133e | 24.30a | 431.13a | 5.22a | 0.99d |
| 18 DAS | 30 DAS | |||||
|---|---|---|---|---|---|---|
| Parameters | Source | Source | ||||
| Trt | Cul | Trt × Cul | Trt | Cul | Trt × Cul | |
| Plant height, cm | NS | *** | NS | *** | *** | NS |
| Node no., plant−1 | NS | NS | NS | *** | NS | NS |
| Leaf area, cm2 | *** | ** | NS | *** | *** | NS |
| Leaf weight, g plant−1 | *** | ** | NS | *** | NS | NS |
| Stem weight, g plant−1 | *** | * | NS | *** | NS | NS |
| Total dry matter, g plant−1 | * | *** | NS | *** | ** | NS |
| Root weight, g plant−1 | *** | *** | NS | *** | NS | NS |
| Root length, cm | *** | *** | NS | *** | ** | NS |
| Root surface area, cm2 | *** | *** | NS | *** | ** | NS |
| Average diameter, mm | *** | NS | NS | *** | NS | NS |
| Root volume, mm3 | *** | NS | NS | *** | ** | NS |
| Root tips no., plant−1 | * | ** | NS | * | ** | NS |
| Root forks no., plant−1 | *** | *** | NS | *** | *** | NS |
| Root crossings no., plant−1 | *** | *** | NS | *** | *** | NS |
| Photosynthesis, µmol CO2 m−2 s−1 | ** | NS | NS | *** | NS | NS |
| gs, mol H2O m−2 s−1 | * | * | NS | *** | * | NS |
| E, mmol H2O m−2 s−1 | *** | NS | NS | *** | NS | NS |
| WUE, mmol CO2mol−1 H2O | ** | NS | NS | ** | NS | NS |
| Ci/Ca | NS | NS | NS | NS | NS | NS |
| Fv’/Fm’ | NS | NS | NS | NS | NS | NS |
| ETR, µmol m−2 s−1 | * | * | NS | * | * | NS |
| Plant Parameter | Regression Parameters | p Value | Coefficient of Determination (r2) | Critical Soil Moisture Content, m3 m−3 | |
|---|---|---|---|---|---|
| a | b | ||||
| Plant height | 0.47 | 2.51 | 0.955 | 0.83 | 0.167 |
| Node no. | 0.64 | 1.74 | 0.831 | 0.79 | 0.130 |
| Leaf area | −0.36 | 6.33 | 0.662 | 0.89 | 0.196 |
| Leaf weight | |||||
| Stem weight | −0.39 | 6.46 | 0.562 | 0.89 | 0.197 |
| Total dry weight | |||||
| Root length | 0.23 | 3.73 | 0.281 | 0.83 | 0.200 |
| Root surface area | 0.27 | 3.49 | 0.350 | 0.85 | 0.203 |
| Root diameter | 0.65 | 1.79 | 0.751 | 0.60 | 0.159 |
| Root volume | |||||
| Root tips no. | 0.24 | 3.6 | 0.918 | 0.87 | 0.203 |
| Root forks no. | |||||
| Root crossings no. | |||||
| Photosynthesis | −0.08 | 5.21 | 0.274 | 0.71 | 0.196 |
| gs | 0.34 | 3.67 | 0.030 | 0.53 | 0.175 |
| Transpiration | 0.34 | 3.47 | 0.328 | 0.45 | 0.185 |
| WUE | 0.47 | 2.52 | 0.976 | 0.83 | 0.188 |
| Fv’/Fm’ | 0.70 | 1.45 | 0.625 | 0.84 | 0.133 |
| ETR | |||||
| Ci/Ca | 0.79 | 1.12 | 0.284 | 0.33 | 0.124 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijewardana, C.; Alsajri, F.A.; Irby, J.T.; Krutz, L.J.; Golden, B.R.; Henry, W.B.; Reddy, K.R. Water Deficit Effects on Soybean Root Morphology and Early-Season Vigor. Agronomy 2019, 9, 836. https://doi.org/10.3390/agronomy9120836
Wijewardana C, Alsajri FA, Irby JT, Krutz LJ, Golden BR, Henry WB, Reddy KR. Water Deficit Effects on Soybean Root Morphology and Early-Season Vigor. Agronomy. 2019; 9(12):836. https://doi.org/10.3390/agronomy9120836
Chicago/Turabian StyleWijewardana, Chathurika, F.A. Alsajri, J.T. Irby, L.J. Krutz, B.R. Golden, W.B. Henry, and K.R. Reddy. 2019. "Water Deficit Effects on Soybean Root Morphology and Early-Season Vigor" Agronomy 9, no. 12: 836. https://doi.org/10.3390/agronomy9120836





