Contribution of Proximal and Distal Grains Within Spikelets in Relation to Yield and Yield Components in the Winter Wheat Production Region of China From 1948 to 2012
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Sampling and Measurements
2.2.1. Shoot Trait Measurements
2.2.2. Accumulation and Transformation of Dry Matter
2.2.3. Yield and Yield-Related Traits
2.3. Statistical Analysis
3. Results
3.1. Yield and Related Traits
3.2. Accumulation and Transformation of Dry Matter
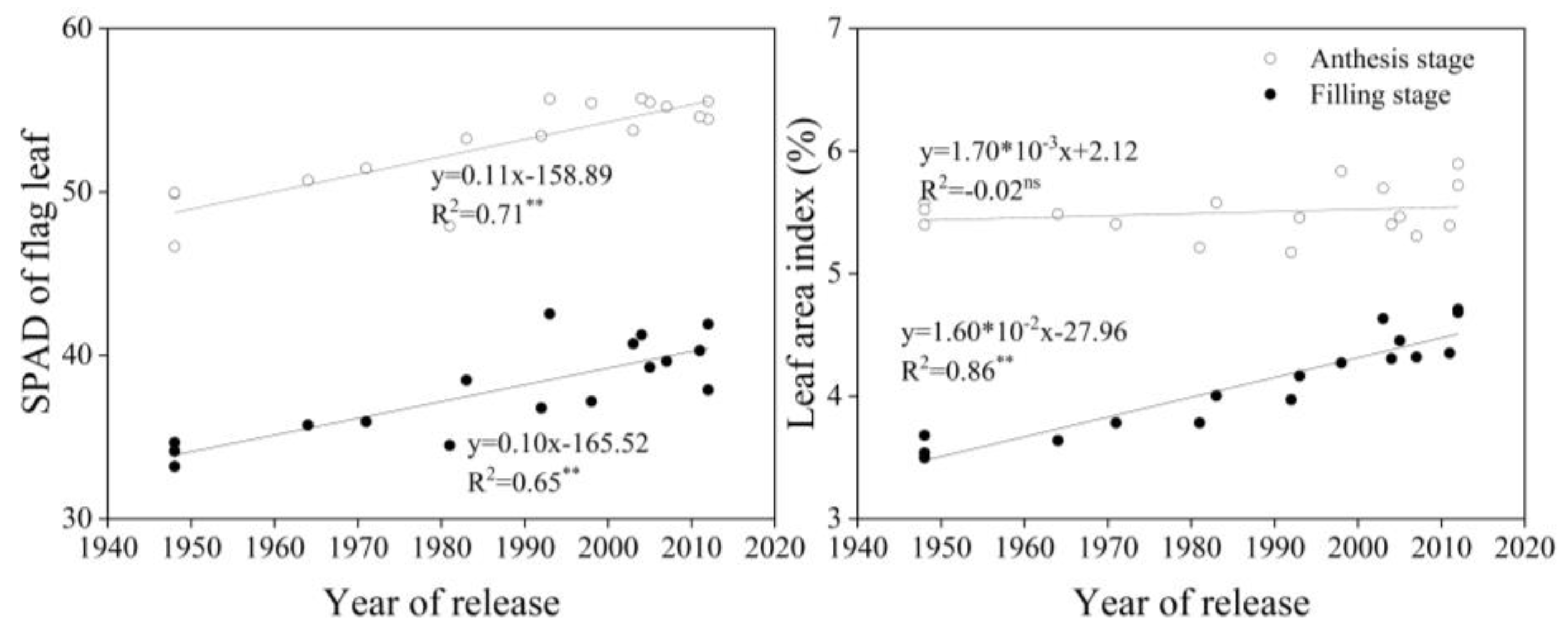
3.3. Shoot Traits at Different Growth Stages
3.4. Change in Related Traits for G1, G2, G3, and G4
3.5. Correlation Between the Performance of G1, G2, G3, and G4 and Yield, TGW, GN Per m2
3.6. Grain Number and Weight in Spikelet
4. Discussion
4.1. Evolution of Yield and Yield-Related Traits
4.2. Accumulation and Translocation of Dry Matter and Photosynthesis During Grain Filling Contribute to Yield
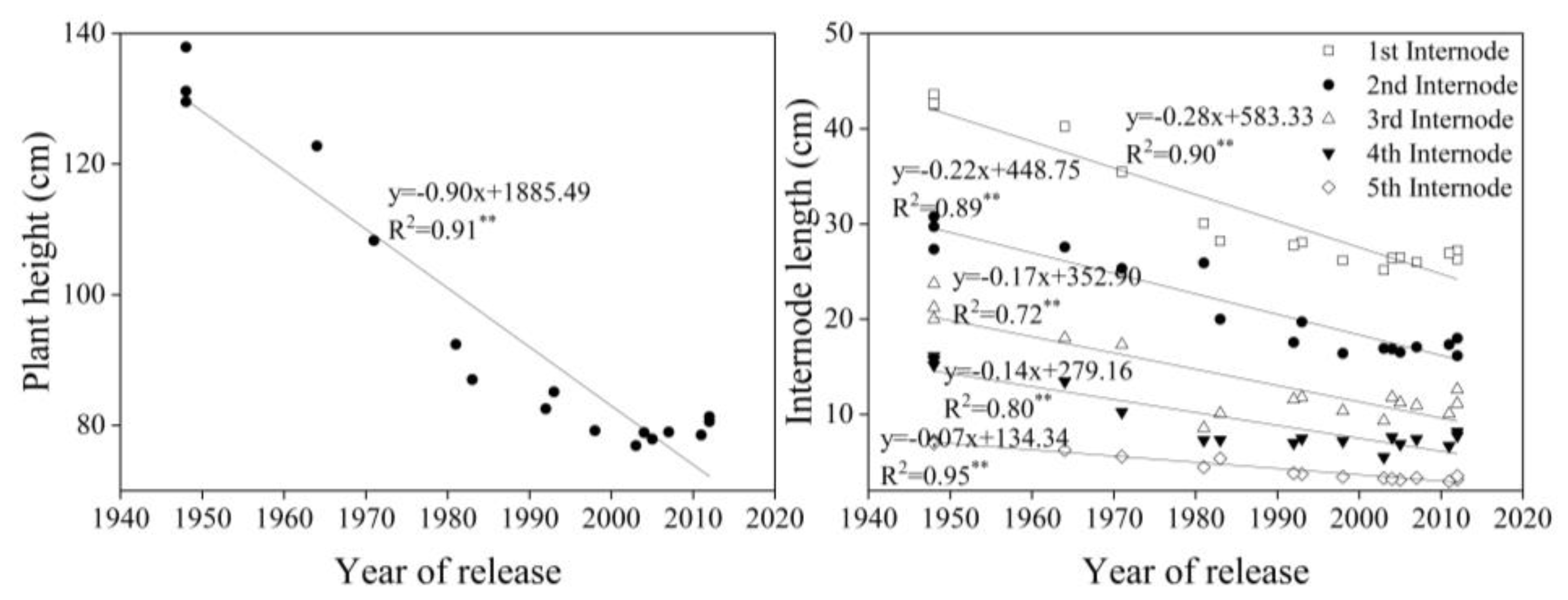
4.3. Plant Height and Internode Length
4.4. Relationship Between Stem Numbers, Spike Number Per Area, and Yield
4.5. Contribution and Influence of Proximal and Distal Grains to Yield, TGW, and GN
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ghose, B. Food security and food self-sufficiency in China: From past to 2050. Food Energy Secur. 2014, 3, 86–95. [Google Scholar] [CrossRef]
- Lal, R.; Uphoff, N.; Hansen, D.O. Food Security and Environmental Quality in the Developing World; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Reynolds, M.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.; Snape, J.W.; Angus, W.J. Raising yield potential in wheat. J. Exp. Botany 2009, 60, 1899–1918. [Google Scholar] [CrossRef] [Green Version]
- Tyczewska, A.E.; Woźniak, J.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards food security: Current state and future prospects of agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, F.; Liu, C.; Yu, H.; Cao, B.; Tian, S.; Liao, Y.; Siddique, K.H. Wheat yield improvements in China: Past trends and future directions. Field Crops Res. 2015, 177, 117–124. [Google Scholar] [CrossRef]
- Langer, R.H.M.; Hanif, M. A study of floret development in wheat (Triticum aestivum L.). Ann. Botany 1973, 37, 743–751. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol. 2006, 171, 293–303. [Google Scholar] [CrossRef]
- Ferrante, A.; Savin, R.; Slafer, G.A. Relationship between fruiting efficiency and grain weight in durum wheat. Field Crops Res. 2015, 177, 109–116. [Google Scholar] [CrossRef]
- Slafer, G.A.; Elia, M.; Savin, R.; García, G.A.; Terrile, I.I.; Ferrante, A.; Miralles, D.J.; González, F.G. Fruiting efficiency: An alternative trait to further rise wheat yield. Food Energy Secur. 2015, 4, 92–109. [Google Scholar] [CrossRef]
- Baillot, N.; Girousse, C.; Allard, V.; Piquet-Pissaloux, A.; Le Gouis, J. Different grain-filling rates explain grain-weight differences along the wheat ear. PLoS ONE 2018, 13, e0209597. [Google Scholar] [CrossRef]
- Ravi, I.; Ghildiyal, M.C. Translocation of 14C-sucrose within ear in durum and aestivum wheat varieties. J. Agron. Crop Sci. 2001, 189, 9–13. [Google Scholar] [CrossRef]
- Calderini, D.F.; Ortiz-Monasterio, I. Crop physiology & metabolism: Grain position affects grain macronutrient and micronutrient concentrations in wheat. Crop Sci. 2003, 43, 141–151. [Google Scholar]
- Rajala, A.; Hakala, K.; Mäkelä, P.; Muurinen, S.; Peltonen-Sainio, P. Spring wheat response to timing of water deficit through sink and grain filling capacity. Field Crops Res. 2009, 114, 263–271. [Google Scholar] [CrossRef]
- Feng, F.; Han, Y.; Wang, S.; Yin, Z.; Peng, M.; Zhou, M.; Gao, W.; Wen, X.; Qin, X.; Siddique, K.H.M. The effect of grain position on genetic improvement of grain number and thousand grain weight in winter wheat in North China. Front. Plant Sci. 2018, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Siddique, K.H.M.; Kirby, E.J.M.; Perry, M.W. Ear:stem ratio in old and modern wheat varieties; relationship with improvement in number of grains per ear and yield. Field Crop Res. 1989, 21, 59–78. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Belford, R.K.; Perry, M.W.; Tennant, D. Growth, development and light interception of old and modern wheat cultivars in a Mediterranean-type environment. Austral. J. Agric. Res. 1989, 40, 473–487. [Google Scholar]
- Royo, C.; Alvaro, F.; Martos, V.; Ramdani, A.; Isidro, J.; Villegas, D.; Del Moral, L.F.G. Genetic changes in durum wheat yield components and associated traits in Italian and Spanish varieties during the 20th century. Euphytica 2007, 155, 259–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wang, H.; Dong, H.; Qi, X.; Zhao, M.; Fang, Y.; Gao, C.; Hu, L. Progress in genetic improvement of grain yield and related physiological traits of Chinese wheat in Henan Province. Field Crops Res. 2016, 199, 117–128. [Google Scholar] [CrossRef]
- Flohr, B.M.; Hunt, J.R.; Kirkegaard, J.A.; Evans, J.R.; Swan, A.; Rheinheimer, B. Genetic gains in NSW wheat cultivars from 1901 to 2014 as revealed from synchronous flowering during the optimum period. Eur. J. Agron. 2018, 98, 1–13. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Z.; Sui, X.; Xia, X.; Zhang, X.; Zhang, G. Genetic improvement of grain yield and associated traits in the northern China winter wheat region from 1960 to 2000. Crop Sci. 2007, 47, 245–253. [Google Scholar] [CrossRef]
- Tian, Z.; Jing, Q.; Dai, T.; Jiang, D.; Cao, W. Effects of genetic improvements on grain yield and agronomic traits of winter wheat in the Yangtze River Basin of China. Field Crops Res. 2011, 124, 417–425. [Google Scholar] [CrossRef]
- Xiao, Y.; Qian, Z.; Wu, K.; Liu, J.; Xia, X.; Ji, W.; He, Z. Genetic gains in grain yield and physiological traits of winter wheat in Shandong Province, China, from 1969 to 2006. Crop Sci. 2012, 52, 44–56. [Google Scholar] [CrossRef]
- Qin, X.; Feng, F.; Wen, X.; Siddique, K.H.; Liao, Y. Historical genetic responses of yield and root traits in winter wheat in the Yellow-Huai-Hai River Valley Region of China due to modern breeding (1948–2012). Plant and Soil. 2019, 439, 7–18. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stage of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- SAS Institute. SAS Version 9.1.2, 2003; SAS Institute Inc.: Cary, NC, USA, 2002–2003. [Google Scholar]
- Ortiz-Monasterio, R.; Sayre, K.; Rajaram, S.; McMahon, M. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 1997, 37, 898–904. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.; Cai, S.; He, Z.; Zhang, X.; Xia, X.; Zhang, G. Genetic improvement of grain yield and associated traits in the southern China winter wheat region: 1949 to 2000. Euphytica 2007, 157, 465–473. [Google Scholar] [CrossRef]
- Gao, F.; Ma, D.; Yin, G.; Rasheed, A.; Yan, D.; Xiao, Y.; Wu, X.; Xia, X.; He, Z. Genetic progress in grain yield and physiological traits in Chinese wheat cultivars of Southern Yellow and Huai valley winter wheat zone since 1950. Crop Sci. 2017, 57, 760–773. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wang, H.; Fang, Y.; Dong, H.; Qi, X. Progress in improving stem lodging resistance of Chinese wheat cultivars. Euphytica 2016, 212, 275–286. [Google Scholar] [CrossRef]
- Zheng, T.C.; Zhang, X.K.; Yin, G.H.; Wang, L.N.; Han, Y.L.; Chen, L.; Huang, F.; Tang, J.W.; Xia, X.C.; He, Z.H. Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1981 and 2008. Field Crops Res. 2011, 122, 225–233. [Google Scholar] [CrossRef]
- Beche, E.; Benin, G.; da Silva, C.L.; Munaro, L.B.; Marchese, J.A. Genetic gain in yield and changes associated with physiological traits in Brazilian wheat during the 20th century. Eur. J. Agron. 2014, 61, 49–59. [Google Scholar] [CrossRef]
- Keser, M.; Gummadov, N.; Akin, B.; Belen, S.; Mert, Z.; Taner, S.; Topal, A.; Yazar, S.; Morgounov, A.; Sharma, R.C. Genetic gains in wheat in Turkey: Winter wheat for dryland conditions. Crop J. 2017, 5, 533–540. [Google Scholar] [CrossRef]
- Gegas, V.C.; Nazari, A.; Griffiths, S.; Simmonds, J.; Fish, L.; Orford, S.; Sayers, L.; Doonan, J.H.; Snape, J.W. A genetic framework for grain size and shape variation in wheat. Plant Cell 2010, 22, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Pheloung, P.; Siddique, K.H. Contribution of stem dry matter to grain yield in wheat cultivars. Funct. Plant Biol. 1991, 18, 53–64. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, Z.; Wen, X.; Liao, Y.; Liu, Y. Effect of non-structural carbohydrate accumulation in the stem pre-anthesis on grain filling of wheat inferior grain. Field Crops Res. 2017, 211, 66–76. [Google Scholar] [CrossRef]
- Tang, Y.; Rosewarne, G.M.; Li, C.; Wu, X.; Yang, W.; Wu, C. Physiological factors underpinning grain yield improvements of synthetic-derived wheat in Southwestern China. Crop Sci. 2015, 55, 98–112. [Google Scholar] [CrossRef]
- Aggarwal, P.; Fischer, R.; Liboon, S. Source–sink relations and effects of post-anthesis canopy defoliation in wheat at low latitudes. J. Agric. Sci. 1990, 114, 93–99. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R. Source–sink relationships and grain mass at different positions within the spike in wheat. Field Crops Res. 1994, 37, 39–49. [Google Scholar] [CrossRef]
- Parry, M.A.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.-G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Botany 2010, 62, 453–467. [Google Scholar]
- Chairi, F.; Vergara-Diaz, O.; Vatter, T.; Aparicio, N.; Nieto-Taladriz, M.T.; Kefauver, S.C.; Bort, J.; Serret, M.D.; Araus, J.L. Post-green revolution genetic advance in durum wheat: The case of Spain. Field Crops Res. 2018, 228, 158–169. [Google Scholar] [CrossRef]
- Moghaddam, M.E.; Jalal Kamali, M.R.; Soda, N.; Sadre Jahani, S.; Ghodsi, M. Temporal variation in phonological and agronomic traits of some irrigated facultative/winter bread wheat, Triticum aestivum L., cultivars released between 1943 and 2011 in Iran. Crop Breeding J. 2016, 6, 9–16. [Google Scholar]
- Wang, Y.S.; Chen, L.; Du, Y.Y.; Yang, Z.Y.; Condon, A.G.; Hu, Y.G. Genetic effectof dwarfing gene Rht13 compared with Rht-D1b on plant height and someagronomic traits in common wheat, Triticum aestivum L. Field Crops Res. 2014, 162, 39–47. [Google Scholar] [CrossRef]
- Joudi, M.; Ahmadi, A.; Mohammadi, V.; Abbasi, A.; Mohammadi, H. Genetic changes in agronomic and phenologic traits of Iranian wheat cultivars grown in different environmental conditions. Euphytica 2014, 196, 237–249. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Sadras, V.O. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014, 157, 71–83. [Google Scholar] [CrossRef]
- Cai, T.; Xu, H.; Peng, D.; Yin, Y.; Yang, W.; Ni, Y.; Chen, X.; Xu, C.; Yang, D.; Cui, Z.; et al. Exogenous hormonal application improves grain yield of wheat by optimizing tiller productivity. Field Crops Res. 2014, 155, 172–183. [Google Scholar] [CrossRef]
- Qin, X.; Weiner, J.; Qi, L.; Xiong, Y.; Li, F. Allometric analysis of the effects of density on reproductive allocation and Harvest Index in 6 varieties of wheat, Triticum. Field Crops Res. 2013, 144, 162–166. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Reynolds, M.P.; Sylvester-Bradley, R. Genetic improvement of grain crops: Yield potential. In Crop Physiology Applications for Genetic Improvement and Agronomy; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Amsterdam, The Netherlands, 2009; pp. 355–386. [Google Scholar]
- Hoang, K.; Li, X.; Bo, Y.; Zhou, Q.; Cai, J.; Wang, X.; Cai, H.; Dai, T.; Cao, W.; Jiang, D. Accumulation of high-molecular-weight glutenin subunits in superior and inferior grains of a winter wheat, Yangmai 158. Cereal Chem. 2017, 94, 508–512. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Yang, Y.; Chen, X.; Wang, Q.; Zhang, X.; Ran, L.; Xiong, F. Morphology and Physicochemical Properties of Starch in Wheat Superior and Inferior Grains. Starch-Stärke 2018, 70, 1700177. [Google Scholar] [CrossRef]
- Guo, Z.; Schnurbusch, T. Variation of floret fertility in hexaploid wheat revealed by tiller removal. J. Exp. Bot. 2015, 66, 5945–5958. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cui, Z.; Ni, Y.; Zheng, M.; Yang, D.; Jin, M.; Chen, J.; Wang, Z.; Yin, Y. Plant density effect on grain number and weight of two winter wheat cultivars at different spikelet and grain positions. PLoS ONE 2016, 11, e0155351. [Google Scholar] [CrossRef]
- Miralles, D.J.; Slafer, G.A. Individual grain weight responses to genetic reduction in culm length in wheat as affected by source-sink manipulations. Field Crops Res. 1995, 43, 55–66. [Google Scholar] [CrossRef]
- Slafer, G.A.; Andrade, F.H. Physiological attributes related to the generation of grain yield in bread wheat cultivars released at different eras. Field Crops Res. 1993, 31, 351–367. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Grain weight response to increases in number of grains in wheat in a Mediterranean area. Field Crops Res. 2006, 98, 52–59. [Google Scholar] [CrossRef]








| Year of Release | Groups | Origin | Dwarf Gene | |
|---|---|---|---|---|
| Bima1 | 1948 | Old | Mazhamai/Biyumai | – |
| Bima4 | 1948 | Old | Mazhamai/Biyumai | Rht8 |
| Xinong6028 | 1948 | Old | Jingyang60/Zhongnong28 | – |
| Fengchan3 | 1964 | Old | Danmai1/Xinong6028 × Bima1 | – |
| Aifeng3 | 1971 | Middle | Xiannong39/58(18)2//Fengchan3 | Rht-D1b |
| Xiaoyan6 | 1981 | Middle | (ST2422/464)/Xiaoyan96 | Rht-B1b + Rht8 |
| Yumai2 | 1983 | Middle | 65(14)3/Kanghuihong | Rht-D1b + Rht8 |
| Xinong881 | 1992 | Middle | Xiaoyan6/Xinong65/83(2)-3-3 | Rht-D1b |
| Shan229 | 1993 | Middle | Shan7853/80356 | Rht-B1b + Rht8 |
| Xiaoyan22 | 1998 | Middle | (Xiaoyan6 × 775-1) × Xiaoyan107 | Rht-B1b + Rht8 |
| Yanzhan4110 | 2003 | New | [(C39 × Xibei78(6)9-2) × (FR81-3 × Aizao781-4)] × Aizao781-4 | Rht-D1b + Rht8 |
| Zhoumai18 | 2004 | New | Neixiang185/Zhoumai9 | Rht-D1b + Rht8 |
| Xinong979 | 2005 | New | Xinong2611/(918/95Xuan1) F1 | Rht8 |
| Zhoumai22 | 2007 | New | Zhoumai12/Wenmai6//Zhoumai13 | Rht-D1b + Rht8 |
| Shanmai139 | 2011 | New | Xiaoyan22/94156/N9134 | Rht-B1b |
| Luomai18 | 2012 | New | 4336/Zhoumai16 | Rht-D1b + Rht8 |
| Zhoumai26 | 2012 | New | Zhoumai24/Zhoumai 22 | Rht-D1b + Rht8 |
| Cultivar | PreDM (t ha−1) | PostDM (t ha−1) | TDMa (t ha−1) | AGBM (t ha−1) |
|---|---|---|---|---|
| Bima1 | 10.33 | 3.09 | 1.24 | 13.42 |
| Bima4 | 10.92 | 3.47 | 1.17 | 14.39 |
| Xinong6028 | 10.61 | 3.12 | 1.34 | 13.73 |
| Fengchan3 | 11.43 | 3.74 | 1.20 | 15.17 |
| Aifeng3 | 10.95 | 3.36 | 1.74 | 14.31 |
| Xiaoyan6 | 10.43 | 3.63 | 1.68 | 14.06 |
| Yumai2 | 10.83 | 3.39 | 2.12 | 14.22 |
| Xinong881 | 10.22 | 3.34 | 2.03 | 13.56 |
| Shan229 | 9.95 | 3.97 | 2.01 | 13.92 |
| Xiaoyan22 | 10.78 | 3.63 | 2.55 | 14.4 |
| Luomai18 | 10.42 | 3.07 | 2.15 | 13.49 |
| Zhoumai18 | 11.15 | 3.91 | 2.67 | 15.06 |
| Xinong979 | 10.13 | 3.43 | 2.00 | 13.57 |
| Yanzhan4110 | 10.98 | 3.37 | 2.27 | 14.35 |
| Zhoumai22 | 10.83 | 3.76 | 2.63 | 14.59 |
| Shanmai139 | 10.68 | 3.53 | 2.83 | 14.21 |
| Zhoumai26 | 11.59 | 4.09 | 2.75 | 15.68 |
| Genetic Gain | 0.01 ns | 0.25 * | 1.20 ** | 0.05 ns |
| R2 | 0.01 | 0.1 | 0.85 | 0.06 |
| Variable | Correlation with Yield | Direct Effect | Indirect Effect | ||
|---|---|---|---|---|---|
| →×1 | →×2 | →×3 | |||
| Path Analysis 1 | |||||
| TGW (×1) | 0.91 ** | 0.53 * | 0.38 | - | |
| Grain Number per m2 (×2) | 0.90 ** | 0.43 | 0.47 | - | |
| Path Analysis 2 | |||||
| PreDM (×1) | 0.25 | 0.007 | 0.10 | 0.14 | |
| PostDM (×2) | 0.63 ** | 0.30 ** | 0.003 | 0.37 | |
| TDMa (×3) | 0.93 ** | 0.81 ** | 0.001 | 0.12 | |
| Grain Weight Per m2 | Average Single Grain Weight | Grain Number Per m2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | |
| Grain yield | 0.85 ** | 0.38 * | 0.84 ** | 0.79 ** | 0.56 ** | 0.20 | 0.19 | 0.57 ** | 0.77 ** | 0.76 ** | 0.83 ** | 0.82 ** |
| TGW | 0.65 ** | 0.42 * | 0.67 ** | 0.84 ** | 0.91 ** | 0.80 ** | 0.75 ** | 0.76 ** | 0.30 | 0.24 | 0.42 * | 0.82 ** |
| GPM | 0.90 ** | 0.35 * | 0.78 ** | 0.61 ** | 0.33 | –0.08 | –0.07 | 0.33 | 0.97 ** | 0.94 ** | 0.87 ** | 0.74 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, F.; Dang, P.; Pu, X.; Wen, X.; Qin, X.; Chen, Y.; H. M. Siddique, K. Contribution of Proximal and Distal Grains Within Spikelets in Relation to Yield and Yield Components in the Winter Wheat Production Region of China From 1948 to 2012. Agronomy 2019, 9, 850. https://doi.org/10.3390/agronomy9120850
Feng F, Dang P, Pu X, Wen X, Qin X, Chen Y, H. M. Siddique K. Contribution of Proximal and Distal Grains Within Spikelets in Relation to Yield and Yield Components in the Winter Wheat Production Region of China From 1948 to 2012. Agronomy. 2019; 9(12):850. https://doi.org/10.3390/agronomy9120850
Chicago/Turabian StyleFeng, Fan, Pengfei Dang, Xuan Pu, Xiaoxia Wen, Xiaoliang Qin, Yinglong Chen, and Kadambot H. M. Siddique. 2019. "Contribution of Proximal and Distal Grains Within Spikelets in Relation to Yield and Yield Components in the Winter Wheat Production Region of China From 1948 to 2012" Agronomy 9, no. 12: 850. https://doi.org/10.3390/agronomy9120850






