A Review of Studies from the Last Twenty Years on Plant–Arbuscular Mycorrhizal Fungi Associations and Their Uses for Wheat Crops
Abstract
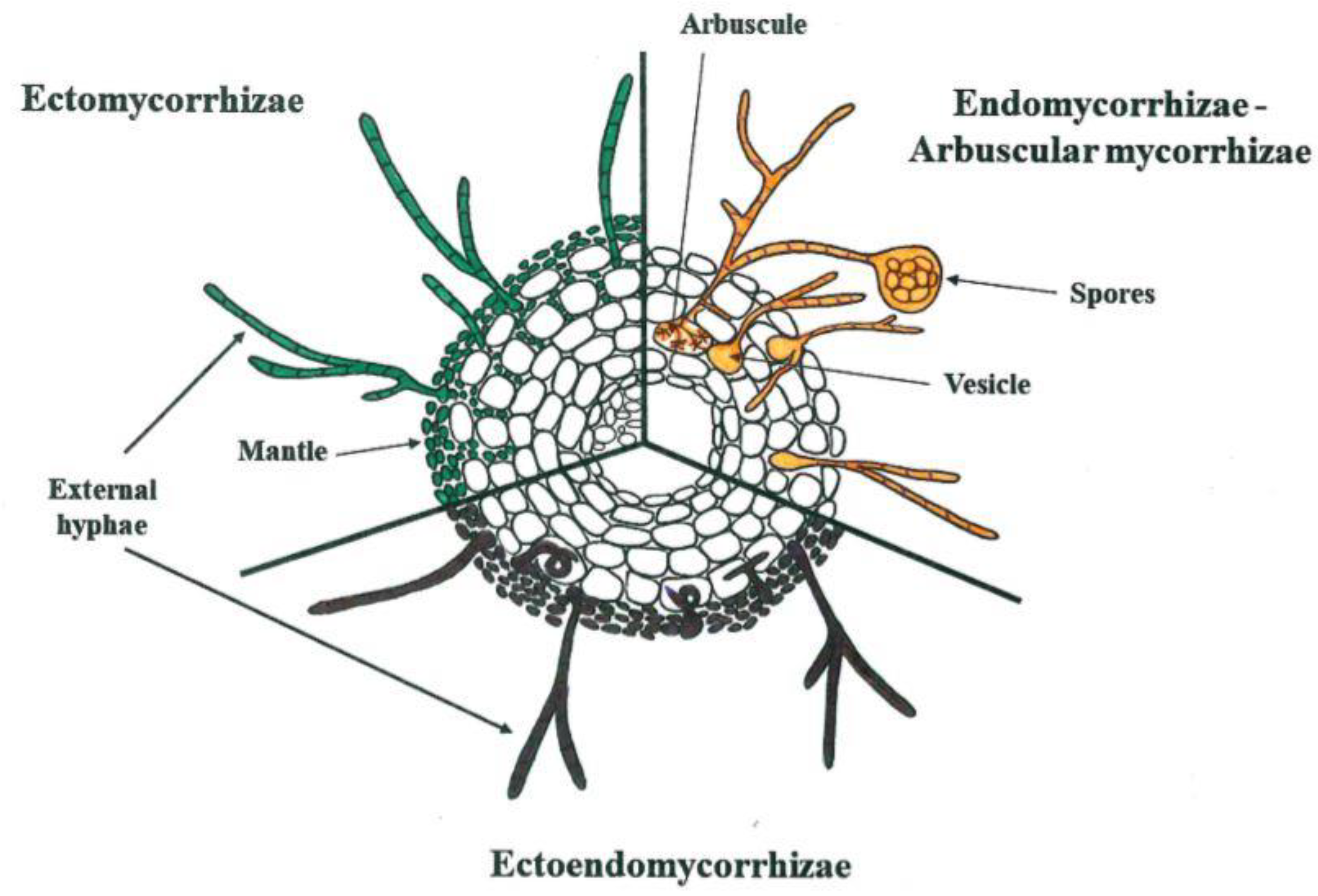
1. Introduction
2. Uptake and Translocation of Soil Nutrients
3. Abiotic Stresses
3.1. Salinity Stress
- (1)
- Higher water uptake: mycorrhizal hyphae can better expand into the soil, taking up more water and preventing plant dehydration and turgor loss—two consequences of salinity compromising the water status of the plant [44].
- (2)
- Increased mineral nutrition linked to maintaining a high K/Na ratio [45]: under osmotic stress conditions, the soil Na+ concentration is very high and negatively interferes with other various transporters in the root plasma membrane, such as K+ selective ion channels [46]. As a consequence, the uptake of mineral nutrients (P, K, Fe, Cu, and Zn) is reduced and, in particular, the Na+/K+ ratio becomes very high, interrupting various enzymatic processes and protein synthesis [47]. Plant association with AMF, thanks to their higher soil exploration capacity, showed a total mineral nutrition increase with great K+ accumulation, helping the plants to maintain a lower Na+/K+ ratio and in this way avoid damage to their biological functioning [48].
- (3)
- Intense production of compatible organic solutes: low-molecular-weight and highly soluble compounds, such as proline, glycine, betaine, and soluble sugars, are accumulated to higher levels in AM plants and appear to be positively correlated to fungi plant colonization [49,50]. Production of these solutes can contribute to cellular osmotic adjustment thanks to their key role in detoxifying ROS, protecting membrane integrity, and stabilizing enzymes/proteins [51].
- (4)
- Antioxidant enzyme activity enhancement: several studies have suggested that AM symbiosis intensifies enzyme system activity in ROS detoxification, including that of peroxides, superoxide, hydroxyl radicals, and singlet oxygen and alpha-oxygen, the production of which in plants is strongly influenced by stress factors such as salinity. The effects induced by these compounds on cell metabolism, such as DNA damage, the oxidation of polyunsaturated fatty acids in lipids and of amino acids in proteins, and the deactivation of specific enzymes, appear to be reduced in AM plants, which show generally lower levels of oxidative damage.
3.2. Drought Stress
- (1)
- Maintenance of water uptake: Mycorrhizal hyphae enter deeper into the soil and explore a great volume in search of water, helping to keep the plant watered [66];
- (2)
- Osmotic adjustment: Some processes like stomatal opening, cellular expansion, and growth are maintained by the mycorrhizal fungus activity, allowing the cells to maintain turgor [67];
- (3)
- (4)
- Antioxidant level increase: The concentrations of many antioxidant compounds, such as superoxide dismutase, catalase, and peroxidase, appear to be enhanced in plants colonized by mycorrhizal fungi, resulting in the reduced presence of ROS such as singlet oxygen, superoxides, hydrogen peroxide, and hydroxyl radicals [70].
3.3. Heavy Metal Stress
4. Resistance to Pathogens
- (1)
- Changes in root growth and morphology: AM colonization induces notable changes in root system morphology, altering the dynamics of pathogens and modifying microbial populations, with the possible stimulation of microbiota components with antagonistic activity toward certain root pathogens [96]. Lucini et al. [97] showed significantly different production of exudates in AMF roots, which can influence the microbiota composition;
- (2)
- Changes in host nutrition: the increased nutrient uptake resulting from AM symbiosis makes the plant more vigorous and, consequently, more resistant, compensating for the loss of root biomass or function caused by pathogens [98];
- (3)
- Competition for colonization sites and photosynthates: both the AM fungi and root pathogens depends on host photosynthates, and they compete for the carbon compounds reaching the root [99,100]; however, AM fungi have primary access to photosynthates, and the higher carbon demand may inhibit pathogen growth [101];
- (4)
- Activation of defense mechanisms: with AM colonization, the host plant produces a great number of phytoalexins, enzymes of the phenylpropanoid pathway, chitinases, b-1,3-glucanases, peroxidases, pathogenesis-related (PR) proteins, callose, hydroxyproline-rich glycoproteins (HRGP), and phenolics [102] that can act in biological control [103,104];
5. Soil Aggregation
6. Conclusions
Funding
Conflicts of Interest
References
- Frank, B. Ueber die auf Wurzelsymbiose beruhende Ernahrung gewisser Baume durch unterirdische Pilze. Ber. Der Dtsch. Bot. Ges. 1885, 3, 128–145. [Google Scholar]
- Bonfante, P.; Giovannetti, M. Quaderni di Biologia. Le Micorrize; Piccin: Padova, Italy, 1982; pp. 1–143. [Google Scholar]
- Pozo, M.J.; Azcon-Aguilar, C. Unraveling mycorrhiza-induced resistance. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Read, D.J.; Lewis, D.H.; Fitter, A.H.; Alexander, I.J. Mycorrhizas in Ecosystems; CAB International: Wallingford, Oxon, UK, 1992. [Google Scholar]
- Allen, M.F. The Ecology of Mycorrhizae; Cambridge Univ. Press: Cambridge, UK, 1991; p. 189. [Google Scholar]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant—Fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Peterson, R.L.; Massicotte, H.B.; Melville, L.H. Mycorrhizas: Anatomy and cell biology. Mycologist 2004, 19, 133. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Simon, L.; Bousquet, J.; Levesque, C.; Lalonde, M. Origin and diversification of endomycorrizal fungi and coincidence with vascular land plants. Nature 1993, 363, 67–69. [Google Scholar] [CrossRef]
- Powell, C.L.; Bagyaraj, D.J. Va Mycorrhiza; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Gadkar, V.; David-Schwartz, R.; Kunik, T.; Kapulnik, Y. Arbuscular mycorrhical fungal colonization. Factors involved in host recognition. Plant Physiol. 2001, 127, 1493–1499. [Google Scholar] [CrossRef]
- Montero, H.; Choi, J.; Paszkowski, U. Arbuscular mycorrhizal phenotyping: The dos and don’ts. New Phytol. 2019, 221, 1182–1186. [Google Scholar] [CrossRef]
- Aloui, A.; Recorbet, G.; Lemaître-Guillier, C.; Mounier, A.; Balliau, T.; Zivy, M.; Wipf, D.; Dumas-Gaudot, E. The plasma membrane proteome of Medicago truncatula roots as modified by arbuscular mycorrhizal symbiosis. Mycorrhiza 2018, 28, 1–16. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223. [Google Scholar] [CrossRef]
- Johns, C.D. Agricultural Application of Mycorrhizal Fungi to Increase Crop Yields, Promote Soil Health and Combat Climate Change; Future Directions international: Dalkeith, Australia, 2014. [Google Scholar]
- Pepe, A.; Giovannetti, M.; Sbrana, C. Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan. Sci. Rep. 2018, 8, 10235. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Biol. Annu. Rev. Plant 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef]
- Javaid, A. Arbuscular mycorrhizal mediated nutrition in plants. Plant Nutr. 2009, 32, 1595–1618. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008. [Google Scholar]
- Smith, S.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Drew, E.A.; Murray, R.S.; Smith, S.E.; Jakobsen, I. Beyond the rhizosphere: Growth and function of arbuscular mycorrhizal external hyphae in sands of varying pore sizes. Plant Soil 2003, 251, 105–114. [Google Scholar] [CrossRef]
- García-Garrido, J.M.; Tribak, M.; Rejón-Palomares, A.; Ocampo, J.A.; García-Romera, I. Hydrolitic enzymes and ability of arbuscular mycorrhizal fungi to colonize roots. J. Exp. Bot. 2000, 51, 1443–1448. [Google Scholar] [CrossRef]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2008, 181, 199–207. [Google Scholar] [CrossRef]
- Pellegrino, E.; Opik, M.; Bonari, E.; Ercoli, L. Responses of wheat to arbuscular mycorrhizal fungi: A meta-analysis of field studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Ma, X.; Luo, W.; Li, J.; Wu, F. Arbuscular mycorrhizal fungi increase both concentrations and bioavilability of Zn in wheat (Triticum aestivum L) grain on Zn-spiked soils. Appl. Soil Ecol. 2019, 135, 91–97. [Google Scholar] [CrossRef]
- Ryan, M.H.; Angus, J.F. Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: Increased Zn-uptake but no increase in P-uptake or yield. Plant Soil 2003, 250, 225–239. [Google Scholar] [CrossRef]
- Ryan, M.H.; McInerney, J.K.; Record, I.R.; Angus, J.F. Zinc bioavailability in wheat grain in relation to phosphorus fertiliser, crop sequence and mycorrhizal fungi. J. Sci. Food Agric. 2008, 88, 1208–1216. [Google Scholar] [CrossRef]
- Li, H.Y.; Smith, S.E.; Holloway, R.E.; Zhu, Y.G.; Smith, F.A. Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol. 2006, 172, 536–543. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Field response of garlic inoculated with arbuscular mycorrhizal fungi to phosphorus fertilization. J. Plant Nutr. 2002, 25, 747–756. [Google Scholar] [CrossRef]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A. Synergistic effects of the inoculation with plant growth promoting rhizobacteria and arbuscular mycorrhizal fungus on the performance of wheat. Turk. J. Agric. For. 2007, 31, 355–362. [Google Scholar] [CrossRef]
- Luo, W.; Li, J.; Ma, X.; Niu, N.; Hou, S.; Wu, F. Effect of arbuscular mycorrhizal fungi on uptake of selenate, selenite, and selenomethionine by roots of winter wheat. Plant Soil 2019, 438, 71–83. [Google Scholar] [CrossRef]
- Durán, P.; Acuna, J.J.; Jorquera, M.A. Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: A preliminary study as a potential Se biofortification strategy. J. Cereal Sci. 2013, 57, 275–280. [Google Scholar] [CrossRef]
- Blackwell, P.; Joseph, S.; Munroe, P.; Anawar, H.M.; Storer, P.; Gilkes, R.J.; Solaiman, Z.M. Influences of biochar and biochar-mineral complex on mycorrhizal colonisation and nutrition of wheat and sorghum. Pedosphere 2015, 25, 686–695. [Google Scholar] [CrossRef]
- Saito, M.; Marumoto, T. Inoculation with arbuscular mycorrhizal fungi: The status quo in Japan and the future prospects. Plant Soil 2002, 244, 273–279. [Google Scholar] [CrossRef]
- Plouznikoff, K.; Declerck, S.; Calonne-Salmon, M. Mitigating Abiotic Stresses in Crop Plants by Arbuscular Mycorrhizal Fungi; Springer Nature: Basel, Switzerland, 2016; pp. 341–400. [Google Scholar]
- Kulkarni, S.; Goswami, A. Effect of Excess Fertilizers and Nutrients: A Review on Impact on Plants and Human Population. In Proceedings of the International Conference on Sustainable Computing in Science, Technology and Management (SUSCOM), Jaipur, India, 26–28 February 2019. [Google Scholar] [CrossRef]
- Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2018, 59, 651–681. [Google Scholar] [CrossRef]
- Sairam, R.K.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Miransari, M. The Role of Arbuscular Mycorrhizal Fungi in Alleviation of Salt Stress. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 2, pp. 23–38. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed, Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef]
- Maathuis, F.J.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of Salinity Stress in Plants by Arbuscular Mycorrhizal Symbiosis: Current Understanding and New Challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Jahromi, F.; Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb. Ecol. 2008, 55, 45–53. [Google Scholar] [CrossRef]
- Yokoi, S.; Quintero, F.J.; Cubero, B.; Ruiz, M.T.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002, 30, 529–539. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Siahpoosh, M.R.; Roessner, U.; Udvardi, M.; Kopka, J. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol. Plant. 2007, 132, 209–219. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Abdel-Fattah, G.M.; Asrar, A.A. Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L.) plants grown in saline soil. Acta Physiol. Plant. 2012, 34, 267–277. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Abdel-Fattah, G.M.; Eman, F.M.; Abd El_Aziz, M.H.; Shohr, A.E. Arbuscular mycorrhizal fungi and spermine alleviate the adverse effects of salinity stress on electrolyte leakage and productivity of wheat plants. New Phytol. 2011, 51, 261–276. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2018, 139, 227–238. [Google Scholar] [CrossRef]
- Daei, G.; Ardekani, M.; Rejali, F.; Teimuri, S.; Miransari, M. Alleviation of salinity stress on wheat yield components, and nutrient uptake using arbuscular mycorrhizal fungi underfield conditions. J. Plant Physiol. 2009, 166, 217–225. [Google Scholar] [CrossRef]
- Mardukhi, B.; Rejali, F.; Daei, G.; Ardakani, M.R.; Malakouti, M.J.; Miransari, M. Arbuscular mycorrhizas enhance nutrient uptake in different wheat genotypes at high salinitylevels under field and greenhouse conditions. C. R. Biol. 2011, 334, 564–571. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic: New York, NY, USA, 1995. [Google Scholar]
- Abbaspour, H.; Saeidi-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef]
- Saraswathi, S.G.; Paliwal, K. Drought induced changes in growth, leaf gas exchange and biomass production in Albizia lebbeck and Cassia siamea seedlings. J. Environ. Biol. 2011, 32, 173–178. [Google Scholar]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, A.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell. Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Rapparini, F.; Penuelas, J. Mycorrhizal fungi to alleviate drought stress on plant growth. In Use of Microbes for the Alleviation of Soil Stress; Springer: New York, NY, USA, 2014; pp. 21–42. [Google Scholar]
- Calvo-Polanco, M.; Sánchez-Castro, I.; Cantos, M.; García, J.L.; Azcón, R.; Ruiz-Lozano, J.M.; Beuzón, C.R.; Aroca, R. Effects of different arbuscular mycorrhizal fungal backgrounds and soils on olive plants growth and water relation properties under well-watered and drought conditions. Plant. Cell. Environ. 2016, 39, 2498–2514. [Google Scholar] [CrossRef]
- Symanczik, S.; Lehmann, M.F.; Wiemken, A.; Boller, T.; Courty, P.E. Effects of two contrasted arbuscular mycorrhizal fungal isolates on nutrient uptake by Sorghum bicolor under drought. Mycorrhiza 2018, 28, 779–785. [Google Scholar] [CrossRef]
- Dar, Z.M.; Masood, A.; Asif, M.; Malik, M.A. Review on arbuscular mycorrhizal fungi: An approach to overcome drought adversities in plants. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1040–1049. [Google Scholar] [CrossRef][Green Version]
- Jiménez Zacarías, J.J.; Altamirano-Hernández, J.; Peña Cabriales, J.J. Nitrogenase activity and trehalose content of nodules of drought-stressed common beans infected with effective (Fix+) and ineffective (Fix−) rhizobia. Soil Biol. Biochem. 2004, 36, 1975–1981. [Google Scholar] [CrossRef]
- Farías-Rodríguez, R.; Mellor, R.B.; Arias, C.; Peña Cabriales, J.J. The accumulation of trehalose in nodules of several cultivars of common bean (Phaseolus vulgaris) and its correlation with resistance to drought stress. Physiol. Plant 1998, 102, 353–359. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.L.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef]
- Jia-Dong, H.; Tao, D.; Hui-Hui, W.; Zou, Y.N.; Wu, Q.S.; Kamil, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar] [CrossRef]
- Amanifar, S.; Khodabandeloo, M.; Fard, E.M.; Askari, M.S.; Ashrafi, M. Alleviation of salt stress and changes in glycyrrhizin accumulation by arbuscular mycorrhiza in liquorice (Glycyrrhiza glabra) grown under salinity stress. Environ. Exp. Bot. 2019, 160, 25–34. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeha, M.; Ferrandino, A.; Khemiraac, H.; Schubert, A.; Secchi, F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by abscisic acid (ABA) in drought-stressed olive plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef]
- Beltrano, J.; Ronco, M. Improved tolerance of wheat plants (Triticum aestivum L.) to drought stress and rewatering by the arbuscular mycorrhizal fungus Glomus claroideum: Effect on growth and cell membrane stability. Braz. J. Plant Physiol. 2008, 20, 29–37. [Google Scholar] [CrossRef]
- Al-Karaki, G.; McMichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Al-Omoush, M. Wheat response to phosphogypsum and mycorrhizal fungi in alkaline soil. J. Plant Nutr. 2002, 25, 873–883. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Blackwell, P.; Abott, L.K.; Storer, P. Direct and residual effect of biochar application on mycorrhizal root colonization, growth and nutrition of wheat. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Srinivas, J.; Purushotham, A.V.; Murali Krishna, K.V.S.G. The effects of heavy metals on seed germination and plant growth on Coccinia, Mentha and Trigonella plant seeds in Timmapuram. Int. Res. J. Environ. Sci. 2013, 2, 20–24. [Google Scholar]
- Nichols, P.B.; Couch, J.D.; Al Hamdani, S.H. Selected physiological responses of Salvinia minima to different chromium concentrations. Aquat. Bot. 2000, 68, 313–319. [Google Scholar] [CrossRef]
- Mehmood, A.; Mirza, M.A.; Choudhary, M.A.; Kim, K.H.; Raza, W.; Raza, N.; Lee, S.S.; Zhang, M.; Lee, J.H.; Sarfraz, M. Spatial distribution of heavy metals in crops in a wastewater irrigated zone and health risk assessment. Environ. Res. 2019, 168, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Joner, E.J.; Briones, R.; Leyval, C. Metal-binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Gomathy, M.; Sabarinathan, K.G.; Thangaraju, M.; Subramanian, K.S.; Sivashankari Devi, T.; Ananthi, K. The effect of mycorrhizae inoculated maize root exudates in alleviation of chromium toxicity in chromium polluted environments. Insight Microbiol. 2011, 1, 20–30. [Google Scholar] [CrossRef][Green Version]
- Rabie, G.H. Contribution of arbuscular mycorrhizal fungus to red kidney and wheat plants tolerance grown in heavy in metal polluted soil. Afr. J. Biotechnol. 2005, 4, 332–345. [Google Scholar]
- Shahabivand, S.; Maivan, H.Z.; Goltapeh, E.M.; Sharifi, M.; Aliloo, A.A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Biochem. 2012, 60, 53–58. [Google Scholar] [CrossRef]
- Sonmez, O.; Aydemir, S.; Kaya, C. Mitigation effects of mycorrhiza on boron toxicity in wheat (Triticum durum) plants. N. Z. J. Crop Hortic. Sci. 2009, 37, 99–104. [Google Scholar] [CrossRef]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular Mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef]
- Berruti, A.; Bianciotto, V.; Lumini, E. Seasonal variation in winter wheat field soil arbuscular mycorrhizal fungus communities after non-mycorrhizal crop cultivation. Mycorrhiza 2018, 28, 535–548. [Google Scholar] [CrossRef]
- Bagy, H.M.M.K.; Hassan, E.A.; Nafady, N.A.; Dawood, M.F.A. Efficacy of arbuscular mycorrhizal fungi and endophytic strain Epicoccum nigrum ASU11 as biocontrol agents against blackleg disease of potato caused by bacterial strain Pectobacterium carotovora subsp. atrosepticum PHY7. Biol. Control 2019, 134, 103–113. [Google Scholar] [CrossRef]
- Nguvo, K.J.; Gao, X. Weapons hidden underneath: Bio-control agents and their potentials to activate plant induced systemic resistance in controlling crop Fusarium diseases. J. Plant Dis. Prot. 2019, 126, 177–190. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, K. Mycorrhizal colonization and phosphorus uptake in presence of PGPRs along with nematode infection. Symbiosis 2018, 77, 185–187. [Google Scholar] [CrossRef]
- De la Peña, E.; Echeverría, S.R.; van der Putten, W.H.; Freitas, H.; Moens, M. Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. New Phytol. 2006, 169, 829–840. [Google Scholar] [CrossRef]
- Barea, J.M.; Pozo, M.J.; Azcon, R.; Aguilar, C.A. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef]
- Lucini, L.; Colla, G.; Miras Moreno, M.B.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphaelf, Y. Inoculation of Rhizoglomus irregulare or Trichoderma atroviride differentially modulates metabolite profiling of wheat root exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef]
- Tahat, M.M.; Sijam, K.; Othman, R. Mycorrhizal fungi as a biological control agent. Plant Pathol. J. 2010, 9, 198–207. [Google Scholar] [CrossRef][Green Version]
- Smith, G.S. Interactions of nematodes with mycorrhizal fungi. In Vistas on Nematology; Society of Nematology: Hyattsville, MD, USA, 1987; pp. 292–300. [Google Scholar]
- Linderman, R.G. Role of VAM fungi in biocontrol. In Mycorrhizae and Plant Health; APS: St Paul, Brazil, 1994; pp. 1–26. [Google Scholar]
- Azcòn-Aguilar, C.; Barea, J.M. Arbuscular mycorrhizas and biological control of soil-borne plant pathogens: An overview of the mechanisms involved. Mycorrhiza 1996, 6, 457–464. [Google Scholar] [CrossRef]
- Gianinazzi-Pearson, V.; Gollotte, A.; Dumas-Gaudot, E.; Franken, P.; Gianinazzi, S. Gene expression and molecular modifications associated with plant responses to infection by arbuscular mycorrhizal fungi. In Advances in Molecular Genetics of Plant-Microbe Interactions; Daniels, M., Downic, J.A., Osbourn, A.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 179–186. [Google Scholar]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–654. [Google Scholar] [CrossRef]
- Siddiqui, Z.A. PGPR: Biocontrol and Biofertilization; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Behn, O. Influence of Pseudomonas fluorescens and arbuscular mycorrhiza on the growth, yield, quality and resistance of wheat infected with Gaeumannomyces graminis. J. Plant Dis. Prot. 2008, 115, 4–8. [Google Scholar] [CrossRef]
- Fiorilli, V.; Vannini, C.; Ortolani, F.; Garcia-Seco, D.; Chiapello, M.; Novero, M.; Domingo, G.; Terzi, V.; Morcia, C.; Bagnaresi, P.; et al. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 9625. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Randoux, B.; Tisserant, B.; Fontaine, J.; Magnin-Robert, M.; Sahraoui, A.L.; Reignault, P.H. Phosphorus supply, arbuscular mycorrhizal fungal species, and plant genotype impact on the protective efficacy of mycorrhizal inoculation against wheat powdery mildew. Mycorrhiza 2016, 26, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Khong, N.G.; Tisserant, B.; Randoux, B.; Fontaine, J.; Magnin-Robert, M.; Reignault, P.; Sahraoui, A.L. Defence mechanisms associated with mycorrhiza-induced resistance in wheat against powdery mildew. Funct. Plant Biol. 2017, 44, 443–454. [Google Scholar] [CrossRef]
- Falahian, F.; Ardebili, Z.O.; Fahimi, F.; Khavarinejad, R. Effect of Mycorrhizal Fungi on Some Defense Enzymes against Gaeumannomyces gaminis in Wheat. Pak. J. Biol. Sci. 2007, 10, 2418–2422. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal fungi influence soil structure. In Arbuscular Mycorrhizas: Molecular Biology and Physiology; Kapulnik, Y., Douds, D.D., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 3–18. [Google Scholar]
- Rilling, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.M.; Sa, J.C.; Albrecht, A. Soil organic matter, biota and aggregation in temperate and tropical soils—Effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Kuyper, T.W. Mycorrhizas and tropical soil fertility. Agric. Ecosyst. Environ. 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 1, 563–569. [Google Scholar] [CrossRef]
- Bai, C.; He, X.; Tang, H.; Shan, B.; Zhao, L. Spatial distribution of arbuscular mycorrhizal fungi, glomalin and soil enzymes under the canopy of Astragalus adsurgens Pall. in the Mu Us sandland, China. Soil Biol. Biochem. 2009, 41, 941–947. [Google Scholar] [CrossRef]
- Nichols, K.A.; Wright, S.F. Contributions of soil fungi to organic matter in agricultural soils. In Functions and Management of Soil Organic Matter in Agroecosystems; Magdoff, F., Weil, R., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 179–198. [Google Scholar]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2001, 238, 325–333. [Google Scholar] [CrossRef]
- Miransari, M.; Bahrami, H.A.; Rejali, F.; Malakouti, M.J. Using arbuscular mycorrhiza to reduce the stressful effects of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol. Biochem. 2008, 40, 1197–1206. [Google Scholar] [CrossRef]
- De Vita, P.; Avio, L.; Sbrana, C.; Laidò, G.; Marone, D.; Mastrangelo, A.M.; Cattivelli, L.; Giovannetti, M. Genetic markers associated to arbuscular mycorrhizal colonization in durum wheat. Sci. Rep. 2018, 8, 10612. [Google Scholar] [CrossRef]
- Lehnert, H.; Serfling, A.; Enders, M.; Friedt, W.; Ordon, F. Genetics of mycorrhizal symbiosis in winter wheat (Triticum aestivum). New Phytol. 2017, 215, 779–791. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganugi, P.; Masoni, A.; Pietramellara, G.; Benedettelli, S. A Review of Studies from the Last Twenty Years on Plant–Arbuscular Mycorrhizal Fungi Associations and Their Uses for Wheat Crops. Agronomy 2019, 9, 840. https://doi.org/10.3390/agronomy9120840
Ganugi P, Masoni A, Pietramellara G, Benedettelli S. A Review of Studies from the Last Twenty Years on Plant–Arbuscular Mycorrhizal Fungi Associations and Their Uses for Wheat Crops. Agronomy. 2019; 9(12):840. https://doi.org/10.3390/agronomy9120840
Chicago/Turabian StyleGanugi, Paola, Alberto Masoni, Giacomo Pietramellara, and Stefano Benedettelli. 2019. "A Review of Studies from the Last Twenty Years on Plant–Arbuscular Mycorrhizal Fungi Associations and Their Uses for Wheat Crops" Agronomy 9, no. 12: 840. https://doi.org/10.3390/agronomy9120840
APA StyleGanugi, P., Masoni, A., Pietramellara, G., & Benedettelli, S. (2019). A Review of Studies from the Last Twenty Years on Plant–Arbuscular Mycorrhizal Fungi Associations and Their Uses for Wheat Crops. Agronomy, 9(12), 840. https://doi.org/10.3390/agronomy9120840




