To Stop Nitrogen Overdose in Soilless Tomato Crop: A Way to Promote Fruit Quality without Affecting Fruit Yield
Abstract
:1. Introduction
2. Results
2.1. Nitrogen Supply Effects on Tomato Plant Growth and Architecture
2.2. Distribution of the Photosynthetic Activity Throughout Plant Architecture and Impact of Nitrogen Treatments
2.3. Influence of Nitrogen Supply on the Yield of a Soilless Tomato Crops
2.4. The Nitrogen Content of the Vegetative Parts as a Function of Nitrogen Supply
2.5. Impact of the Nitrogen Supply on Tomato Fruit Quality
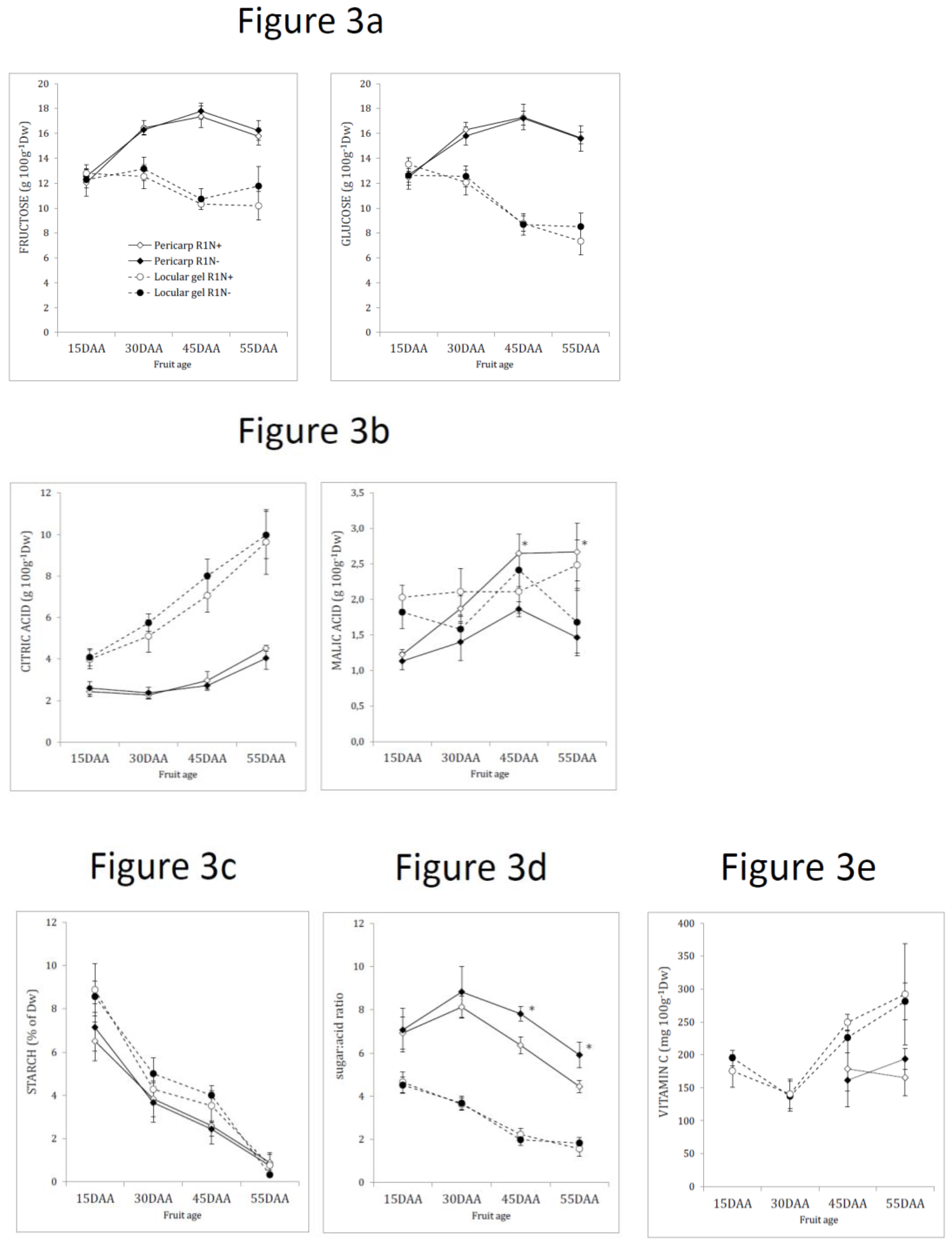
2.6. Variation in the Biochemical Compounds in the Inner Tissues and Pericarp during Tomato Ripening
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Nitrogen Management
4.3. Agronomic Performance
4.4. Plant Architecture
4.5. Leaf Potential Photosynthetic Activity
4.5.1. Photosynthetic Activity of All Individual Leaves in the Control Treatment
4.5.2. Photosynthetic Activity and Impact of the Nitrogen Treatments
4.6. Plant and Fruit Sampling
4.6.1. Vegetative Material Sampling
4.6.2. Fruit Sampling
4.6.3. Biochemical Analyses
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanthoor, B.H.E.; Stanghellini, C.; van Henten, E.J.; de Visser, P.H.B. A methodology for model-based greenhouse design: Part 1, a greenhouse climate model for a broad range of designs and climates. Biosyst. Eng. 2011, 110, 363–377. [Google Scholar] [CrossRef]
- Boulard, T.; Roy, J.-C.; Pouillard, J.-B.; Fatnassi, H.; Grisey, A. Modelling of micrometeorology, canopy transpiration and photosynthesis in a closed greenhouse using computational fluid dynamics. Biosyst. Eng. 2017, 158, 110–133. [Google Scholar] [CrossRef]
- Truffault, V.; Le Quillec, S.; Brajeul, E. Insights into the potential of semi-closed greenhouses and future perspectives for tomato crops. Acta Horti. 2018, 1227, 141–150. [Google Scholar] [CrossRef]
- Nitrate Directive: Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A31991L0676 (accessed on 20 November 2018).
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Schwarz, D.; Thompson, A.J.; Kläring, H.-P. Guidelines to use tomato in experiments with a controlled environment. Front. Plant Sci. 2014, 5, 625–641. [Google Scholar] [CrossRef]
- Hoagland, D.R. Inorganic nutrition of plants; Chronica Botanica Co.: Waltham, UK, 1944. [Google Scholar]
- De Kreij, C.; Voogt, W.; Van den Bos, A.; Baas, R. Voedingsoplossingen voor de teelt van tomaat in gesloten teeltsystemen; Brochure VG: Naaldwijk, The Netherlands, 1997. [Google Scholar]
- Bénard, C.; Gautier, H.; Bourgaud, F.; Grasselly, D.; Navez, B.; Caris-Veyrat, C.; Weiss, M.; Génard, M. Effects of low nitrogen supply on tomato (Solanum lycopersicum) fruit yield and quality with special emphasis on sugars, acids, ascorbate, carotenoids, and phenolic compounds. J. Agric. Food Chem. 2009, 57, 4112–4123. [Google Scholar] [CrossRef]
- Turcotte, G.; Larouche, R.; Carrier, A.; Lambert, L. Production de la tomate de serre au Québec (guide technique). Available online: https://www.agrireseau.net/documents/95590/production-de-la-tomate-de-serre-au-quebec-guide-technique-de-297-pages (accessed on 15 August 2017).
- Le Bot, J.; Adamowicz, S.; Robin, P.; Andriolo, J.L.; Gary, C. Modelling nitrate uptake by greenhouse tomato crops at the short and long time scales. Acta Horti. 1998, 456, 237–246. [Google Scholar] [CrossRef]
- Adamowicz, S.; Le Bot, J. Trends in modelling nitrate uptake. Acta Horti. 1991, 507, 231–240. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Nutrient Solutions for Soilless Cultures. In Plant Nutrition of Greenhouse Crops; Springer: Houten, The Netherlands, 2009; pp. 257–275. [Google Scholar]
- Incrocci, L.; Massa, D.; Pardossi, A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Davies, J.N.; Hobson, G.E.; McGlasson, W.B. The constituents of tomato fruit—the influence of environment, nutrition, and genotype. CRC Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Scott, J.W.; Einstein, M.A.; Malundo, T.M.M.; Carr, B.T.; Shewfelt, R.L.; Tandon, K.S. Relationship between Sensory and Instrumental Analysis for Tomato Flavor. J. Amer. Soc. Hort. Sci. 1998, 123, 906–915. [Google Scholar] [CrossRef]
- Bhowmik, D.; Kumar, K.S.; Paswan, S.; Srivastava, S. Tomato-a natural medicine and its health benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How Does Tomato Quality (Sugar, Acid, and Nutritional Quality) Vary with Ripening Stage, Temperature, and Irradiance? J. Agr. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits—making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Bertin, N. The potential of the magic tom parental accessions to explore the genetic variability in tomato acclimation to repeated cycles of water deficit and recovery. Front. Plant Sci. 2016, 6, 1187. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Li, S.-X.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops. J. Sci. Food Agr. 2007, 88, 7–23. [Google Scholar] [CrossRef]
- Wang, C.; Gu, F.; Chen, J.; Yang, H.; Jiang, J.; Du, T.; Zhang, J. Assessing the response of yield and comprehensive fruit quality of tomato grown in greenhouse to deficit irrigation and nitrogen application strategies. Agr. Water Manage. 2015, 161, 9–19. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y. Evaluation of the effects of irrigation and fertilization on tomato fruit yield and quality: A principal component analysis. Sci. Rep. 2017, 7, 350. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of genetic material and current technologies on product quality of selected greenhouse vegetables – A review. Eur. J. Horticul. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Larbat, R.; Olsen, K.M.; Slimestad, R.; Løvdal, T.; Bénard, C.; Verheul, M.; Bourgaud, F.; Robin, C.; Lillo, C. Influence of repeated short-term nitrogen limitations on leaf phenolics metabolism in tomato. Phytochemistry 2012, 77, 119–128. [Google Scholar] [CrossRef]
- Parisi, M.; Giordano, L.; Pentangelo, A.; D’Onofrio, B.; Villari, G. Effects of different levels of nitrogen fertilization on yield and fruit quality in processing tomato. Acta Horticulturae. 2006, 700, 129–132. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Tan, C.S.; Liu, K.; Drury, C.F.; Papadopoulos, A.P.; Warner, J. Yield and Economic assessments of fertilizer nitrogen and phosphorus for processing tomato with drip fertigation. Agron. J. 2010, 102, 774–780. [Google Scholar] [CrossRef]
- Albornoz, F. Crop responses to nitrogen overfertilization: A review. Scientia Horticulturae. 2016, 205, 79–83. [Google Scholar] [CrossRef]
- Sarlikioti, V.; De Visser, P.H.; Marcelis, L.F.M. Exploring the spatial distribution of light interception and photosynthesis of canopies by means of a functional—structural plant model. Ann. Bot. 2011, 107, 875–883. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.W.; Ahn, T.I.; Shin, J.H.; Park, K.S.; Son, J.E. Sweet Pepper (Capsicum annuum L.) Canopy photosynthesis modeling using 3D plant architecture and light ray-tracing. Front. Plant Sci. 2016, 7, 1321–1331. [Google Scholar] [CrossRef]
- Sarlikioti, V.; De Visser, P.H.; Buck-Sorlin, G.H.; Marcelis, L.F.M. How plant architecture affects light absorption and photosynthesis in tomato: Towards an ideotype for plant architecture using a functional–structural plant model. Ann. Bot. 2011, 108, 1065–1073. [Google Scholar] [CrossRef]
- Field, C. Allocating leaf nitrogen for the maximization of carbon gain: Leaf age as a control on the allocation program. Oecologia. 1989, 56, 341–347. [Google Scholar] [CrossRef]
- Prieto, J.A.; Louarn, G.; Peña, J.P.; Ojeda, H.; Simonneau, T.; Lebon, E. A leaf gas exchange model that accounts for intra-canopy variability by considering leaf nitrogen content and local acclimation to radiation in grapevine (Vitis vinifera L.). Plant Cell Environ. 2012, 35, 1313–1328. [Google Scholar] [CrossRef]
- Hikosaka, K. Optimality of nitrogen distribution among leaves in plant canopies. J. Plant Res. 2016, 129, 299–311. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.-R.; Krapp, A. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Le Bot, J.; Jeannequin, B.; Fabre, R. Growth and Nitrogen Status of Soilless Tomato Plants Following Nitrate Withdrawal from the Nutrient Solution. Ann. Bot. 2001, 88, 361–370. [Google Scholar] [CrossRef]
- Vos, J.; van der Putten, P.E.L. Effect of nitrogen supply on leaf growth, leaf nitrogen economy and photosynthetic capacity in potato. Field Crop. Res. 1998, 59, 63–72. [Google Scholar] [CrossRef]
- Vos, J.; Putten, P.E.L.; van der Birch, C.J. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crop. Res. 2005, 93, 64–73. [Google Scholar] [CrossRef]
- Guidi, L.; Lorefice, G.; Pardossi, A.; Malorgio, F.; Tognoni, F.; Soldatini, G.F. Growth and photosynthesis of Lycopersicon esculentum (L.) plants as affected by nitrogen deficiency. Biol. Plantarum 1997, 35, 235. [Google Scholar] [CrossRef]
- Hirose, T.; Werger, M.J.A. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 1987, 72, 520–526. [Google Scholar] [CrossRef]
- Hörtensteiner, S.; Feller, U. Nitrogen metabolism and remobilization during senescence. J. Exp. Bot. 2002, 53, 927–937. [Google Scholar] [CrossRef]
- Hocking, P.J.; Steer, B.T. The distribution and identity of assimilates in tomato with special reference to stem reserves. Ann. Bot. 1994, 73, 315–325. [Google Scholar] [CrossRef]
- Ende, W.V.; den Peshev, D. Sugars as antioxidants. In Crop improvement under adverse conditions; Springer: New York, NY, USA, 2013; pp. 285–307. [Google Scholar]
- Scheible, W.-R.; Lauerer, M.; Schulze, E.-D.; Caboche, M.; Stitt, M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997, 11, 671–691. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Paul, M.J.; Driscoll, S.P. Sugar repression of photosynthesis: The role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 1997, 20, 110–116. [Google Scholar] [CrossRef]
- Muñoz, P.; Antón, A.; Paranjpe, A.; Ariño, J.; Montero, J.I. High decrease in nitrate leaching by lower N input without reducing greenhouse tomato yield. Agron. Sustain. Dev. 2008, 28, 489–495. [Google Scholar] [CrossRef]
- Le Bot, J.; Jeannequin, B.; Fabre, R. Impacts of N-deprivation on the yield and nitrogen budget of rockwool grown tomatoes. Agronomie. 2001, 21, 341–350. [Google Scholar] [CrossRef]
- Bénard, C.; Bourgaud, F.; Gautier, H. Impact of temporary nitrogen deprivation on tomato leaf phenolics. Int. J. Mol. Sci. 2011, 12, 7971–7981. [Google Scholar] [CrossRef]
- Hoffland, E.; Jeger, M.J.; van Beusichem, M.L. Effect of nitrogen supply rate on disease resistance in tomato depends on the pathogen. Plant Soil 2000, 218, 239–247. [Google Scholar] [CrossRef]
- Le Bot, J.; Adamowicz, S. Nitrogen nutrition and use in horticultural crops. J. Crop Improv. 2006, 15, 323–367. [Google Scholar] [CrossRef]
- Wang, L.; Qian, C.; Bai, J.; Luo, W.; Jin, C.; Yu, Z. Difference in volatile composition between the pericarp tissue and inner tissue of tomato (Solanum lycopersicum) fruit. J. Food Process Pres. 2017, 42, e13387. [Google Scholar] [CrossRef]
- Wang, F.; Smith, A.G.; Brenner, M.L. Temporal and spatial expression pattern of sucrose synthase during tomato fruit development. Plant Physiol. 1994, 104, 535–540. [Google Scholar] [CrossRef]
- Brown, M.M.; Hall, J.L.; Ho, L.C. Sugar uptake by protoplasts isolated from tomato fruit tissues during various stages of fruit growth. Physiol. Plantarum 1997, 101, 533–539. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Wang, T.-T.; Chen, J.-H.; Lin, T.-T. Spatial–temporal analyses of lycopene and sugar contents in tomatoes during ripening using chemical shift imaging. Postharvest Biology and Technology. 2011, 62, 17–25. [Google Scholar] [CrossRef]
- Van de Poel, B.; Vandenzavel, N.; Smet, C.; Nicolay, T.; Bulens, I.; Mellidou, I.; Vandoninck, S.; Hertog, M.L.; Derua, R.; Spaepen, S.; et al. Tissue specific analysis reveals a differential organization and regulation of both ethylene biosynthesis and E8 during climacteric ripening of tomato. BMC Plant Biol. 2014, 14, 11. [Google Scholar] [CrossRef]
- Biais, B.; Bénard, C.; Beauvoit, B.; Colombié, S.; Prodhomme, D.; Ménard, G.; Bernillon, S.; Gehl, B.; Gautier, H.; Ballias, P.; et al. Remarkable Reproducibility of Enzyme Activity Profiles in Tomato Fruits Grown under Contrasting Environments Provides a Roadmap for Studies of Fruit Metabolism. Plant Physiol. 2014, 164, 1204–1221. [Google Scholar] [CrossRef]
- Colombié, S.; Nazaret, C.; Bénard, C.; Biais, B.; Mengin, V.; Solé, M.; Fouillen, L.; Dieuaide-Noubhani, M.; Mazat, J.-P.; Beauvoit, B.; et al. Modelling central metabolic fluxes by constraint-based optimization reveals metabolic reprogramming of developing Solanum lycopersicum (tomato) fruit. Plant J. 2015, 81, 24–39. [Google Scholar] [CrossRef]
- Rambla, J.L.; Tikunov, Y.M.; Monforte, A.J.; Bovy, A.G.; Granell, A. The expanded tomato fruit volatile landscape. J Exp Bot. 2014, 65, 4613–4623. [Google Scholar] [CrossRef]
- Wang, L.; Baldwin, E.A.; Bai, J. Recent Advance in aromatic volatile research in tomato fruit: The metabolisms and regulations. Food Bioproc. Technol. 2016, 9, 203–216. [Google Scholar] [CrossRef]
- Fishman, S.; Génard, M. A biophysical model of fruit growth: Simulation of seasonal and diurnal dynamics of mass. Plant Cell Environ. 1998, 21, 739–752. [Google Scholar] [CrossRef]
- Fanwoua, J.; Visser, P.H.B.; de Heuvelink, E.; Yin, X.; Struik, P.C.; Marcelis, L.F.M. A dynamic model of tomato fruit growth integrating cell division, cell growth and endoreduplication. Funct. Plant Biol. 2013, 40, 1098–1114. [Google Scholar] [CrossRef]
- Beauvoit, B.; Belouah, I.; Bertin, N.; Cakpo, C.B.; Colombié, S.; Dai, Z.; Gautier, H.; Génard, M.; Moing, A.; Roch, L.; et al. Putting primary metabolism into perspective to obtain better fruits. Ann. Bot. 2018, 122, 1–21. [Google Scholar] [CrossRef]
- Mozafar, A. Nitrogen fertilizers and the amount of vitamins in plants: A review. J. Plant Nutr. 1993, 16, 2479–2506. [Google Scholar] [CrossRef]
- Gautier, H.; Massot, C.; Stevens, R.; Sérino, S.; Génard, M. Regulation of tomato fruit ascorbate content is more highly dependent on fruit irradiance than leaf irradiance. Ann. Bot. 2009, 103, 495–504. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F.M. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Rubio, E.; Auge, M. A new procedure for extraction and measurement of soluble sugars in ligneous plants. J. Sci. Food Agr. 2002, 82, 360–369. [Google Scholar] [CrossRef]
- Truffault, V.; Riqueau, G.; Garchery, C.; Gautier, H.; Stevens, R.G. Is monodehydroascorbate reductase activity in leaf tissue critical for the maintenance of yield in tomato? J. Plant Physiol. 2018, 222, 1–8. [Google Scholar] [CrossRef] [PubMed]


| R1N+ | R1N− | R2N+ | R2N− | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sd | Tukey’s group | sd | Tukey’s group | sd | Tukey’s group | sd | Tukey’s group | |||||
| Stem growth (cm week−1) | 25.5 | ±2.9 | a | 25.0 | ±2.5 | a | 25.6 | ±2.6 | a | 25.7 | ±2.5 | a |
| Final length leaf 10 (cm) | 47.3 | ±3.0 | a | 45.6 | ±3.1 | b | 47.0 | ±3.5 | ab | 46.1 | ±3.0 | ab |
| Final width leaf 10 (cm) | 53.3 | ±2.9 | a | 49.9 | ±3.2 | c | 52.8 | ±3.5 | ab | 51.0 | ±3.1 | bc |
| Leaf photosynthetic activity (µmol CO2 m−2 s−1) | 18.5 | ±4.6 | a | 20.1 | ±1.4 | a | 17.2 | ±2.5 | a | 21.6 | ±1.6 | a |
| Marketable yield (kg m−2) | 49.8 | ±5.1 | a | 47.1 | ±1.9 | a | 48.7 | ±1.4 | a | 46.8 | ±2.6 | a |
| Average fruit mass (g) | 122.3 | ±3.3 | a | 117.8 | ±3.9 | b | 116.2 | ±3.5 | bc | 114.6 | ±3.3 | c |
| Marketable fruits (number) | 407 | ±35 | a | 400 | ±13 | a | 419 | ±5 | a | 408 | ±14 | a |
| Unmarketable fruits (number) | 27.2 | ±3.1 | ab | 25.4 | ±2.2 | b | 30.1 | ±2.5 | a | 24.4 | ±3.4 | b |
| Nitrogen Content | ||||
|---|---|---|---|---|
| Month | Treatment | µmol N g−1Dw | sd | Tukey’s Group |
| May | N+ | 942.6 | ±148.2 | a |
| N− | 375.8 | ±186.4 | b | |
| June | N+ | 752.4 | ±164.6 | a |
| N− | 376.5 | ±180.5 | b | |
| July | N+ | 932.6 | ±170.3 | a |
| N− | 294.9 | ±150.5 | bc | |
| September | N+ | 871.7 | ±271.6 | a |
| N− | 303.6 | ±126.3 | bc | |
| October | N+ | 455.8 | ±127.7 | b |
| N− | 62.8 | ±32.3 | c | |
| Spring horde | Fructose | Glucose | Citric acid | Malic acid | sugar: acid ratio | Vitamin C | ||||||||||||
| g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | sd | Tukey’s group | mg 100g−1Fw | sd | t-test | ||
| R1N+ | 77.0 | ±5.8 | a | 77.9 | ±6.1 | a | 18.4 | ±1.8 | ab | 1.8 | ±0.6 | a | 7.7 | ±0.6 | ab | 12.7 | ±1.2 | ns |
| R1N− | 77.8 | ±2.3 | a | 76.0 | ±3.7 | a | 17.7 | ±1.8 | ab | 1.5 | ±0.9 | a | 8.2 | ±1.3 | ab | 11.6 | ±1.2 | ns |
| R2N+ | 74.1 | ±9.8 | a | 75.7 | ±11 | a | 20.4 | ±1.9 | a | 2.1 | ±0.3 | a | 6.7 | ±1.0 | b | |||
| R2N− | 77.8 | ±5.9 | a | 76.9 | ±6.8 | a | 15.9 | ±1.4 | b | 1.3 | ±0.6 | a | 9.0 | ±0.9 | a | |||
| Summer horde | Fructose | Glucose | Citric acid | Malic acid | sugar:acid ratio | Vitamin C | ||||||||||||
| g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | sd | Tukey’s group | mg 100g−1Fw | sd | t-test | ||
| R1N+ | 78.6 | ±3.8 | a | 78.6 | ±3.9 | a | 21.6 | ±1.9 | ab | 5.8 | ±0.5 | a | 5.8 | ±0.5 | b | 9.1 | ±0.7 | ns |
| R1N− | 84.5 | ±5.8 | a | 80.6 | ±5.5 | a | 17.7 | ±1.4 | b | 2.2 | ±0.4 | b | 8.4 | ±0.6 | a | 10.0 | ±1.1 | ns |
| R2N+ | 77.3 | ±3.7 | a | 77.7 | ±4.6 | a | 22.5 | ±2.8 | a | 3.7 | ±1.3 | ab | 6.0 | ±0.4 | b | |||
| R2N− | 79.3 | ±3.9 | a | 75.2 | ±3.0 | a | 17.4 | ±2.6 | b | 2.3 | ±0.5 | b | 8.0 | ±1.0 | a | |||
| Autumn horde | Fructose | Glucose | Citric acid | Malic acid | sugar:acid ratio | Vitamin C | ||||||||||||
| g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | g kg−1Fw | sd | Tukey’s group | sd | Tukey’s group | mg 100g−1Fw | sd | t-test | ||
| R1N+ | 71.5 | ±6.3 | b | 70.9 | ±5.5 | a | 20.5 | ±1.6 | ab | 12.0 | ±1.7 | a | 4.5 | ±0.3 | c | 7.5 | ±1.5 | ns |
| R1N− | 77.8 | ±6.5 | ab | 74.6 | ±7.2 | a | 19.3 | ±2.6 | ab | 7.0 | ±0.8 | b | 5.9 | ±0.6 | b | 9.3 | ±1.1 | ns |
| R2N+ | 77.7 | ±6.1 | ab | 76.2 | ±7.4 | a | 24.1 | ±2.6 | a | 6.9 | ±2.3 | b | 5.1 | ±0.5 | bc | |||
| R2N− | 85.1 | ±4.8 | a | 80.4 | ±4.5 | a | 18.5 | ±1.3 | b | 4.8 | ±2.4 | b | 7.2 | ±0.7 | a | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truffault, V.; Ristorto, M.; Brajeul, E.; Vercambre, G.; Gautier, H. To Stop Nitrogen Overdose in Soilless Tomato Crop: A Way to Promote Fruit Quality without Affecting Fruit Yield. Agronomy 2019, 9, 80. https://doi.org/10.3390/agronomy9020080
Truffault V, Ristorto M, Brajeul E, Vercambre G, Gautier H. To Stop Nitrogen Overdose in Soilless Tomato Crop: A Way to Promote Fruit Quality without Affecting Fruit Yield. Agronomy. 2019; 9(2):80. https://doi.org/10.3390/agronomy9020080
Chicago/Turabian StyleTruffault, Vincent, Marlene Ristorto, Eric Brajeul, Gilles Vercambre, and Hélène Gautier. 2019. "To Stop Nitrogen Overdose in Soilless Tomato Crop: A Way to Promote Fruit Quality without Affecting Fruit Yield" Agronomy 9, no. 2: 80. https://doi.org/10.3390/agronomy9020080
APA StyleTruffault, V., Ristorto, M., Brajeul, E., Vercambre, G., & Gautier, H. (2019). To Stop Nitrogen Overdose in Soilless Tomato Crop: A Way to Promote Fruit Quality without Affecting Fruit Yield. Agronomy, 9(2), 80. https://doi.org/10.3390/agronomy9020080





