Abstract
The present study aimed to evaluate the potentiality of three seaweeds, which belong to different algal taxa (green alga Ulva lactuca Linnaeus, brown alga Cystoseira spp., and red alga Gelidium crinale (Hare ex Turner) Gaillon) as bio-fertilizers to improve the growth and yield of canola (Brassica napus L.) plants under greenhouse conditions. Furthermore, the impact of seaweeds in alleviating the effects of salt stress (75 and 150 mM NaCl) on canola plants was also investigated. The three examined seaweeds (applied as soil amendments) successfully alleviated the harmful effects of salinity on canola plants by significantly reducing the inhibition of chlorophyll a, b, total carbohydrate accumulation, and growth promoting hormones, while increasing antioxidative compounds, such as phenols, flavonoids, anthocyanin, and osmoprotectants, including total carbohydrates and proline. Phytochemical analysis of the three examined seaweeds suggests that their stimulatory effect on growth and productivity under normal and salinity growth conditions may be linked to their constituents of a wide variety of growth promotive hormones, including indole acetic acid, indole butyric acid, gibberellic acid, cytokinins, total carbohydrates, and phenolic compounds. U. lactuca was found to be the best candidate to be used as a bio-fertilizer to improve canola growth, yield, and salt stress tolerance.
Keywords:
bio-stimulant; Brassica napus; carbohydrates; phytohormones; salinity; seaweeds; secondary metabolites; yield 1. Introduction
Seaweeds are thallophytic, macro- aquatic algae belonging to the plant kingdom [1]. Seaweeds have the ability to flourish within a large range of extreme environmental habitats. However, when adapting to new environmental surroundings, they produce a wide variety of hygienic primary and secondary metabolites [2]. Therefore, seaweeds are regarded as a treasure trove of untapped natural biologically active compounds [3]. They have been used as renewable quality and quantitative bio-resources in sustainable botanical applications [4]. Chemical fertilizers, pesticides, fungicides, and herbicides give immediate results; however, their continuous use has an adverse impact on the quality of the soil, the beneficial soil microbial communities, the soil’s fertility, and on the plants cultivated in these soils.
Seaweeds are effectively used as bio-fertilizers because they include high levels of organic matter, which leads to soil nutrient enrichment [5,6]. In addition, they were found to be a better and more suitable alternative to chemical and mineral fertilizers when used in adequate quantities [7]. Many recent studies have discovered wide applications of these marine macro algae (in the form of finely powdered or aqueous extracts) as eco-friendly fertilizers in modern agriculture and horticulture crops improvements [8]. The application of seaweeds as a soil drench was found to be more effective on plant vigor than the foliar spray application [9].
Salinity stress is a major abiotic stress that has significant negative effects on plant growth and yield by causing osmotic and ionic stresses that affect various primary metabolic processes in plants [10]. These negative effects include interference with the root function in absorbing water, as well as detrimental effects on physiological and biochemical processes, such as nutrient uptake and assimilation [11]. Unfortunately, soil salinity is a current growing issue in a number of areas around the world. It is estimated that at least 0.3 million hectares of farmland are becoming unusable annually, and another 20–46 million hectares are suffering decreases in production potential each year [12]. The development of strategies to alleviate the adverse effects of salinity stress on plants has received considerable attention.
Rapeseed (Brassica napus L.), which is also known as rapeseed oil or canola, is the third largest source of vegetable oil in the world. Its seeds contain about 40%–45% oil and about 25% protein, and the oil is edible [13]. Canola is frequently grown in arid and semiarid regions of the world where salinity is or will be a huge issue. Therefore, studies on improving the salt tolerance of canola are necessary. The objectives of the current study are to: (i) Investigate the possible effect of seaweeds as a potentially promising technique to mitigate the harmful effect of salt stress on the canola plants’ growth and yield; (ii) make a comparative performance evaluation between three seaweeds of different algal taxa; and (iii) highlight the possible mechanisms underlying the beneficial effect of seaweed treatment. The novelty of the current study is in the comparison between the efficiency of three different algal taxa not only as bio-fertilizers, but also as protective agents against environmental stress. This is the first study that uses seaweeds to improve canola plants’ salt stress tolerance.
2. Materials and Methods
2.1. Plant Materials
Brassica napus ‘Pactole’, a French cultivar, was obtained from Oil Crops Council, Ministry of Agriculture, Giza, Egypt.
2.2. Algae Collection
In the current study, seaweeds of green alga Ulva lactuca Linnaeus (family: Ulvaceae), brown alga Cystoseira spp. (family: Fucaceae), and red alga Gelidium crinale (Hare ex Turner) Gaillon (family: Gelidiaceae) were selected and harvested by hand during low tide from Al- Agamy (31°5′55″ N and 29°45′59″ E), Alexandria, Mediterranean coast. These were identified in accordance with Aleem [14]. The collected algal samples were washed thoroughly with sea-water and hard brushed to remove macroscopic epiphytes and sand particles. Then, they were washed with tap water to remove adhering salt. Some of the algal samples were preserved in a freezer for fresh weight evaluations, while the others were blotted and air-dried in room temperature for 6 d to remove excess water. The dried seaweed samples were ground into a fine powder by a mechanic grinder and kept in plastic tubes for further analyses.
2.3. Application of the Powdered Seaweeds in Planting Soils
About 15 g of each examined powdered seaweed was amended in five kg of soil, at a rate of 7 d before planting. During this week, the amended soils with algae were watered twice daily.
2.4. Growth Conditions
The study was performed in the growth seasons (2016–2017) in a greenhouse located in Botany Department, Faculty of Science, Ain Shams University, Egypt. Canola seeds were surface-sterilized with 0.1% mercuric chloride for 5 min and washed thoroughly with sterile distilled water. Homogenous quantities of canola seeds were then sown in pots. Pots were divided into 4 groups, each included 5 kg of soil (2 clay: 1 sand w/w) untreated (to serve as a control) or amended with 15 g of one of the seaweeds under study (Ulva lactuca Linnaeus, brown alga Cystoseira spp., and red alga Gelidium crinale (Hare ex Turner) Gaillon. After emergence, seedlings (15 days old) were thinned to 10 uniform seedlings per pot. Plants were grown inside a growth chamber under these conditions: 15 h photoperiod, 70%–75% relative humidity, and the day/night temperature ranged between 18 °C and 25 °C. One month after sowing, each group of pots was divided into 3 subgroups; each subgroup was treated with one of the following NaCl concentrations (0, 75, and 150 mM). Each pot received 750 mL of the salt treatment (equal to 80% of the water holding capacity of the soil). Afterward, pots were irrigated with tap water to attain 80% of the filled capacity till the end of the experiment.
Samples from each approach were assembled at the vegetative stage (65 days old plants) to measure several growth parameters, including: Shoot length (cm plant−1), root length (cm plant−1), number of leaves per plant, mean leaf area per plant (cm2 plant−1) along with the fresh and dry weights of shoots and roots (g plant−1). In addition, photosynthetic pigment elements (chlorophyll a, b, chlorophyll a/b ratio, carotenoids, and photosynthetic pigments) were determined as well as selected biochemical compounds, including: Total carbohydrates, proline, phenols, flavonoids, and anthocyanin. Furthermore, 10 g of shoot apex were collected to estimate endogenous phytohormone components, including: Indole acetic acid (IAA), indole butyric acid (IBA), gibberellic acid (GA3), jasmonic acid (JA), abscisic acid (ABA), and cytokinins (CKs; zeatin (Z) and benzyl adenine (BA)). In addition, one gram of fresh leaves was used to determine the total antioxidant activity as a 2.2 diphenyl to 1 picrylhydrazyl. At harvest (140 day old plants), samples were collected to determine the yield characteristics and the seed oil’s percentage. The yield characteristics included the shoot and root length, stem circumference, number of siliqua plant−1, number of seeds siliqua−1, seed index (dry weight of 1000 seeds), straw yield plant−1 (dry matter of a plant without seeds), and biological yield plant−1 (the total dry matter accumulation of a plant). Five replicates, 10 plants each, were used to determine the growth parameters and yield components while three replicates, five plants each, were used for the biochemical analyses.
2.5. Methods
2.5.1. Extraction, Separation, and Estimation of Growth Regulating Substances by Gas Chromatography (GC) and High-Performance Liquid Chromatography (HPLC)
The extraction procedure was according to the one used by Shindy and Smith [15]. Identification and determination of acidic hormones (IAA, IBA, GA3, and ABA) were performed using a Helwett Packered Gas Chromatograph 5890 (Helwett-Packered, Orlando, FL, USA). The chromatography was fitted and equipped with a HP-130 m × 0.32 mm × 0.25 μm capillary column coated with methyl silicone. Gases flow rates were 30, 30, and 300 cm s−1 for N2, H2, and air, respectively. While the flow rate within the column was regulated at 2 mL min−1. JA was measured according to Kramell [16] using a NUCLEODEX beta-PM (Macherey-Nagel, Düren, Germany), 200 mm, and 4 mm ID column. The flow rate was tuned at 1 mL min−1, and observed at UV 210 nm. Cytokinin fractions (zeatin and benzyl adenine) were detected by a HPLC system equipped with a variable wave length detector (Agilent, Waldbronn, Germany) 1100, analyses were performed on a C18 reverse phase (BDS 5 μm, Labio, Prague, Czech Republic) packed stainless-steel column (4×250 mm, i.d.), 20 min gradient from 0.1 N acetic acid pH 2.8 to 0.1 N acetic acid in 95% aqueous ethanol; pH 4. The flow rate was: 1 mL min−1, whereas the detection was: UV 254 nm [17].
2.5.2. Phytochemical Analyses
Glycerol extraction and determination were done according to the methods of Lambert and Neish [18]. Photosynthetic pigments chlorophyll a (chl a), chlorophyll b (chl b), and carotenoids in the fresh leaves of canola plants were calculated as explained by Metzner et al. [19]. The total carbohydrate accumulation was determined by the phenol sulfuric acid method [20]. In this method, the concentrated sulfuric acid breaks down polysaccharides, oligosaccharides, and disaccharides to monosaccharides, which are then dehydrated to furfural and hydroxymethyl furfural. These compounds reacted with phenol to produce a yellow color and the absorption was then measured at 490 nm. The methodology of Bates et al. [21] was followed during the evaluation of proline based on proline’s reaction with ninhydrin while phenol was evaluated using the methodology of Malik and Singh [22]. The absorbance was measured at 765 nm using a spectrophotometer (Spectronic 601, Milton Roy Company, Ivyland PA, United States). Flavonoids were determined spectrophotometrically (at 425 nm) using Christ-Muller’s method [23]. Total anthocyanin content (TAC) was determined by the pH-differential method [24]. The technique of Tailor and Goyal [25] was used to identify the total antioxidant activities using a DPPH scavenging assay. These were evaluated spectrophotometrically (at 517 nm) against the absorbance of the indicator, 2.2 diphenyl and 1 picrylhydrazyl (DPPH).
2.6. Statistical Analyses
The experiment utilized a completely randomized design. Statistical analyses of data were done using SAS Windows (SAS Institute Inc., Cary, NC, USA), version 9.3 [26]. The data were subjected to analyses of variance [27] and the significance of the different sources of variation was tested by Duncan’s multiple range test to discriminate significance (defined as p < 0.05).
3. Results
3.1. Phytochemical and Hormonal Analyses of Seaweeds
Phytochemical analyses of the three examined seaweeds revealed that U. lactuca had the highest glycerol and proline content in comparison to those detected in Cystoseira spp. and G. crinale. Whereas, the highest percentage of phenols was detected in Cystoseira spp., and when calculated, its percentage was 152.9% and 287% higher than those of G. crinale and U. lactuca, respectively. The highest amounts of total carbohydrates were found in U. lactuca and G. crinale compared with Cystoseira spp., which had the lowest amount. Total antioxidant activities were the highest in U. lactuca tissues; the ratio was calculated as 74.45% and 179.2% higher than the antioxidant activities of Cystoseira spp. and G. crinale, respectively (Table 1). Regarding phytohormonal content, the three examined seaweeds had detectable amounts of all the determined hormones. U. lactuca had significantly high (p < 0.05) IAA, zeatin, and benzyl adenine when compared with Cystoseira spp. and G. crinale, while the highest IBA and ABA were detected in G. crinale and the highest GA3 was found in Cystoseira spp. Endogenous JA in Cystoseira spp. and G. crinale was significantly higher (p < 0.05) than in U. lactuca.

Table 1.
Phytochemical analyses of the studied marine macro-algae. Data are presented as the mean ± SD (n = 15).
3.2. Growth Parameters of Canola Plant
Canola growth parameters were significantly (p < 0.05) inhibited in response to salt treatment. The inhibitory effect was proportional to the applied concentration of NaCl, except for the root length, which significantly increased by 13.6% in the canola plant treated with 75 mM NaCl over the control value. The inhibition in response to 150 mM NaCl was calculated as 32.35%, 52.37%, 58.6%, 68.94%, 50.9%, and 75.22% in the shoot length, mean leaf area per plant, fresh weights of shoot and root, and the dry weight of shoot and root, respectively (Table 2). Soil amendment with U. lactuca, Cystoseira spp., or G. crinale had a significant stimulatory effect on all the measured growth parameters of the canola under both normal and salt stress conditions compared with the corresponding controls. Treatment with seaweeds completely alleviated the harmful effect of up to 150 mM NaCl on the shoot and root length, number of leaves per plants, fresh, and dry weights of root of canola plants. It was found that at low concentration of NaCl (75 mM), canola plants grown in the soil amended with U. lactuca had significantly higher growth parameters compared to those treated with Cystoseira spp. or G. crinale. Under high salt concentration (150 mM NaCl), the highest values of the root length, number of leaves per plant, fresh weight of shoot, and dry weight of shoot and root were obtained in plants grown in soil treated with U. lactuca, whereas the Cystoseira spp. treated plant had the highest values of shoot length, mean leaf area per plant, and fresh weight of root compared to other algal treatments (Table 2).

Table 2.
The impact of Ulva lactuca L., Cystoseria sp., and Gelidium crinale soil treatment on growth parameters of canola plants (at the vegetative stage) grown under different levels of salinity. Data are presented as the mean ± SD (n = 50).
3.3. Yield Characteristics
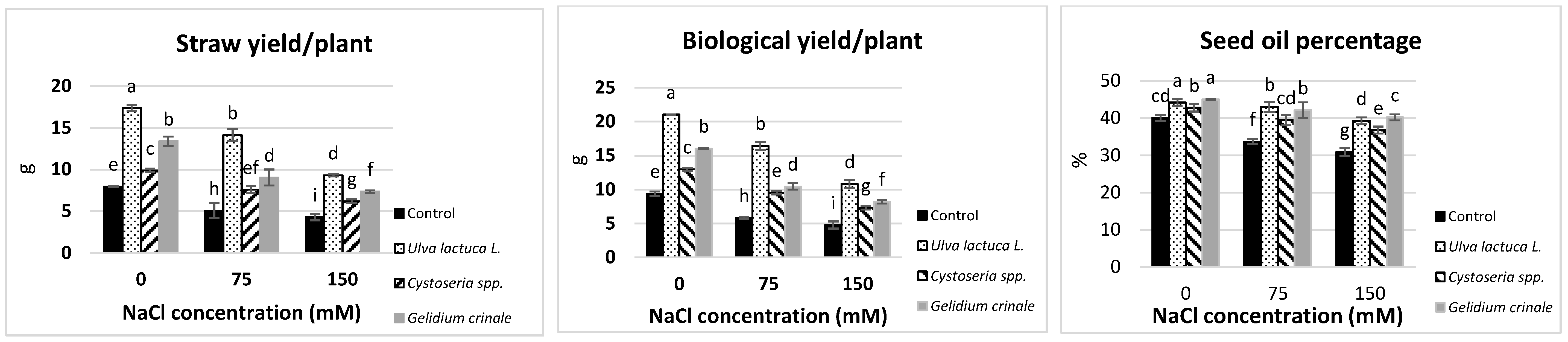
All the measured yield characteristics were significantly (p < 0.05) reduced in canola plants grown under different levels of salinity as compared with the control. The reduction was calculated as 30.2%, 46.17%, 49.1%, and 22.9% below the control values in the seed index, straw yield per plant, biological yield per plant, and the seed oil percentage, respectively (Table 3 and Figure 1). All the measured yield characteristics of the canola plant were significantly (p < 0.05) raised after the seaweed soil treatments regardless of whether the plants were grown under normal or salt stress conditions. Under the normal growth condition, the maximum shoot length, number of siliqua per plant, straw yield, and biological yield were measured after the treatment with U. lactuca, next by G. crinale, then Cystoseira spp., followed by the untreated control. The increases were calculated as 32.6%, 40%, 117.8%, and 123.9% more in response to green alga U. lactuca as compared to the control value in shoot length, number of siliqua per plant, straw yield, and biological yield, respectively. Whereas red alga (G. crinale) had the most promotive effect on the root length and stem circumference, brown alga (Cystoseira spp.) treatment caused the highest increase in the seed index in the canola plant grown under normal conditions as compared to other seaweeds treatments.

Table 3.
The impact of Ulva lactuca L., Cystoseria sp., and Gelidium crinale soil treatment on the yield characteristics of canola plants grown under different levels of salinity. Data are presented as the mean ± SD (n = 50).
Figure 1.
The impact of Ulva lactuca L., Cystoseria sp., and Gelidium crinale soil treatment on the yield and seed oil percentage of canola plants grown under different levels of salinity. Data are presented as the mean ± SD (n = 50). Straw yield/plant = dry matter of a plant without seeds. The biological yield refers to the total dry matter accumulation of a plant (straw yield + weight of seeds). Different letters indicate significant differences based on a comparison of means with Duncan’s multiple range test at p < 0.05.
Seaweeds treatments significantly (p < 0.05) improved the canola yield grown under salinity stress compared to the untreated plants growing under the same salt levels. Interestingly, the treatment with U. lactuca alleviated the inhibitory effect of 150 mM NaCl on the shoot length, number of seeds per siliqua, straw yield, biological yield, and the seed oil percentage of the canola plants. Whereas G. crinale treatment ameliorated the inhibitory effect of 150 mM NaCl on the shoot length, stem circumference, and the seed oil percentage. Brown alga (Cystoseira spp.) was able to overcome the negative effect of 150 mM on the seed index.
3.4. Photosynthetic Pigments
Salt treatment significantly decreased chlorophyll a, chlorophyll b, and the total amount of pigments in canola leaves, and this decrease was directly related to the applied salt level. In contrast, carotenoids increased by 5.67% and 62.89% over the control when the canola plant was treated with 75 and 150 mM NaCl, respectively (Table 4). Under normal conditions, soil treatment with seaweeds significantly (p < 0.05) increased chlorophyll a, b, carotenoids, and the total photosynthetic pigments over the control value. The most promotive treatment was U. lactuca followed by G. crinale, and then Cystoseira spp. All the seaweed treatments enabled the canola plants to totally overcome the inhibitory effect of up to 150 mM NaCl on chlorophyll b and the photosynthetic pigments, while only green and red algae could alleviate the harmful effect of the same concentration on chlorophyll a.

Table 4.
The impact of Ulva lactuca L., Cystoseria sp., and Gelidium crinale soil treatment on the photosynthetic pigment content of canola plants (at the vegetative stage) grown under different levels of salinity. Values expressed as μg g−1 fresh weight (FW) of leaves. Data are presented as the mean ± SD (n = 15).
3.5. Phytohormones
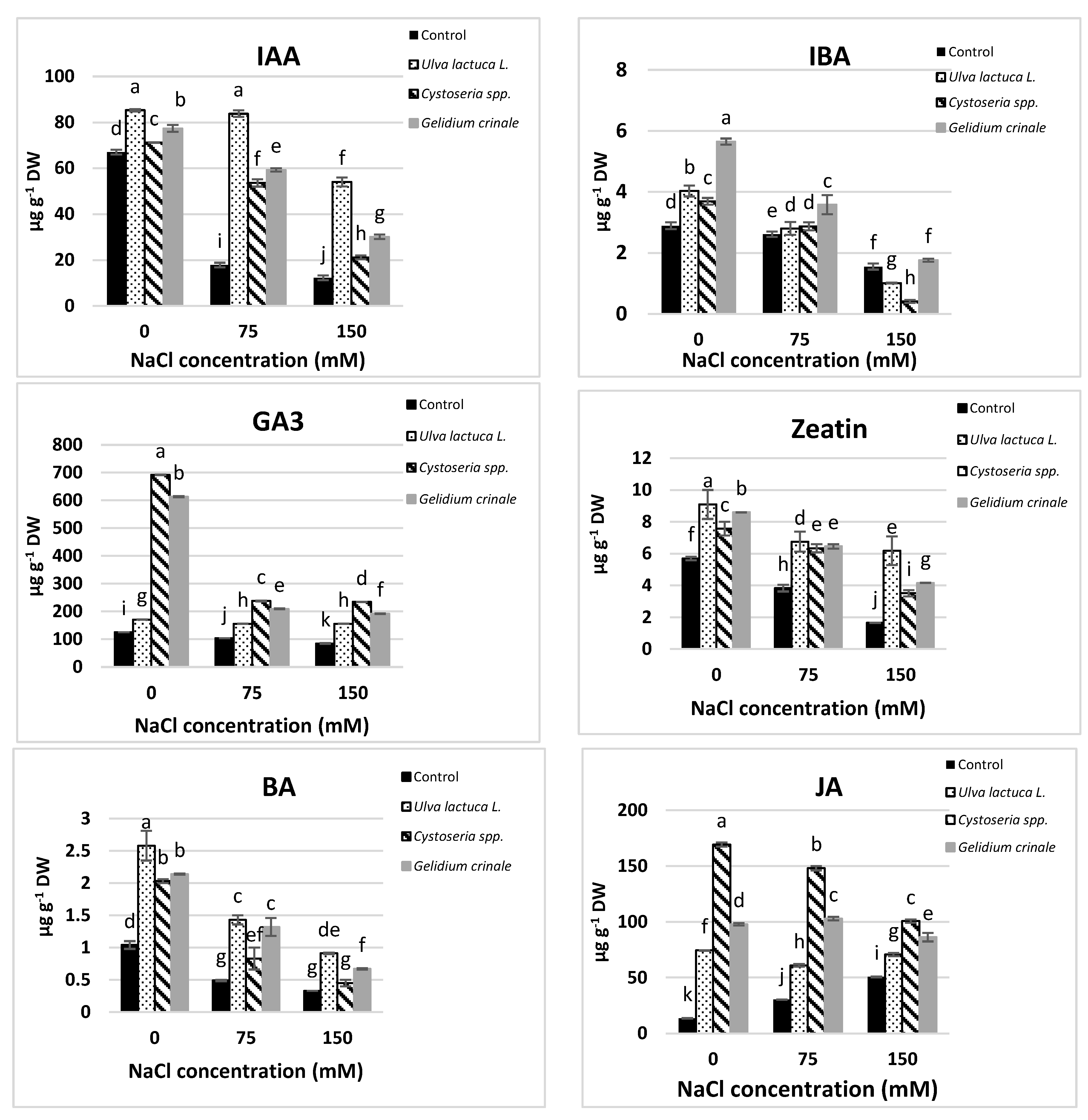
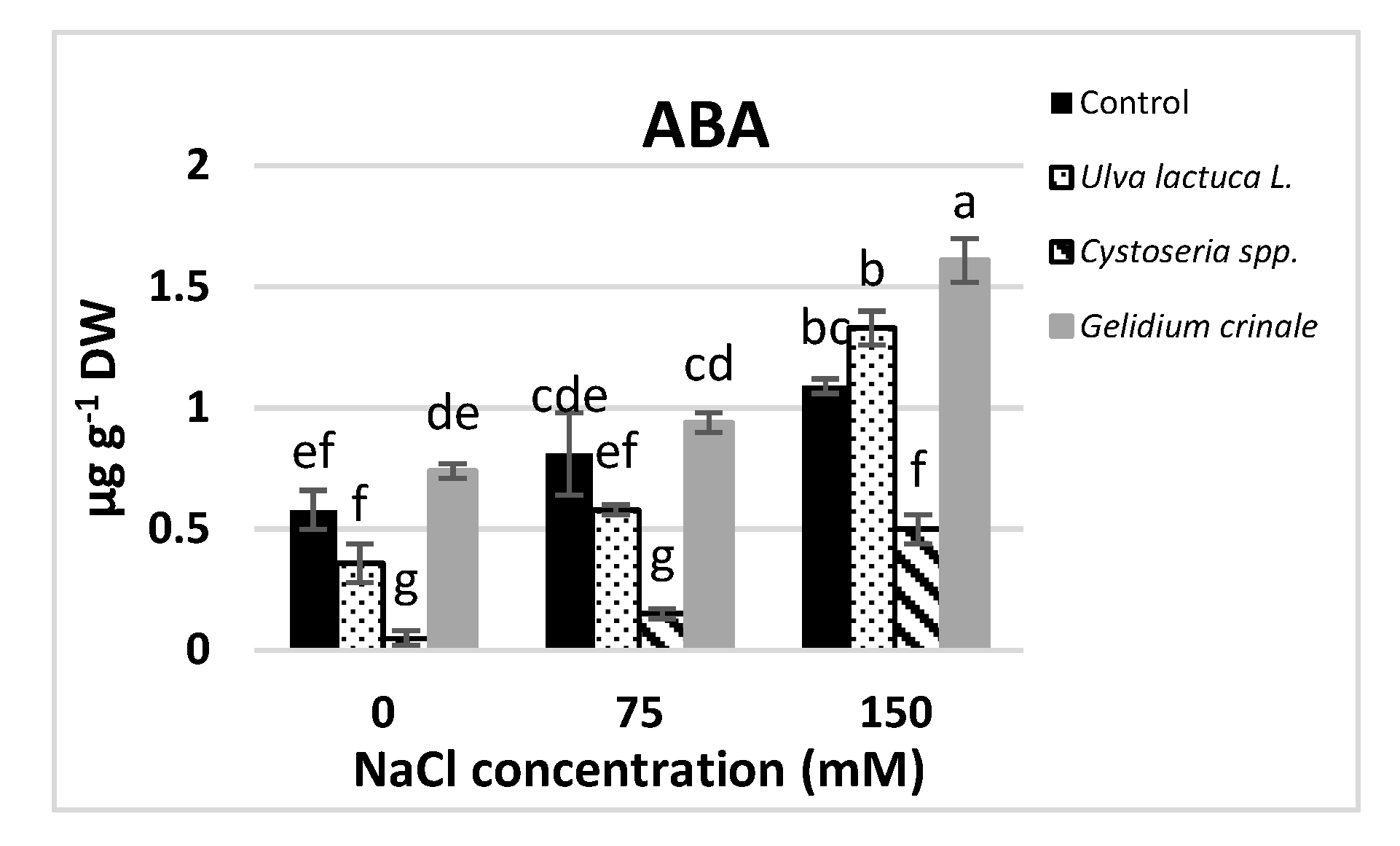
The results showed a significant difference (p < 0.05) between the hormonal content of the canola plants grown under normal conditions and those subjected to salinity stress (Figure 2). Growth promoters, including IAA, IBA, GA3, zeatin, and BA, were significantly decreased in response to salt stress. The decreases were directly proportional to the applied salt level, and were evaluated as 81.7%, 46.26%, 32.5%, 71%, and 68.46%, respectively, below the control value in a plant that received 150 mM NaCl. On the other hand, JA and ABA were significantly increased in response to salt stress.
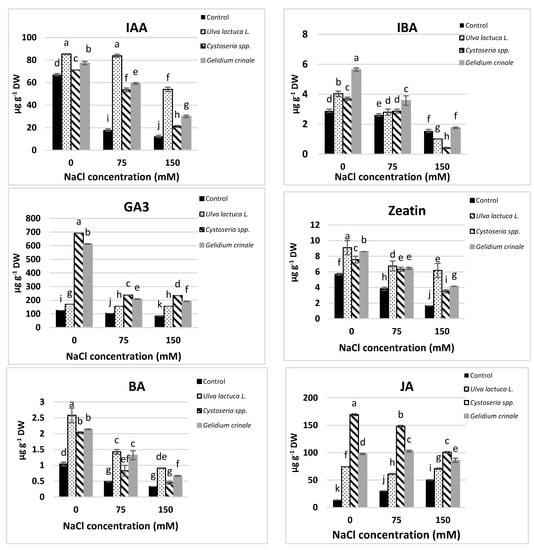
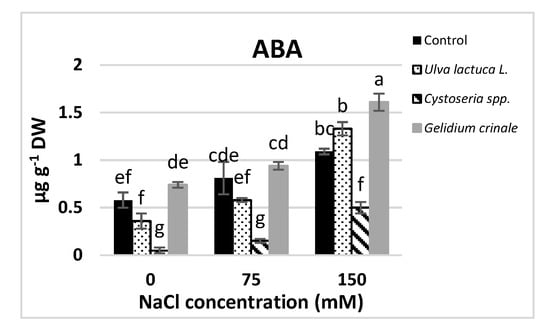
Figure 2.
The impact of Ulva lactuca L., Cystoseria spp., and Gelidium crinale soil treatment on endogenous phytohormones (IAA, IBA, GA3, Z, BA, ABA, and JA) of the canola shoot apex (at the vegetative stage) grown under different levels of salinity. Data are presented as the mean ± SD (n = 15). Different letters indicate significant differences based on a comparison of means with Duncan’s multiple range test at p < 0.05.
Under normal conditions, plants grown in the soil treated with seaweeds had significantly higher amounts of all the detected hormones compared to the untreated plants. U. lactuca’s application had the most promotive effect on IAA and cytokinins (zeatin and BA), whereas plants treated with G. crinale had the highest IBA and ABA rate and those treated with Cystoseira spp. had higher amounts of GA3 and JA compared to plants treated with other seaweeds. The detected amounts of these hormones in canola plants after the treatment with the three examined algae are in good agreement with the phytochemical analyses results of the three algae (Table 1). All the examined seaweeds completely alleviated the inhibitory effect of 150 mM NaCl on GA3 and 75 mM NaCl on IBA and zeatin, whereas only treatments with U. lactuca completely mitigated the inhibitory effect of up to 150 mM NaCl on cytokinins.
3.6. Primary Metabolites
Total carbohydrate accumulation in canola plants was significantly reduced in response to salt stress. The reduction was evaluated as 85.9% and 93.75% in plants treated with 75 and 150 mM NaCl, respectively (Table 5). Canola plants treated with different seaweeds had significantly higher amounts of total carbohydrates in comparison to the untreated plants. The highest carbohydrate level was detected in the plants treated with U. lactuca, followed by those treated with G. crinale, then Cystoseira spp. The treatment with U. lactuca and G. crinale completely alleviated the inhibitory effect of up to 150 mM NaCl on the total carbohydrate accumulation, with a 26.56% and 14.1% increase, respectively, over the untreated plants grown under unstressed conditions. According to the present study, proline levels significantly increased in the canola plant when subjected to salt stress. The increase was calculated as 103.33% in response to 150 mM NaCl. All the applied seaweeds caused an additional accumulation of proline in canola plants.

Table 5.
The impact of Ulva lactuca L., Cystoseria spp., and Gelidium crinale soil treatment on total carbohydrates, proline, total phenols, total flavonoids, anthocyanins, and total antioxidant activity of canola plants (at the vegetative stage) grown under different levels of salinity. Data are represented as the mean ± SD (n = 15).
3.7. Secondary Metabolites
Phenols, flavonoids, and anthocyanin were significantly increased in the canola plants grown under salt stress conditions compared to the control. The increase was much more pronounced in response to 150 mM NaCl and was evaluated as 30.95%, 289.9%, and 58.8% in the case of phenols, flavonoids, and anthocyanin, respectively, as compared to the control values (Table 5).
Under normal growth conditions, plants treated with U. lactuca and G. crinale had significantly lower amounts of phenols and flavonoids compared to the control. Whereas, the brown alga (Cystoseira spp.) significantly increased flavonoids (4-folds of the control), and non-significantly reduced phenols. Anthocyanins content was higher in plants treated with Cystoseira spp. and G. crinale (15.6% and 8.4%, respectively, more than the control value), followed by the control plants, and those treated with U. lactuca had the lowest anthocyanin content (75.2% below the control value). Plants grown under salt stress (75 or 150 mM) and treated with U. lactuca had higher amounts of phenols, and those treated with Cystoseira spp. had the highest amounts of flavonoids and anthocyanins compared to the control or other seaweed treatments.
3.8. Total Antioxidant Activity
Canola plants grown under salt stress (75 and 150 mM) had significantly higher total antioxidant activity compared to the control value (with no significant difference between low and high NaCl concentrations). All the used seaweed treatments significantly increased the total antioxidant activity over those of the corresponding control values. The maximum antioxidant activity was detected in the canola plants treated with Cystoseira spp., and the increase was calculated as 45.35%, 39.2%, and 48.9% more than the untreated plants under 0, 75, and 150 mM NaCl, respectively.
4. Discussion
The present phytochemical analyses results indicate that the three studied marine algae have considerable amounts of proline, total carbohydrates, and phytohormones, but with significantly different amounts. Marine macroalgae are considered valuable resources for plant improvement due to their high content of polysaccharides, glycerol, and plant growth regulators, including auxins, cytokinins, and gibberellins, that have a broad range of biological activities [28]. Khan et al. [4] indicated that the fertilizing efficiency of seaweed liquid extract was due to the existence of micro and macro nutrients, and growth hormones at preferential levels. However, under salt stress conditions, the algal production of additional amounts of metabolites beyond those detected should also be considered.
Salt stress had harmful effects on all the measured growth parameters of canola plants. This reduction may be attributed to a direct inhibition of cell division and expansion [29]. Treatment with marine algae (U. lactuca, Cystoseira spp., and G. crinale) overcame the NaCl-induced growth inhibition. Our findings are consistent with those of past research performed on Salvia officinalis [30], Glycine max [31], and Vigna sinensis L. [32]. In this respect, Hernandez-Herrera et al. [9] proposed that seaweed treatment can stimulate root proliferation, thereby making plants more able to mine adequate nutrients from the deeper soil layer, which leads to improvements in the plants’ sprouting and growth.
The promotive effect of the three examined seaweeds on canola growth under both normal and stress conditions compared with the untreated plants could be attributed to the presence of growth-promoting substances, total carbohydrates, proline, and phenolic compounds. This indicated that seaweeds are efficient bio-fertilizers under normal growth conditions and noticeably increased their plant tolerance under stressful conditions. Khan et al. [4] and Mattner et al. [33] reported positive outcomes when seaweeds were used. These outcomes included root development, leaf quality, general plant vigor, and resistance to pathogens. Moreover, Rao and Chatterjee [34] found that the growth rate (in all parameters) was increased in some vegetable crops (brinjal, tomato, and chili) when treated with seaweed liquid fertilizer (SLF) from red algae Gracilaria verrucose. Generally, U. lactuca was the most effective seaweed treatment, causing the maximum stimulation in canola growth under normal or stress condition. The observed benefit of U. lactuca over the other used seaweeds could be explained by its higher level of total carbohydrates, proline, IAA, and cytokinins. Whereas, the distinctive effect of Cystoseira spp., at high salt concentrations (150 mM), could be attributed to its significantly higher phenols compared to the other examined seaweeds.
It was reported that salt stress has a significant negative impact on canola yield and seed oil percentage. Our results confirmed the data of Musyimi et al. [35], who reported that the impact of salinity on plants is a complicated condition that comprises osmotic stress, ion toxicity, and mineral deficiencies, leading to the reduction of crop yields. Seaweed treatment tended to alleviate the inhibitory effect of up to 150 mM NaCl on canola yield. High productivity in response to the treatment with seaweeds was indicated in many plants, such as tomato, chili [34], maize [36], and brinjal [37].
According to the obtained data, chlorophyll a, b and total photosynthetic pigments were very sensitive to salt stress. Smirnoff [38] declared that the reduction in chlorophyll levels in salt-stressed plants is a conventional indication of oxidative stress; this was associated with the inhibition of chlorophyll synthesis, along with the activation of its degradation by the enzyme, chlorophyllase [39]. Soil amendment with U. lactuca or G. crinale protects chlorophylls from the harmful effect of salinity. The observed enhancement effect of algal treatments on chlorophyll, under normal and salt stress conditions, indicated that these treatments resulted in the maintenance of higher photosynthetic activities over the control. Similar results were recorded by Ramarajan et al. [40], who found that soybean plants treated with SLF had higher amounts of chlorophyll a and b than the untreated control. Mastafa and Skeekh [41] reported that the plant growth substances present in SLF enhance the chlorophyll in plant leaves. Carotenoids were increased in salt stressed canola leaves compared to the control and additional amounts of carotenoids were accumulated when plants were treated with marine algae. Christaki et al. [42] suggested that carotenoids could protect plant cells from deleterious oxidative stresses by reducing the harm induced by reactive oxygen species (ROS) as a result of different defense mechanisms.
Plant hormones are active members of the signal compounds involved in the induction of plants’ salt stress response [43]. Canola plants grown in soil amendments with seaweeds had significantly elevated levels of endogenous phytohormones compared to the control. This indicated that the detected stimulatory effect of seaweed treatments on canola plant growth could be related to their hormonal contents, which positively affect the endogenous hormonal level of the treated plants, hence promoting their growth and development. Generally, all the used seaweed treatments significantly increased the growth-promoting hormone in canola plants subjected to salt stress compared to untreated plants growing under the same stressful conditions. Only U. Lactuca completely alleviated the inhibitory effect of high salt levels on canola plants’ endogenous cytokinins content. Cytokinin is a fundamental growth boosting element in chlorophyll biosynthesis and it plays a role in plant growth enhancement [37]. This could explain the higher efficiency of U. lactuca treatment as a bio-stimulant of growth and the chlorophylls’ content in canola plants under normal and stress conditions compared to other algal treatments.
Total carbohydrate accumulation at all NaCl concentrations used in this study were found to be significantly lower than that of the control. Meanwhile, canola plants treated with different seaweeds had significantly higher amounts of total carbohydrates compared to untreated plants. This finding agrees with the detected reduction in the chlorophyll a and b amounts, which follow the same order, in response to the seaweed treatments. This implies that seaweeds increased the total carbohydrates by stimulating chlorophylls’ biosynthesis and/or inhibiting their degradation, which promotes photosynthesis and increases the amount of carbohydrates. Similar results were obtained by Haroun and Hussein [44], who tested Lupinus termis, and Lozano et al. [45], who examined potatoes. U. lactuca and G. crinale treatments were the most efficient treatments in alleviating the inhibitory effect of high salt concentrations on carbohydrate content. The growth enhancing potential of seaweeds may be attributed to the increased amounts of carbohydrates that are known to play an important role in osmoregulation and also improve the plant growth in a way that is similar to that of hormones [46].
The observed increase in the proline content of canola plants grown under salt stress compared to the control indicates that proline is one of the defense mechanisms exhibited by the canola plant to cope with oxidative and osmotic stresses resulting from high salt levels. Osmoprotectants, such as amino acids—proline—, are able to stabilize proteins and cellular structures and/or maintain cell turgor by osmotic adjustment, and use redox metabolism to scavenge excess levels of ROS and re-establish the cellular redox balance under salt stress [47]. Additional amounts of proline were accumulated in plants grown in soil supplemented with seaweeds. It is well known that a high accumulation of proline is correlated with an increased tolerance of stress [48]. Canola plants also accumulated higher amounts of phenols, flavonoids, and anthocyanin when subjected to salt stress compared to those detected in unstressed control plants, which are thought to increase the ability of the plant to tolerate high salt levels. Phenolic compounds perform a vital function in plant growth and development, particularly in defense mechanisms. Most phenolic compounds have potent antioxidant properties, neutralizing the effects of oxidative stress [49]. Marine algae were found to increase the amounts of phenols, flavonoids, and anthocyanins in salt stressed canola plants.
Seaweeds have lately been examined intensively for their ability to work as natural antioxidants [50]. A correlation between the antioxidants’ capacity and NaCl tolerance was demonstrated in several plant species. Similarly, we found that canola plants treated with seaweeds had significantly elevated levels of antioxidant activity compared with the control. Maximum antioxidant activity was detected in response to Cystoseira spp., even though the phytochemical analysis showed that U. lactuca had significantly higher antioxidant activity. This suggested that the application of algae as a bio-fertilizer not only added nutrients to the soil, directly transmitting nutrients to the plant for better growth and development, but also caused significant changes to the plants’ metabolism for better adaptation to adverse environmental conditions.
5. Conclusions
All the applied algal treatments (U. lactuca, Cystoseira spp., and G. crinale) caused significant changes in the morphological, biochemical, and physiological parameters of the canola plants. They all had a significant promotive effect on the canola plants’ growth and yield, and their use as bio-fertilizers is thus recommended. The three examined seaweeds successfully alleviated the harmful effects of salt stress (up to 150 mM NaCl) on canola plants by significantly reducing the inhibition of the affected parameters, namely chlorophyll a, b, total carbohydrate accumulation, as well as growth promoting hormones, and reinforced the natural defense mechanisms exhibited by canola plants, such as proline, phenolics, and anthocyanin accumulation. Different seaweeds accelerated the canola growth and alleviated the effect of high salt levels to different degrees. Trends were observed, such as that U. lactuca treated canola had increased IAA, cytokinins, chlorophyll a, b, and carbohydrates. Meanwhile, treatment with Cystoseira spp. was associated with increased JA, GA3, flavonoids, and antioxidant activity while treatment with G. crinale was associated with effects on the canola IBA and ABA content. After comparing the growth and yield of differently treated canola plants, the application of U. lactuca was the most effective treatment under normal or salt stress conditions.
Author Contributions
Conceptualization, H.A.H., H.A.M., S.A.E.-K. and R.A.H.; Methodology, H.H.A, H.A.M., S.A.E.-K. and R.A.H.; Experimental Analysis, H.A.H., H.A.M. and S.A.E.-K.; Data Curation, H.A.H. and H.A.M.; Writing—Original Draft Preparation, H.H.A; Writing—Review & Editing, H.A.H., H.A.M., S.A.E.-K. and R.A.H.; Supervision, R.A.H.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Applications of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef]
- Chojnacka, K.; Saeid, A.; Witkowska, Z.; Tuhy, L. Biologically active compounds in seaweed extracts—the prospects for the application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2016, 14, 1119–1134. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Kumareswari, T.; Rani, M.S. Utilization of seaweeds to enhance growth and nutritive status of Amaranthus caudatus L. Int. J. Res. Stud. Biosci. 2015, 3, 9–15. [Google Scholar]
- Layek, J.; Das, A.; Idapuganti, R.G.; Sarka, D.; Ghosh, A.; Zodape, S.T.; Lal, R.; Yadav, G.S.; Panwar, A.S.; Ngachan, S.; et al. Seaweed extract as organic bio-stimulant improves productivity and quality of rice in eastern Himalayas. J. Appl. Phycol. 2018, 30, 547–558. [Google Scholar] [CrossRef]
- Mirparsa, T.; Ganjali, H.R.; Dahmardeh, M. The effect of biofertilizers on yield and yield components of sunflower oil seed and nut. Int. J. Agric. Biosci. 2016, 5, 46–49. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Briceño-Domínguez, D.; Di-Filippo-Herrera, D.A.; Hernández-Carmona, G. Seaweed as potential plant growth stimulants for agriculture in Mexico. Hidrobiológica 2018, 28, 129–140. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2013, 26, 619–628. [Google Scholar] [CrossRef]
- Khan, N.; Syeed, S.; Masood, A.; Nazar, R.; Iqbal, N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010, 1, e1. [Google Scholar] [CrossRef]
- Carillo, P.; Annunziata, M.G.; Pontecorvo, G.; Fuggi, G.; Woodrow, P. Salinity stress and salt tolerance. In Abiotic Stress in Plants; Shanker, A., Venkateswarlu, B., Eds.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils (FAO & ITPS). Status of the World’s Soil Resources (SWSR)—Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015. [Google Scholar]
- Amin, R.; Khalil, S.K. Effect of pre and post-emergence herbicides and row spacing on Canola. Sarhad J. Agric. 2005, 21, 165–170. [Google Scholar]
- Aleem, A.A. The Marine Algae of Alexandria; Alexandria Privately Published: Alexandria, Egypt, 1993; p. 139. [Google Scholar]
- Shindy, W.W.; Smith, O. Identification of plant hormones from cotton ovules. Plant Physiol. 1975, 55, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Kramell, R. HPLC separation of jasmonic acid methyl ester enantiomers. Phytochem. Anal. 1996, 7, 738–743. [Google Scholar]
- Müller, P.; Hilgenberg, W. Isomers of zeatin and zeatin riboside in club root tissue: Evidence for trans-zeatin biosynthesis by Plasmodiophora brassica. Physiol. Plant. 1986, 66, 245–250. [Google Scholar] [CrossRef]
- Lambert, M.; Neish, A.C. Rapid method for estimation of glycerol in fermentation solutions. Can. J. Med. Sci. 1950, 28, 83–89. [Google Scholar] [CrossRef]
- Metzner, H.; Rau, H.; Senger, H. Untersuchungen Zur Synchronisier barkeep ein Zelner pigmentmangel Mutanten Von chlorella. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Be Miller, J.N. Carbohydrate analysis. In Food Analysis, 4th ed.; Nielsen, S.S., Ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histoenzymology; Kalyani Publishers: New Delhi, India, 1980; p. 53. [Google Scholar]
- Strzelecka, H.; Kaminska, J.; Kowalski, J.; Malinowski, J.; Walewska, E. Chemiczne Metody Badań Roślinnych Surowców Leczniczych; PZWL: Warszawa, Poland, 1987; pp. 82–83. (In Polish) [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; F12.1–F12.13; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Tailor, C.S.; Goyal, A. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. leaves. Am. J. Ethnomed. 2014, 1, 244–249. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 12.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984; p. 680. [Google Scholar]
- Hamed, S.M.; Abd El-Rhman, A.A.; Abdel-Raouf, N.; Ibrahim, I.B.M. Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 104–110. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- El-Kaoua, M.; Chernane, H.; Benaliat, A.; Neamallah, L. Seaweed liquid extracts effect on Salvia officinalis growth, biochemical compounds and water deficit tolerance. Afr. J. Biotech. 2013, 12, 4481–4489. [Google Scholar]
- Rathorea, S.S.; Chaudharyb, D.R.; Borichab, G.N.; Ghoshb, A.; Bhatta, B.P.; Zodapeb, S.T.; Patoliab, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Sivasankari, S.; Chandrasekaran, M.; Kannathasan, K.; Venkatesalu, V. Studies on the biochemical constituents of Vigna radiata Linn treated with seaweed liquid fertilizer. Seaweed Res. Util. 2006, 28, 151–158. [Google Scholar]
- Mattner, S.W.; Milinkovic, M.; Arioli, T. Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J. Appl. Phycol. 2018, 30, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.M.N.; Chatterjee, R. Effect of seaweed liquid fertilizer from Gracilaria textorii and Hypnea musciformis on seed germination and productivity of some vegetable crops. Univ. J. Plant Sci. 2014, 2, 115–120. [Google Scholar]
- Musyimi, D.M.; Netondo, G.W.; Ouma, G. Effects of salinity on growth and photosynthesis of avocado seedling. Int. J. Bot. 2007, 3, 78–84. [Google Scholar]
- Mzibra, A.; Aasfar, A.; El Arroussi, H.; Khouloud, M.; Dhiba, D.; Kadmiri, I.M.; Bamouh, A. Polysaccharides extracted from Moroccan seaweed: A promising source of tomato plant growth promoters. J. Appl. Phycol. 2018, 30, 2953–2962. [Google Scholar] [CrossRef]
- Ramya, S.S.; Vijayanand, N.; Rathinavel, S. Foliar application of liquid biofertilizer of brown alga Stoechospermum marginatum on growth, biochemical and yield of Solanum melongena. Int. J. Recycl. Org. Waste Agric. 2015, 4, 167–173. [Google Scholar] [CrossRef]
- Smirnoff, N. The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hort. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Ramarajan, S.; Joseph, L.H.; Ganthi, A.S. Effect of seaweed liquid fertilizer on the germination and pigment concentration of soybean. J. Crop Sci. Technol. 2012, 1, 1–5. [Google Scholar]
- Mastafa, M.E.; Skeekh, L. Effect of seaweed extracts on seed germination, seedling growth and some metabolic process of Vicia faba L. Cytobios 1999, 100, 23–25. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Pedranzani, H.; Racagni, G.; Alemano, S.; Miersch, O.; Ramírez, I.; Peña-Cortés, H.; Taleisnik, E.; Machado-Domenech, E.; Abdala, G. Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul. 2003, 41, 149–158. [Google Scholar] [CrossRef]
- Haroun, A.S.; Hussein, M.H. The promotive effect of algal biofertilizers on growth, protein pattern and some metabolic activities of Lupins terms plant grown in siliceous soil. Asian J. Plant Sci. 2003, 2, 944–951. [Google Scholar]
- Lozano, M.S.; Verde Star, J.; Maitic, P.K.; Orandy, C.A.; Gaona, R.H.; Aranada, H.E.; Rojas, G.M. Effect of algal extract and several plant growth regulators on the nutritive value of potatoes (Solanum tuberosum L. Var. Gigant). Arch. Latinoam. Nutr. 1999, 49, 166–170. [Google Scholar]
- Rolland, F.; Moore, B.; Sheen, J. Sugar sensing and signalling in plants. Plant Cell 2002, 14, 185–205. [Google Scholar] [CrossRef]
- Hashem, H.A.; Hassanein, R.A. Plant metabolites expression. In Mathematical Advances towards Sustainable Environmental Systems; Furze, J.N., Swing, K., Gupta, A.K., McClatchey, R., Reynolds, D., Eds.; Springer International Publishing: Berlin, Germany, 2017; pp. 151–180. [Google Scholar]
- Hokmabadi, H.; Arzani, K.; Grierson, P.F. Growth, chemical composition, and carbon isotope discrimination of pistachio (Pistacia vera L.) rootstock seedlings in response to salinity. Aust. J. Agric. Res. 2005, 56, 135–144. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Rajasulochana, P.; Krishnamoorthy, P. Marine algae for agricultural sector for high yield. J. Chem. Pharm. Sci. 2014, 7, 369–372. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).