Abstract
As natural plant growth stimulators, amino acids are widely used to improve the yield and quality of crops. Several studies have illustrated the effects of different amino acids on lettuce plant parts. However, the effects of applying single amino acids on root growth remain elusive. The objective of this study was to evaluate the effect of root application of L-methionine on the growth of lettuce. In this study, two successive experiments on butterhead lettuce were conducted under hydroponic conditions. Three amino acids, L-methionine (20 mg/L), L-glycine (210 mg/L), and L-tryptophan (220 mg/L), were applied separately. L-methionine significantly increased the growth performance by 23.60%, whereas growth using L-tryptophan and L-glycine decreased by 98.78% and 27.45%, respectively. Considering the results of the first experiment, a second experiment was established with different concentrations of L-methionine (2200 mg/L, 220 mg/L, 22 mg/L, 2.2 mg/L, 0.2 mg/L, and 0.02 mg/L). The plants were allowed to grow for four weeks. Leaf width, plant area, leaf area, chlorophyll contents, etc., were evaluated. The results show that plant growth significantly improved by applying L-methionine at the lowest concentrations of 0.2 mg/L and 0.02 mg/L, which can, therefore, improve hydroponic production of lettuce and, accordingly, human nutrition.
1. Introduction
Lettuce (Lactuca sativa L.) is one of the main vegetable crops widely cultivated in China and consumed as a salad throughout the world. Due to its high nutritional value provided by mineral elements, vitamins, and folate, which play significant roles in human nutrition and diet, lettuce has become the focal point of several studies [1,2,3,4].
Nitrogen is an essential element for lettuce plants, an integral component of protein, phospholipid, and chloroplast [5,6]. Nitrogen uptake, assimilation, and utilization play essential roles in plant growth and development [7,8]. The plants mainly take up nitrogen in the form of nitrate (NO3−) and ammonium (NH4+) or N2 from the atmosphere through nitrogen-fixing bacteria [9,10,11]. The application of a large amount of chemical fertilizer to ensure high crop yield causes serious issues for agricultural products [12] and the environment [13,14]. Hence, there is a need to look for sustainable horticultural practices to counteract chemical-based agribusiness. In this respect, the application of amino acids, as a type of growth-promoting substance, supplies plant nutrients but also improves plant quality, which ultimately boosts the yield and commercial output of crops [15]. Therefore, it has become popular in sustainable agriculture [16,17,18,19,20]
Amino acids as biostimulants (substances that promote plant growth, improve nutrient availability, and enhance plant quality) [20,21] are not only getting popular for mitigating injuries caused by abiotic stresses [22] but also serve as hormone precursors [20,23,24,25]; signaling factors of different physiological progressions, such as glutamate receptors (GRLs) [24,25,26]; and regulators of nitrogen uptake [27], root development [25,28,29,30], and antioxidant metabolism [25,30,31,32]. Better root development supported by the addition of amino acids can boost nitrogen fixation, which induces an enhanced root surface for nutrient uptake [29,33].
Direct application of amino acids and their products could modulate N uptake and assimilation; this phenomenon is mediated by enzymes engaged with N assimilation [15,25,30]. In addition, it could be a follow-up to the flagging pathway that controls N securing amino acids in roots, which are mostly accessible as supplements [30]. Additionally, application of amino acids was also found to increase K+ in plants both in the presence of salt stress and without salt application [25,30,32]
A recent study [31] showed that seed treatment or foliar application of amino acids had different effects on soybean crops. An amino acid applied individually acts as a signaling component, i.e., increases antioxidant enzyme activity and causes efficient nutrient uptake [25,34,35]. Different investigations have demonstrated a positive impact of foliar application of amino acid mixtures on plants, for example, increased production in Solanum lycopersicum L. [36] and accumulation of dry matter, chlorophyll [37], starch, and polysaccharides in Vicia faba L. [38].
Previous studies have indicated an association of L-methionine with the biosynthesis of growth regulating substances such as cytokinins, auxins, and brassinosteroids in plants [39,40]. L-methionine functions as a precursor of a significant number of essential biomolecules such as vitamins, polyamine, cofactors [41], and antioxidants such as glutathione, which are considered to be significant determinants of cellular redox homeostasis and many defense compounds [19]. All of these biomolecules contain sulfur moieties that act as functional groups and are derived from L-methionine. In plants, L-methionine biosynthesis plays a central role in fixing inorganic sulfur from the environment, providing the only metabolic sulfide donor for the generation of glutathione, phytochelatins, iron–sulfur clusters, vitamin cofactors, and multiple secondary metabolites [42,43].
However, there is little data on the impact of isolated amino acids, particularly in root application. Additionally, the majority of investigations have been carried out utilizing a mixture of amino acids and other methods of application, such as foliar application and seed treatment. Hence, this study is based on the hypothesis that the root application of individual amino acids can improve the uptake of nitrogen and other growth-related factors, which can lead to increased productivity of lettuce plants. Therefore, the objective of the present work was to evaluate the effect of the separate application of L-tryptophan, L-glycine, and L-methionine in nutrient solution on the growth, yield, and physiology of lettuce plants.
2. Materials and Methods
The research study was carried out at the Vegetables and Flowers Institute, Chinese Academy of Agricultural Sciences, Beijing, China, in 2017–2018 to determine the regulation of lettuce plant growth response under different amino acids and concentrations.
2.1. Plant Material and Growth Conditions
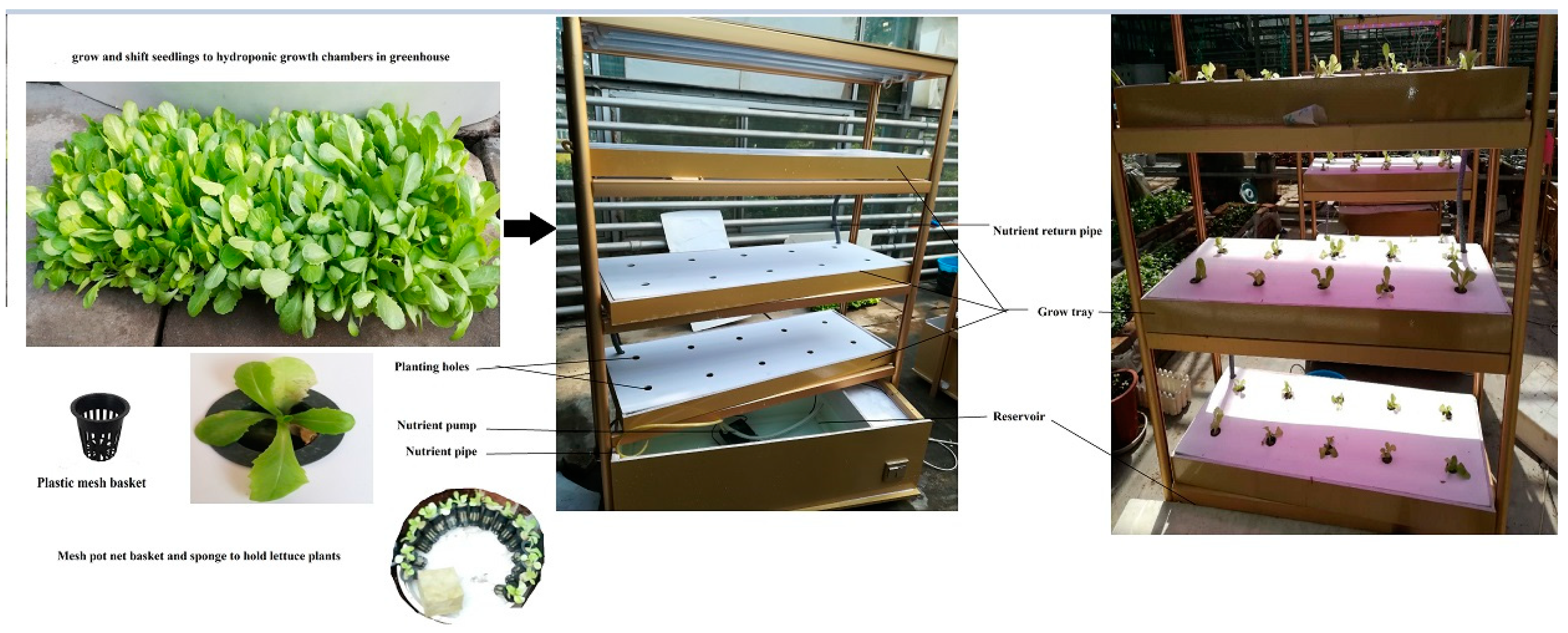
Sowing of butterhead lettuce seeds was done under controlled conditions using a mixture of peat moss with an average of 2–3 seeds per hole. All cultural practices were maintained in order to have a good plant stand. Average minimum and maximum monthly temperatures were set to 24 °C and 34 °C. Plants were provided with natural sunlight with a light intensity of approximately 900–1000 µmoles m−2/s. At pre-emergence stages, the nutrient solution was applied once a week. Plants with at least 2 fully expanded leaves 30 days after sowing were transferred to a closed-loop hydroponic system (Figure 1).

Figure 1.
The hydroponic system, mesh basket, and other materials used to grow lettuce plants.
Briefly, the hydroponic system consisted of 3 growing nutrient trays (used as 3 replicates) with 10 holes each, with a distance of 21.2 × 20.5 cm between the holes. The height of the hydroponic growing stand was 160 cm from the ground, while the length and width of the growing tray were 102 cm and 38 cm, respectively. The capacity of the water reservoir was 80 L with 6 cm depth and recyclable. Plastic mesh used to cover the plants was 6 cm long and 2 cm wide.
A pH of 6.0–6.3 and electrical conductivity (EC) of 1.5–2.0 mS cm−1 of nutrient solution were maintained regularly for optimal plant growth.
Plantlets were grown with 75% strength Hoagland nutrient solution containing the following nutrients (in mg/L), as previously described [44]: Ca(NO3)2 = 1122; KNO3 = 910; KH2PO4 = 272; NH4NO3 = 40; MgSO4 = 247; EDTA (Ethylenediamine tetraacetic acid Ferric Sodium Salt) = 16.80; ZnSO4 = 1.20; Na2B4O7 = 0.28; Na2MoO4 = 0.20; CuSO4 = 0.10; and MnSO4 = 0.86.
2.2. Application of Three Amino Acids on Lettuce
The experiment was conducted from December 2017 to February 2018 with 3 replications. The concentration of 3 amino acids, L-methionine, L-tryptophan, and L-glycine, was kept at 20 mg/L, 210 mg/L, and 220 mg/L, respectively. This gave 4 treatment combinations (3 amino acids and 1 control treatment). The amino acid treatment was started 8 days after transplanting into the nutrient solution to prevent plants from undergoing nutrient shock. Data were recorded every week and the crop was harvested after 30 days of treatment.
2.3. Application of L-Methionine Concentrations on Lettuce
The second experiment was conducted from January to March 2018 with a single amino acid, L-methionine (selected from experiment 1), in 6 concentrations, as 3 treatments and 3 replications. Plants were transplanted to the nutrient solution and treated with the amino acid after 8 days. The concentration of L-methionine applied was 2200 mg/L, 220 mg/L, and 22 mg/L for the treatment and 2.2 mg/L, 0.2 mg/L, and 0.02 mg/L for the control. All other experimental conditions were the same as in the first experiment. The tanks of nutrient solution were refreshed weekly.
2.4. Data Collection and Analysis
Data were recorded for the following morphological and physiological parameters: root length, leaf length, leaf width, leaf area, plant area, chlorophyll content, and fresh and dry mass of root and shoot.
2.4.1. Vegetative Growth Parameters
The number of leaves, plant height, plant diameter, and leaf area were measured every 7 days following standard procedures as proposed in [45,46,47].
Leaf length and width were measured by using a measuring tape/scale.
Root length was measured by separating roots from plants and placing them on paper and blotting them, then using a measuring tape (recorded in cm). Fresh weight per plant was square root transformed to normalize the error distribution before the analysis, as described [48], using an electronic balance (S = 0.1 g) (Acculab V-1200). The harvested plants were rinsed with distilled water, then the roots were blotted on filter paper and dried completely in an oven at 60–65 °C to determine dry weight [49]. The following formulas were used to calculate the index of growth traits [45].
Relative growth rate (RGR) was calculated by the following formula:
where W2 and W1 denote the plant’s dry mass (g) at time t2 and t1, respectively.
The net assimilation rate was calculated by the following formula:
where A is the area of assimilation organs (cm2), dW is the dry mass increment (g), and dt is the time of cultivation (days). Root mass ratio (RMR; root mass per unit total plant mass) was calculated as described in [49].
2.4.2. Physiological Measurements
Total chlorophyll content was estimated by using a portable The Soil Plant Analysis Development (SPAD) chlorophyll apparatus (SPAD-502 Plus, Konica Minolta, Tokyo, Japan). The leaf net photosynthesis rate for 3 independent lettuce seedlings per experimental replicate was determined using a portable LI-6400 photosynthesis system (Li-Cor 6400-18, Lincoln, NE, USA) [40,50]. The set values used were as follows: photosynthetic photon flux density, 500 μmol·m−2·s−1; air flow rate inside the sample chamber, 400 μmol·s−1.
The nutrient contents were measured in dried leaves ground by an electric mortar (multipurpose high-speed disintegrator, Dingia), sieved, weighed out to 0.2 g, and digested by concentrated nitric acid (HNO3, 5–6 mL) carefully under a laminar flow hood cabinet. All nutrients were analyzed using an optical emission spectrometer (Optima 5300 DV Spectrometer, Shelton, CT, USA).
The total N was determined by the Kjeldahl method [51].
2.5. Statistical Analysis
The recorded data were subjected to analysis of variance (ANOVA) and fixed-factor models [52], and Duncan’s multiple range test was used to assess the significance of treatment differences by means of IBM SPSS Statistics for Windows (version 20.0, IBM Corp., Armonk, NY, USA).
3. Results
3.1. Application of Three Amino Acids on Lettuce
The effects of different amino acids were studied for vegetative growth. The plants grown in the modified nutrient solution (Hoagland and amino acids) showed varied responses for metric traits.
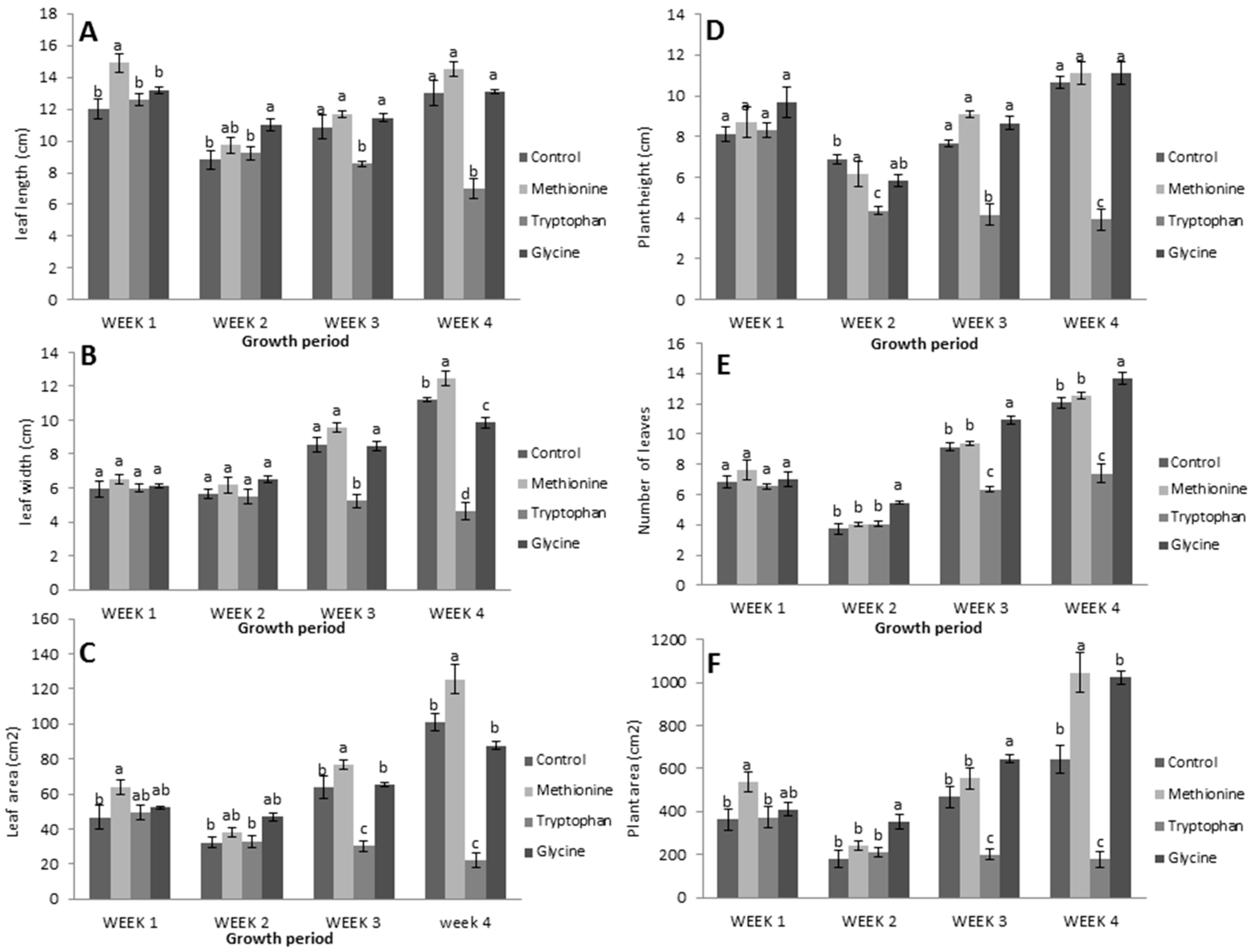
The vegetative indicators responded positively and significantly to all applied L-methionine concentrations (Figure 2A–F). Leaf length, width, and the number increased in response to L-methionine application, and decreased with L-glycine and L-tryptophan. The leaf length of L-methionine treated plants increased by 11.41% compared to control plants, while it decreased by 13.76% and 61.92% in L-glycine and L-tryptophan treated plants, respectively (Figure 2A). A significant increase in leaf width (17.46%) was also found with L-methionine, but there was an 18.25% and 63.49% decrease in response to L-glycine and L-tryptophan, respectively (Figure 2B). Similarly, leaf area and leaf numbers also increased under L-methionine treatment (31.41% and 50.4%, respectively), while leaf area decreased under L-glycine and L-tryptophan (29.67% and 86.25%, respectively) and leaf numbers decreased under L-tryptophan (50.36%) compared to control (Figure 2C,D).

Figure 2.
(A) Leaf length, (B) leaf width, (C) leaf area, (D) plant height, (E) leaf number, and (F) plant area with L-methionine (20 mg/L), L-glycine (220 mg/L), and L-tryptophan (210 mg/L). Means followed by the same lowercase letters do not differ significantly from each other in the comparison between amino acid treatments each week using Duncan’s multiple range test (p < 0.05).
Furthermore, plant height and area also had an encouraging response to L-methionine application (Figure 2E,F). The results revealed that there was an abrupt change in plant height and area, which were reduced by 82.91% and 90.78%, respectively, upon L-tryptophan application.
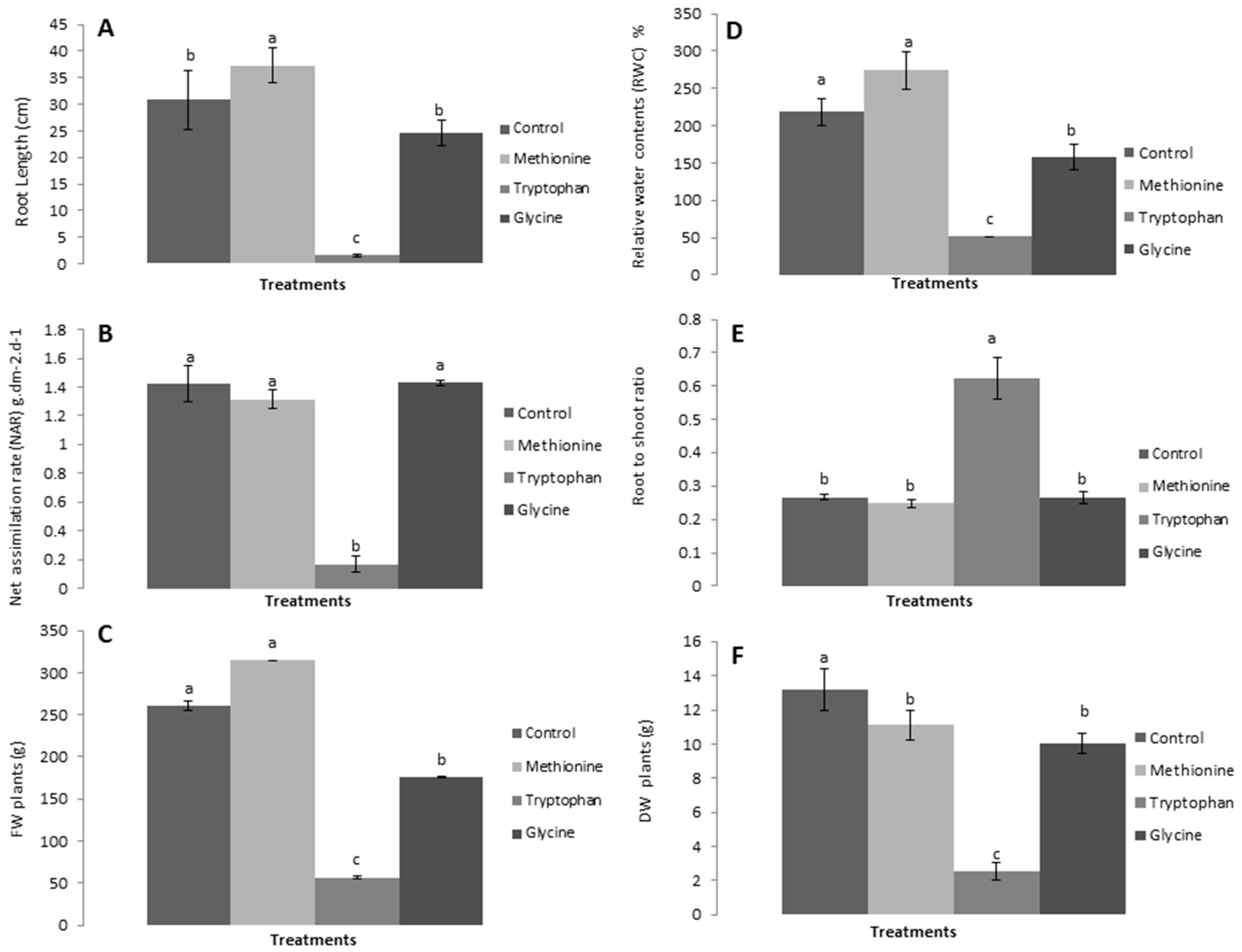
Likewise, root length, shoot-to-root ratio, relative water content, net assimilation rate, and fresh and dry biomass were positively affected by L-methionine treatment and negatively by L-tryptophan and L-glycine (Figure 3). The results revealed that lettuce plants showed a significant increase in root length (Figure 3A), relative water content (Figure 3D), and net assimilation rate (Figure 3C) in response to L-methionine application, and a decrease with the other two amino acids. Interestingly, the shoot-to-root ratio was found to be higher in response to L-tryptophan, which suggests that amino acids other than L-methionine also have an important role in plant growth. Moreover, a relative increase in fresh and dry biomass was observed with L-methionine application, by 20.88% and 15.71%, respectively, and a decrease with L-tryptophan and L-glycine treated plants (Figure 3D). Taken together, these results indicate that biostimulants, specifically the amino acid L-methionine, play a critical role in the growth and development of lettuce plants.
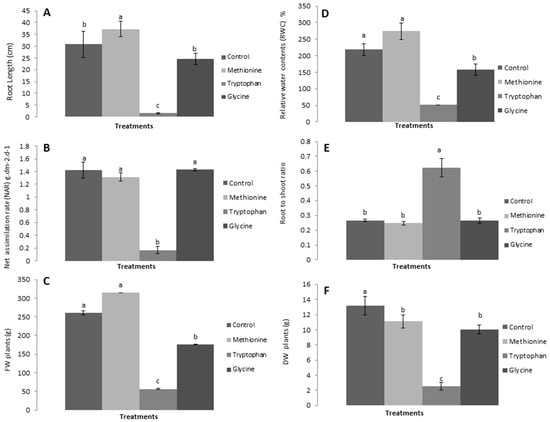
Figure 3.
Effects of amino acids on (A) root length, (B) net assimilation rate (NAR), (C) plant fresh weight (FW), (D) relative water content (RWC), (E) shoot-to-root ratio (S:R), and (F) plant dry weight (DW). Means followed by the same lowercase letters do not differ significantly from each other according to Duncan’s multiple range test (p < 0.05).
3.1.1. Photosynthetic Measurements
Photosynthetic measurements of lettuce leaves (Table 1) included amino acid application effect, rate of net photosynthesis, stomatal conductance, and transpiration rate. They were significantly affected by applied amino acids compared to control, except for total chlorophyll content. Transpiration rate and intracellular CO2 were relatively higher among all amino acid treated plants. The high levels of photosynthesis and chlorophyll content suggest that amino acids are important regulators of photosynthesis in lettuce, ultimately leading to higher yield and biomass.

Table 1.
Effects of amino acids on physiological indicators of lettuce plants.
In addition, relative growth parameters including leaf area index, leaf dry matter content, root mass ratio, specific leaf area, and leaf area ratio were higher in response to L-tryptophan treatment (Table 2). The relative growth rate was found to be higher with L-glycine application. L-methionine and L-glycine treated plants had a nonsignificant association with all other traits compared to control plants. These results show that along with L-methionine, L-tryptophan is also an important player, especially for relative growth enhancement in lettuce.

Table 2.
Effects of amino acids on growth indices of lettuce plants.
However, vitamin C content was not significantly affected by the applied concentrations, although there was a tendency for it to be higher in L-methionine and L-glycine compared to control (Table 3). In contrast, dry matter percentages were higher in L-tryptophan treated plants.

Table 3.
Effects of amino acids on vitamin C content and dry matter percentage of lettuce leaves.
3.1.2. Nutrient Contents
Nutrient content analysis (Table 4) revealed a dynamic change in response to all amino acid concentrations. The content of essential elements such as nitrogen, phosphorus, and potassium varied among treatments.

Table 4.
Effects of amino acids on essential elements of lettuce.
3.2. Application of Different L-Methionine Concentrations on Lettuce
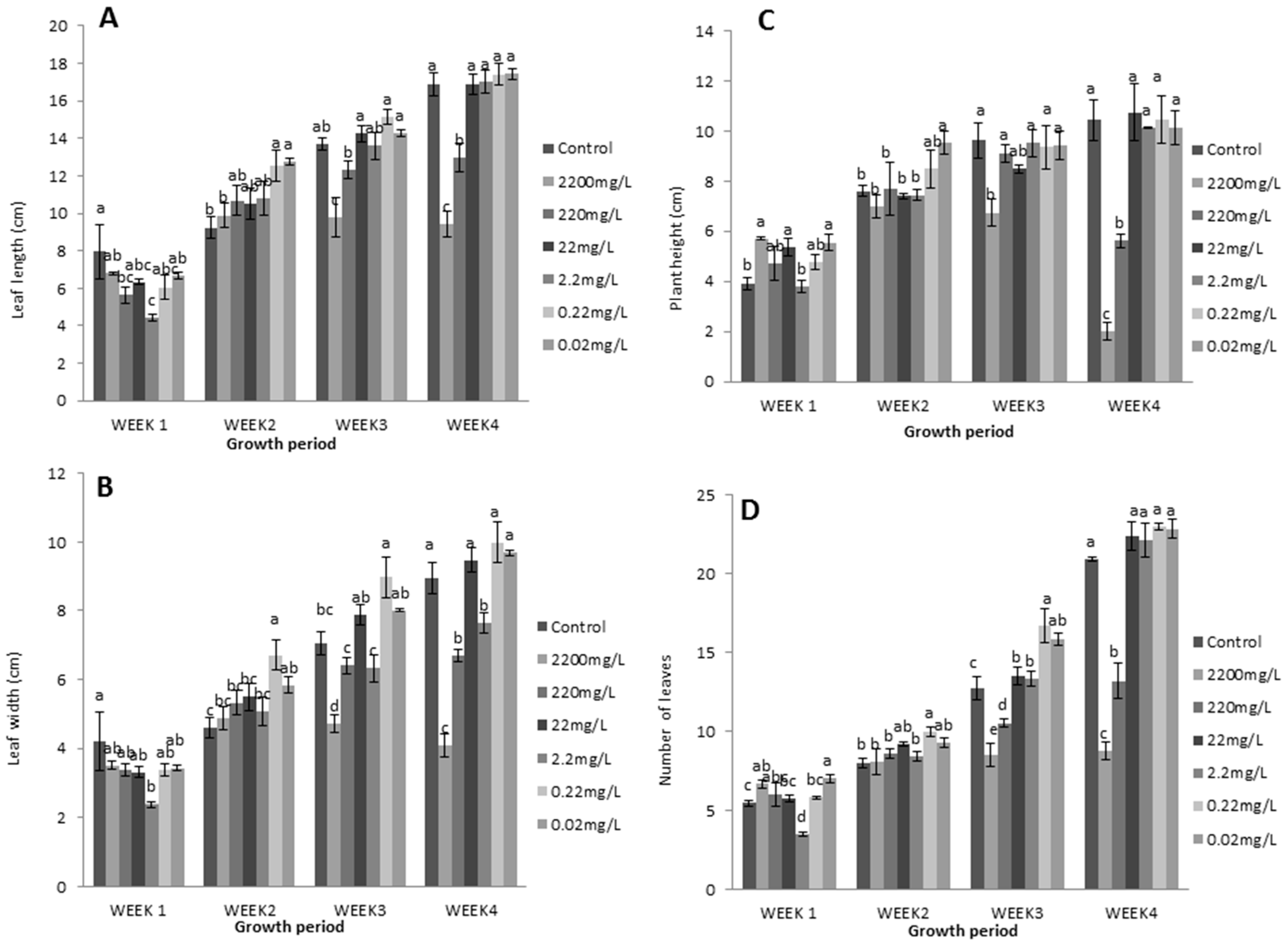
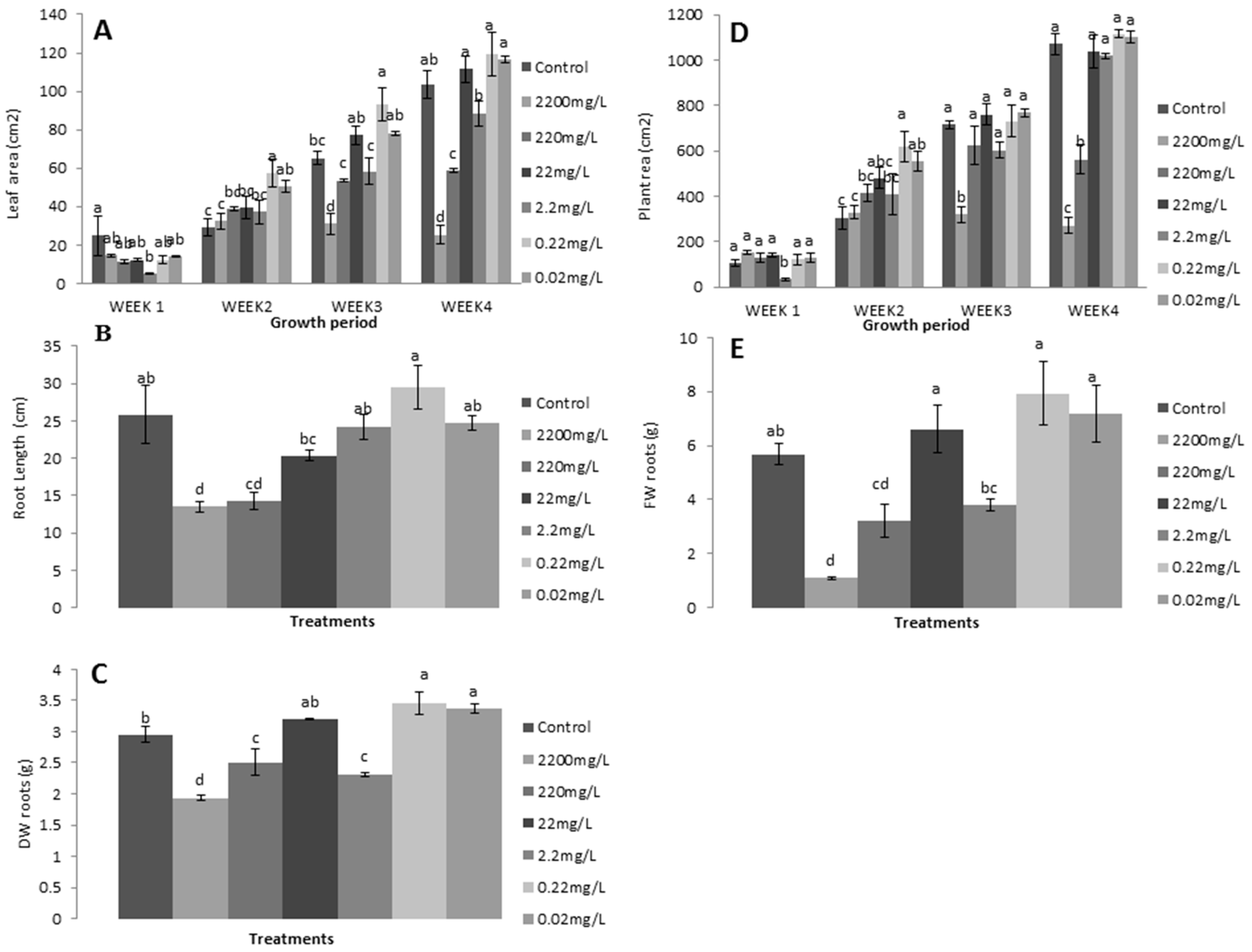
In the second trial, L-methionine at a higher concentration had a reduced effect on plant growth and physiology. The results show that lower levels of L-methionine significantly contributed to enhancing the number of leaves, plant height, and leaf length and width (Figure 4). Remarkably, 0.22 mg/L concentration of L-methionine resulted in a gradual increase in vegetative growth compared to control plants during all weeks. In contrast, higher levels were negatively associated with the corresponding measurements. Lettuce plants had short stature and fewer leaves in response to 2200 mg/L and 220 mg/L of L-methionine (Figure 4B,D). Concomitantly, more than 80% and 50% decreases in these two traits were found with increasing amino acid levels.
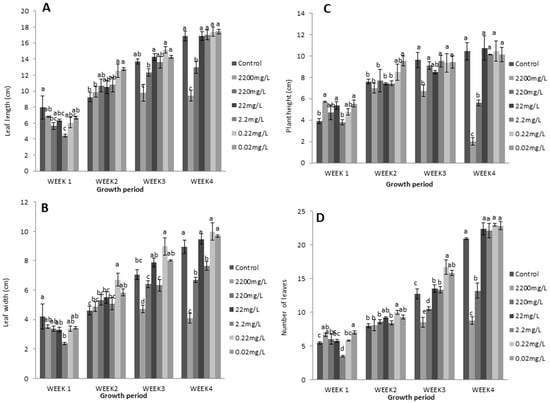
Figure 4.
Effects of L-methionine concentrations on (A) leaf length, (B) plant height, (C) leaf width, and (D) the number of leaves per plant. Means followed by the same letters are not statistically different from each other, according to Duncan’s multiple range test at (p < 0.05).
Similarly, relatively decreased leaf length (42.24% and 23.3%, respectively) and width (54.5% and 25.23%, respectively) were observed with increased treatment levels (Figure 4A,C). A strong increasing trend was also found for both traits in response to 0.2 mg/L of L-methionine. Overall, these results indicate that higher levels of L-methionine have an inhibiting effect on plant growth.
Leaf and plant area, root length, and fresh and dry weight of lettuce plants were improved by lower L-methionine concentrations (especially 0.2 mg/L) in advanced growth stages (Figure 5). However, mixed growth patterns were also present with different amino acid levels at each plant stage. A maximum reduction in leaf and plant area (75.5% and 74.653%, respectively) was found in 2200 mg/L treated plants, and a minimum (15.6% and 4.03%, respectively) in 0.2 mg/L plants (Figure 5A,B). Moreover, root length was found to be reduced at all levels except 0.2 mg/L, which caused a relative increase by 14.8% (Figure 5C). At the same time, the fresh and dry weight of roots were also lower with more concentrated treatment (Figure 5D), which suggests that amino acids are essential elements required as micronutrients for plant growth. An increased concentration leads to restricted plant biomass. Moreover, the decreased fresh and dry weight of lettuce plants indicates that nutrient stress and reduced photosynthetic activity were responsible for the lower accumulation of leaf organic material and growth rate.
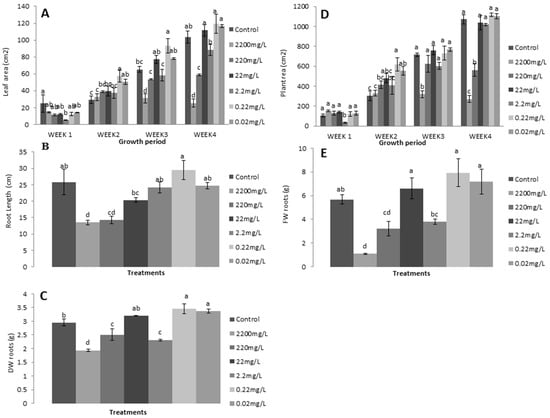
Figure 5.
Effects of L-methionine concentrations on (A) leaf area, (B) plant area, (C) root length, (D) root fresh weight, (E) and root dry weight. Means followed by the same letters are not statistically different from each other, according to Duncan’s multiple range test at (p < 0.05).
3.2.1. Relative Growth Measurements
Relative growth parameters measured in the second trial revealed that most of the traits were substantially increased by decreased L-methionine concentrations (Table 5). Surprisingly, root mass and leaf area ratios increased (p < 0.05) under higher concentration, which shows that plants can respond to a stress environment by maintaining their growth patterns. However, all other measured parameters had a decreasing tendency under a nutrient stress environment.

Table 5.
Effects of concentrations of L-methionine on growth indices of lettuce plants.
3.2.2. Photosynthetic Measurements
Enhanced photosynthetic activity, transpiration, and total chlorophyll content (Table 6) at lower L-methionine concentrations suggest that plants require this nutrient only in small amounts. More moderate transpiration activity occurred with all applied treatments, and reduced accumulation of chlorophyll in leaves causes restricted photosynthetic activity. There was no significant difference observed for stomatal conductance but it was relatively higher with 22 mg/L of L-methionine.

Table 6.
Effects of L-methionine levels on net physiological and growth indicators of lettuce leaves.
3.2.3. Nutrient Contents
Data in Table 7 describe the effects of L-methionine on macro- and microelements. The increasing trend of essential element accumulation including N, P, and K at reduced L-methionine levels indicates that these elements affect plant metabolism and help to adapt to modified environmental cues, which directly or indirectly affects plant metabolism. For example, a significant increase in nitrogen (N) content in leaf tissues increases photosynthesis efficiency, which is key to increasing crop yield. Plant metabolism is maintained by these elements with lower fractions of amino acids to regulate plant growth and development. Mixed fractions of other elements at different concentrations signify their importance in plant health regulation. For example, in addition to essential elements, S, Mg, Fe, Cu, Mn, and Na accumulation was higher at all levels.

Table 7.
Effects of L-methionine concentrations on essential macro- and microelements of lettuce leaves.
Any change in amino acid concentration leads to stress conditions, and plants respond differently at different levels by changing their growth patterns.
Moreover, a significant (p < 0.05) decrease in vitamin C content and leaf dry matter content and percentage also highlights the importance of micronutrients in plant metabolism.
Table 8 shows that vitamin C content decreased significantly (p < 0.05) with 2200 mg/L, but increased by 14.21% with 0.22 mg/L as compared to control. In contrast, a significant increase in leaf dry matter content was found with 2200 mg/L and in dry matter percentage (p < 0.05) with L-methionine application of 2200 mg/L and 220 mg/L. Moreover, a decrease in the fresh and dry weight of lettuce plants indicates that reduced photosynthetic activity was responsible for the lower accumulation of leaf organic material and reduced growth.

Table 8.
Effects of L-methionine concentrations on vitamin C content, leaf dry matter content (LDMC), dry matter percentage (DM%), fresh weight (FW), and dry weight (DW) of lettuce leaves.
As indicated by the outcomes shown in Table 3 and Table 8, there was no significant change (p < 0.05) observed in plants with or without amino acid treatment. It was observed that all individual amino acid treatments, except for one L-methionine concentration, led to no significant (p < 0.05) impact on vitamin C content. The special case was L-methionine at 2200 mg L−1, which prompted a decrease in vitamin C content, essentially contrasting with the control plants (p < 0.05).
4. Discussion
Amino acid application is a common practice for horticultural crops worldwide, with the majority of treatments making use of biostimulants with a mixture of amino acids [53]. In our study, we checked the activity of a single amino acid that regulates the nutrient contents involved in growth variables.
Previous investigations have demonstrated that plant developmental cues respond distinctively to the provision of amino acids [54,55,56]. It is likely that the effects of amino acids on plants rely on the kind of amino acids supplied [56] and the plant cultivars [57]
From our results, we can presume that amino acids (L-methionine, L-tryptophan, and L-glycine) not only make nutrients available to plants but also act as signal transducing molecules [31], as small doses are sufficient for plant development response, while these molecules can act as signals of several beneficial plant physiological processes. Studies demonstrate that amino acids in the form of a foliar spray on plants is a promising technique [38]. In this manner, L-methionine induces more prominent absorption of sulfur and nitrogen in plants, which also depends on the amount applied [26,58,59,60].
Plants utilize amino acids according to their nutritional needs and genetic background, as well as environmental and developmental cues [61,62]. This might be the reason why amino acid reactions were not consistent in both experiments. Therefore, we can assume that the decreasing effect of L-tryptophan on yield might be due to the inhibitory impact of auxin on vegetative growth. In this association, the reduction of lettuce yield per plant caused by the inhibition effect might be due to the detrimental effect of auxin accumulation stress on growth, the aggravation of mineral nutrient uptake, and the improvement of plant respiration [41]. The distinctive response was reported by Abbas et al. [54], in an investigation on L-tryptophan applied to chickpea at rates of 10−1 M, 10−2 M, and 10−3 M. They found random results with different parameters: root length was increased only in the control compared to the three treatments, the number of nodules increased only with 10−1 M, and nodule fresh and dry weight decreased with 10−3 M treatment and increased with the other two compared to control, while control remained nonsignificant. The most pods and highest plant weight were shown with 10−2 M, but pod weight per plant was significantly affected by all treatments due to the production of phytochromes suitable to chickpea. This experiment may provide evidence for the substantiating inconsistency of lettuce observed in our previous experiment.
However, our results contrast those described in [63], in which numbers of strawberry leaves per plant were significantly higher with the application of L-tryptophan than control.
A few reasons can clarify the positive effects of L-methionine. First, it has a role in maintaining the structure of proteins required for cell division, cell differentiation, and growth. Second, it provides sufficient sulfur and nitrogen according to plant needs. Third, the ability of L-methionine to be converted into polyamines and enlarge by entering the hormone structures [64] allows nitrogen movement between cells and organs [65]. It also functions as a buffer and behaves as a source of carbon and energy [66], and as a precursor of spermidine and gibberellin biosynthesis [43,67], growth regulators, and many secondary metabolites [43]. L-methionine also acts as a growth regulator of cytokinin, brassinosteroids, and auxin, increasing the initiation of roots; helps with the absorption of more nutrients by the plant [39,67], which may stimulate endogenous hormone homeostasis [68,69]; and is required for the development of hairy roots [67] at optimum levels.
Increased L-methionine levels influence phytohormones, which ultimately increases the chlorophyll content and chloroplast development or cytokinins [70,71]. An expected requirement for the prompting of L-methionine application might be the proximity of phytohormones (e.g., auxins and cytokinin). The phytohormones and signaling compounds may improve the photosynthetic activity, leading to better yield. Another possible mechanism involved with the amino acid effect could be related to the stimulation of root growth of treated plants, which may improve water and nutrient uptake capability, leading to yield productivity [68,69], as well as enhanced cell formation and increased fresh and dry matter [72], with increased growth behavior [69,70,73].
Our results demonstrate that high L-methionine concentration reduced plant growth due to damage to the photosynthetic apparatus [61] and blocking of nutrient uptake. Higher levels of this nutrient cause blockage of photosynthesis in stressed environments [65]. Padgett (1996) applied L-methionine to the root zones of chrysanthemum plants, producing a physiological disorder called methionosis, with the typical pattern of a metabolite–antimetabolite relationship [47]. It is thought that in this case, L-methionine, especially because of the large amounts applied, may function as an antimetabolite that interferes with normal amino acid metabolism. In other words, amino acids should be meticulously applied, as they could reduce the percentage of dry weight because they cause swollen, water-filled tissues due to depressed vegetative growth [65,74].
Thus, we speculate that application of L-methionine with 0.22 mg/L in the nutrient solution was sufficient, but other concentrations were too high and might have been a source of stress. Our results are consistent with previous studies [42,75] proposing that the improvement of novel “bio-sound items” ought to continue based on a foundational approach established in chemical synthesis, natural chemistry or biochemistry, and biotechnology connected to genuine plant physiological, agrarian, and environmental constraints. It was proposed that these items should work at low dosages, be biologically and ecologically friendly, and have reproducible advantages in horticultural plant development. The high amount of L-methionine also reduced vitamin C content. Therefore, we conclude that plant metabolism is affected by external N and thus can reflect the changes in N absorption, transport, and metabolism [76]. Similar findings have been reported in Chinese cabbage and lettuce [77]. The optimal required concentration is essential for optimal growth. It was confirmed from the previous study that when amino acids are added alone, care must be taken, as they can inhibit cell growth [63]. In general, the use of amino acids on plants can improve their capacity of transporting mineral components [66].
In view of the synthetic pathway of vitamin C and the synthesis of ascorbic acid requiring plant climatic changes and the conditions of plant sustenance, it may be hypothesized that whatever factor builds the sugar (or glucose) content in plant tissues can thus increase the vitamin C content [78]. It has been reported that amino acids [43] and nitrogen fertilizers do not impact the vitamin C content in broccoli. Conversely, in cauliflower, when nitrogen fertilizers are extended from 80 kg to 120 kg per ha, the ascorbic acid content is reduced by 7% [79].
Optimizing the amino acid content can bring about different morphogenetic responses; higher concentrations generally inhibit growth in Cicer arietinum [80]. The available information from various studies suggests that optimal levels of various amino acids may be species- or genotype-dependent, which needs to be determined before recommending their use [81]. Increasingly, plant ecologists working at all levels have become interested in the role of amino acid nutrition in the lives of plants and determining the proper amount of amino acids suitable for plant growth [82].
The depressive effect of L-tryptophan and high amounts of L-methionine on yield may be attributed to the inhibitory effect of auxin accumulation on vegetative growth, the disturbance in mineral uptake, and/or the enhancement of plant respiration [83].
5. Conclusions
Taking into consideration the discussion above, it can be inferred that L-methionine increases the chlorophyll content of plants and contributes to the saving of energy, thus boosting the plant yield. L-methionine led to significant increases in observed physiological factors in lettuce leaves at lower concentrations because at high concentrations it affects auxin uptake, which can kill plants. In brief, L-methionine at a concentration of 0.2 mg/L showed the best effect on the growth of lettuce plants. Therefore, we can say that L-methionine can contribute as a suitable substitute for fertilizers to increase crop yield. Future research should concentrate on assessing the mechanisms of how amino acids can influence the genetic transcription of various parameters, including supplement transporters, hormone production, and antioxidant metabolism. Along these lines, it will be possible to acquire the best understanding of the role of amino acids as biostimulants in lettuce plants.
Author Contributions
Conceptualization, W.J.; methodology, Q.L. and Y.G.; software, S.K.; validation, S.K.; formal analysis, S.K. and B.N.S.; investigation, S.K.; data curation, S.K.; writing—original draft preparation, S.K.; writing—review and editing, H.W., P.L., H.Y., and Q.L.; visualization, H.Y.; S.K.; supervision, W.J.; project administration, Y.G.; funding acquisition, W.J.
Funding
Demonstration of innovative and resource-efficient urban agriculture for multiple benefits in China (National key R&D program of China, (2017YFE0118500).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Samuolienė, G.; Urbonavičiūtė, A.; Duchovskis, P.; Bliznikas, Z.; Vitta, P.; Žukauskas, A. Decrease in nitrate concentration in leafy vegetables under a solid-state illuminator. HortScience 2009, 44, 1857–1860. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar]
- Christopoulou, M.; Wo, S.R.C.; Kozik, A.; McHale, L.K.; Truco, M.J.; Wroblewski, T.; Michelmore, R.W. Genome-wide architecture of disease resistance genes in lettuce. G3 Genes Genomes Genet. 2015, 5, 2655–2669. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Wang, L.; Jiao, Y.; Chen, C.; Zhao, L.; Mei, M.; Yu, Y.; Bie, Z.; Huang, Y. Pumpkin rootstock improves nitrogen use efficiency of watermelon scion by enhancing nutrient uptake, cytokinin content, and expression of nitrate reductase genes. Plant Growth Regul. 2017, 82, 233–246. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Chen, C.; Shireen, F.; Zheng, Z.; Sohail, H.; Afzal, M.; Ali, M.A.; Bie, Z.; Huang, Y. Genome-wide expression profiling of leaves and roots of watermelon in response to low nitrogen. BMC Genom. 2018, 19, 456. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.-R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Du, J.; Sun, J.; Guo, S. Effects of 24-epibrassinolide on nitrogen metabolism in cucumber seedlings under Ca(NO3)2 stress. Plant Physiol. Biochem. 2012, 61, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Amalfitano, C.; Gomez, L.D.; Frendo, P.; De Pascale, S.; Pepe, O.; Simister, R.; Ventorino, V.; Agrelli, D.; Borrelli, C.; McQueen-Mason, S.J.; et al. Plant–Rhizobiumsymbiosis, seed nutraceuticals, and waste quality for energy production of Vicia faba L. as affected by crop management. Chem. Biol. Technol. Agric. 2018, 5, 15. [Google Scholar] [CrossRef]
- Jämtgård, S.; Näsholm, T.; Huss-Danell, K. Nitrogen compounds in soil solutions of agricultural land. Soil Biol. Biochem. 2010, 42, 2325–2330. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- Caruso, G.; Conti, S.; La Rocca, G. Influence of crop cycle and nitrogen fertilizer form on yield and nitrate content in different species of vegetables. Adv. Hort. Sci. 2011, 25, 81–89. [Google Scholar]
- Silva, L.F.; Hower, J.C.; Izquierdo, M.; Querol, X. Complex nanominerals and ultrafine particles assemblages in phosphogypsum of the fertilizer industry and implications on human exposure. Sci. Total Environ. 2010, 408, 5117–5122. [Google Scholar] [CrossRef]
- Verger, P.J.; Boobis, A.R. Reevaluate pesticides for food security and safety. Science 2013, 341, 717–718. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation. Plant Physiol. 2005, 138, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, X.; Li, X.; Zhao, X.; Zang, L.; Pan, W. Development of prolonged release microspheres of metformin hydrochloride using ion exchange resins. J. Chin. Pharm. Sci. 2006, 15, 155. [Google Scholar]
- Pang, J.; Ross, J.; Zhou, M.; Mendham, N.; Shabala, S. Amelioration of detrimental effects of waterlogging by foliar nutrient sprays in barley. Funct. Plant Biol. 2007, 34, 221–227. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Thierry, G.; Lonhienne, A.; Doris, R.; Nicole, R.; Michael, C.; Richard, I.W.; Gamage, H.K.; Bernard, J.C.; Peer, M.S.; et al. Plants can use protein as a N source without assistance from other organisms. Proc. Natl. Acad. Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-Throughput Plant Phenotyping for Developing Novel Biostimulants: From Lab to Field or From Field to Lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Zielony, T.; Gajewski, M. Effect of Aminoplant and Asahi on yield and quality of lettuce grown on rockwool. Biostimulators Mod. Agric. Veg. Crops 2008, 35–43. [Google Scholar]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Forde, B.G.; Roberts, M.R. Glutamate receptor-like channels in plants: A role as amino acid sensors in plant defence? F1000Prime Rep. 2014, 6, 37. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J. Exp. Bot. 2007, 59, 111–119. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Forde, B.G. L-Glutamate as a novel modifier of root growth and branching: What’s the sensor? Plant Signal. Behav. 2007, 2, 284–286. [Google Scholar] [CrossRef]
- Weiland, M.; Mancuso, S.; Baluska, F. Signalling via glutamate and GLRs in Arabidopsis thaliana. Funct. Plant Biol. 2016, 43, 1–25. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: New York, NY, USA, 2015; Volume 130, pp. 141–174. [Google Scholar]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghelio, D.; Altissimo, A.; Nardi, S. Use of meat hydrolyzate derived from tanning residues as plant biostimulant for hydroponically grown maize. J. Plant Nutr. Soil Sci. 2013, 176, 287–296. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.-P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Persson, J.; Högberg, P.; Ekblad, A.; Högberg, M.N.; Nordgren, A.; Näsholm, T. Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 2003, 137, 252–257. [Google Scholar] [CrossRef]
- Gioseffi, E.; de Neergaard, A.; Schjørring, J.K. Interactions between uptake of amino acids and inorganic nitrogen in wheat plants. Biogeosciences 2012, 9, 1509–1518. [Google Scholar] [CrossRef]
- Koukounaras, A.; Tsouvaltzis, P.; Siomos, A.S. Effect of root and foliar application of amino acids on the growth and yield of greenhouse tomato in different fertilization levels. J. Food Agric. Environ. 2013, 11, 644–648. [Google Scholar]
- El-Aal, M.A.; Eid, R.S. Effect of foliar spray with lithovit and amino acids on growth, bioconstituents, anatomical and yield features of soybean plant. Plant Biotechnol. 2018, 187–201. [Google Scholar]
- SH SADAK, M.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2014, 20, 141–152. [Google Scholar]
- El-Awadi, M.; El-Bassiony, A.; Fawzy, Z.; El-Nemr, M. Response of snap bean (Phaseolus vulgaris L) plants to nitrogen fertilizer and foliar application with methionine and tryptophan. Nat. Sci. 2011, 9, 87–94. [Google Scholar]
- Yong, J.W.H.; Letham, S.D.; Wong, C.S.; Graham, D.F. Rhizobium-induced elevation in xylem cytokinin delivery in pigeon pea induces changes in shoot development and leaf physiology. Funct. Plant Biol. 2014, 41, 1323–1335. [Google Scholar] [CrossRef]
- Paciorek, T.; Zazímalová, E.; Ruthardt, N.; Petrásek, J.; Stierhof, Y.D.; Kleine-Vehn, J.; Morris, D.A.; Emans, N.; Jürgens, G.; Geldner, N.; et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005, 435, 1251–1256. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Aleksandr, A.L.; Ildus, A.Y.; Patrick, H.B. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Shekari, G.; Javanmardi, J. Effects of Foliar Application Pure Amino Acid and Amino Acid Containing Fertilizer on Broccoli (Brassica oleracea L. var. italica) Transplant. Adv. Crop Sci. Tech. 2017, 5, 280. [Google Scholar] [CrossRef]
- Jiang, W.J. New Techniques for Soilless Cultivation of Vegetables, rev. ed.; Beijing Jindun Publishing House: Beijing, China, 2007; pp. 197–200. [Google Scholar]
- Hunt, R. Plant growth curves. In The Functional Approach to Plant Growth Analysis; Edward Arnold Ltd.: London, UK, 1982. [Google Scholar]
- Yoshida, S.; Kitano, M.; Eguchi, H. growth of lettuce plants (Lactuca sativa L.) under control of dissolved O2 concentration in hydroponics. Biotronics 1997, 26, 39–45. [Google Scholar]
- Woltz, S.; Jackson, C. Production of yellow strapleaf of chrysanthemum & similar disorders by amino acid treatment. Plant Physiol. 1961, 36, 197. [Google Scholar] [PubMed]
- León, A.P.; Martín, J.P.; Chiesa, A. Vermicompost application and growth patterns of lettuce (Lactuca sativa L.). Agric. Tropica Subtrop. 2012, 45, 134–139. [Google Scholar] [CrossRef][Green Version]
- Medek, D.E.; Ball, M.C.; Schortemeyer, M. Relative contributions of leaf area ratio and net assimilation rate to change in growth rate depend on growth temperature: Comparative analysis of subantarctic and alpine grasses. New Phytol. 2007, 175, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, H.; Liu, P.; Ma, C.; Li, Q.; Jiang, W. Ending composting during the thermophilic phase improves cultivation substrate properties and increasing winter cucumber yield. Waste Manag. 2018, 79, 260–272. [Google Scholar] [CrossRef]
- Ahn, Y.S. Plant analysis for evaluating plant nutrition. In International Training Workshop on Soil Test and Plant Analysis; RDA& FFTC/ASPAC, 1987. [Google Scholar]
- Snedecor, G.; Cochran, W. Statistical Method, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1980; pp. 39–63. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chen, H.Y.; Ni, Q.X.; Kyu, S.L. Evaluation of the role of mixed amino acids in nitrate uptake and assimilation in leafy radish by using 15N-labeled nitrate. Agric. Sci. China 2008, 7, 1196–1202. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Koukounaras, A.; Siomos, A.S. Application of amino acids improves lettuce crop uniformity and inhibits nitrate accumulation induced by the supplemental inorganic nitrogen fertilization. Int. J. Agric. Biol. 2014, 16, 951–955. [Google Scholar]
- Wang, H.J.; Wu, L.H.; Wang, M.Y.; Zhu, Y.H.; Tao, Q.N.; Zhang, F.S. Effects of amino acids replacing nitrate on growth, nitrate accumulation, and macroelement concentrations in pakchoi (Brassica chinensis L.). Pedosphere 2007, 17, 595–600. [Google Scholar] [CrossRef]
- Mobini, M.; Khoshgoftarmanesh, A.H.; Ghasemi, S. The effect of partial replacement of nitrate with arginine, histidine, and a mixture of amino acids extracted from blood powder on yield and nitrate accumulation in onion bulb. Sci. Hortic. 2014, 176, 232–237. [Google Scholar] [CrossRef]
- Vincill, E.D.; Bieck, A.M.; Spalding, E.P. Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol. 2012, 159, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Price, C.A. Leaf Gui: Analyzing the geometry of veins and areoles using image segmentation algorithms. In High-Throughput Phenotyping in Plants; Springer: New York, NY, USA, 2012; pp. 41–49. [Google Scholar]
- Singh, R.; Parihar, P.; Prasad, S.M. Sulfur and calcium simultaneously regulate photosynthetic performance and nitrogen metabolism status in As-challenged Brassica juncea L. seedlings. Front. Plant Sci. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Prasad, M. Vertisol prevent cadmium accumulation in rice: Analysis by ecophysiological toxicity markers. Chemosphere 2014, 108, 85–92. [Google Scholar] [CrossRef]
- El-Shiaty, O.H.; El-Sharabasy, S.F.; Abd El-Kareim, A.H. Effect of some amino acids and biotin on callus and proliferation of date palm (Phoenix dactylifera L.) Sewy cultivar. Arab. J. Biotechnol. 2004, 7, 265–272. [Google Scholar]
- Kakkar, R.; Nagar, P.; Ahuja, P.; Rai, V. Polyamines and plant morphogenesis. Biol. Plant. 2000, 43, 1–11. [Google Scholar] [CrossRef]
- Padgett, P.E.; Leonard, R.T. Free amino acid levels and the regulation of nitrate uptake in maize cell suspension cultures. J. Exp. Bot. 1996, 47, 871–883. [Google Scholar] [CrossRef][Green Version]
- Shafeek, M.; Helmy, Y.; Magda, A.; Shalaby, F.; Nadia, M.O. Response of onion plants to foliar application of sources and levels of some amino acid under sandy soil conditions. J. Appl. Sci. Res. 2012, 8, 5521–5527. [Google Scholar]
- Davies, P.J. Plant Hormones: Biosynthesis, Signal Transduction, Action! Springer: New York, NY, USA, 2004. [Google Scholar]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dosedependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Bahari, A.; Pirdashti, H.; Yaghubi, M. The effects of amino acid fertilizers spraying on photosynthetic pigments and antioxidant enzymes of wheat (Triticum aestivum L.) under salinity stress. Int. J. Agron. Plant Prod. 2013, 4, 787–793. [Google Scholar]
- Anne, C.; Thomas, S. Regulation of chloroplast development and function by cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar]
- Fawzy, Z.; El-Shal, Z.; Yunsheng, L.; Zhu, O.; Sawan, O.M. Response of garlic (Allium Sativum L.) plants to foliar spraying of some bio-stimulants under sandy soil condition. J. Appl. Sci. Res. 2012, 8, 770–776. [Google Scholar]
- Romero, I.; Téllez, J.; Yamanaka, L.E.; Steindel, M.; Romanha, A.J.; Grisard, E.C. Transsulfuration is an active pathway for cysteine biosynthesis in Trypanosoma rangeli. Parasites Vectors 2014, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.H.; Sohail, M.; Saleem, M.; Mahmood, T.; Aziz, I.; Qamar, M.; Majeed, A.; Arif, M. Effect of L-tryptophan on plant weight and pod weight in chickpea under rainfed conditions. SciTech Dev. 2013, 32, 277–280. [Google Scholar]
- Herve, J.J. Biostimulants, a new concept for the future; prospects offered by the chemistry of synthesis and biotechnology. C. R. Acad. Agric. Fr. 1994, 80, 91–102. [Google Scholar]
- Yunna, Z.; Gang, L.; Houcheng, L.; Guangwen, S.; Riyuan, C.; Shiwei, S. Effects of partial replacement of nitrate with different nitrogen forms on the yield, quality and nitrate content of Chinese kale. Commun. Soil Sci. Plant. Anal. 2018, 1384–1393. [Google Scholar]
- Chen, G.L.; Gao, X.R.; Zhang, X.B. Effect of partial replacement of nitrate by amino acid and urea on nitrate content of non-heading Chinese cabbage and lettuce in hydroponic condition. Agric. Sci. China 2002, 444–449. [Google Scholar]
- Valpuesta, V.; Botella, M. Biosynthesis of L-ascorbic acid in plants: New pathways for an old antioxidant. Trends Plant Sci. 2004, 9, 573–577. [Google Scholar] [CrossRef]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- John, S.J.; Guha-Mukherjee, S. Plant Molecular Biology and Biotechnology, 1st ed.; Narosa Pub. House: New Delhi, India, 1997; pp. 17–28. [Google Scholar]
- El-sharabasy, S.; Fatma, I.; Gehan, H.; El-Dawayaty, M. Effect of different amino acids at different concentrations on multiplication and rooting stage of in vitro propagation of strawberries (Fragaria X Ananassa Duch cv. Chandler). Egypt. J. Genet. Cytol. 2015, 44, 31–34. [Google Scholar]
- Rosati, A.; Day, K.; DeJong, T. Distribution of leaf mass per unit area and leaf nitrogen concentration determine partitioning of leaf nitrogen within tree canopies. Tree Physiol. 2000, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Thimann, K.V. Auxins and the inhibition of plant growth. Biol. Rev. 1939, 14, 314–337. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).