Benefits of Native Mycorrhizal Amendments to Perennial Agroecosystems Increases with Field Inoculation Density
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenhouse Study
2.2. Field Study
2.3. Statistical Analyses
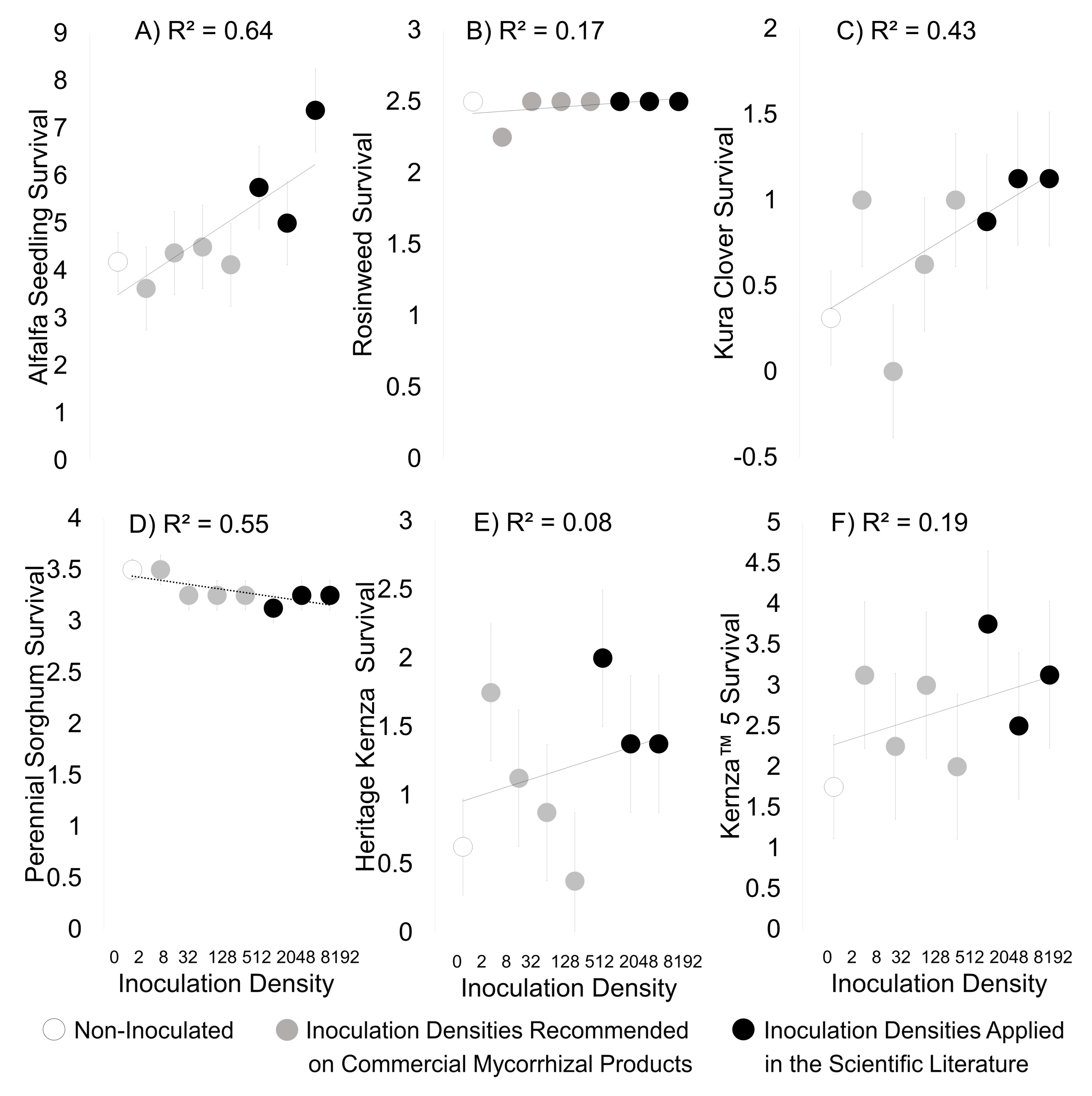
3. Results
3.1. Greenhouse Study
3.2. Field Experiment
3.3. Field Experiment and Greenhouse Correlations
4. Discussion
Density of Mycorrhizal Inocula
5. Conclusions and Implications to Sustainable Cropping Systems
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbott, L.; Robson, A. Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric. Ecosyst. Environ. 1991, 35, 121–150. [Google Scholar] [CrossRef]
- Agarwal, S.; Sinha, R.K.; Sharma, J. Vermiculture for sustainable horticulture agronomic impact studies of earthworms, cow dung compost and vermicompost vis-a-vis chemical fertilisers on growth and yield of lady’s finger (Abelmoschus esculentus). Int. J. Glob. Environ. Issues 2010, 10, 366–377. [Google Scholar] [CrossRef]
- Baer, S.; Kitchen, D.; Blair, J.; Rice, C. Changes in ecosystem structure and function along a chronosequence of restored grasslands. Ecol. Appl. 2002, 12, 1688–1701. [Google Scholar] [CrossRef]
- Bauer, J.T.; Koziol, L.; Bever, J.D. Ecology of Floristic Quality Assessment: Testing for correlations between coefficients of conservatism, species traits, and mycorrhizal responsiveness. AoB Plants 2018, 10, plx073. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.E.; Bever, J.D. Mycorrhizal species differentially alter plant growth and response to herbivory. Ecology 2007, 88, 210–218. [Google Scholar] [CrossRef]
- Bever, J.; Schultz, P.; Miller, R.; Gades, L.; Jastrow, J. Prairie mycorrhizal fungi inoculant may increase native plant diversity on restored sites (Illinois). Ecol. Restor. 2003, 21, 311–312. [Google Scholar]
- Cozzolino, V.; di Meo, V.; Piccolo, A. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. J. Geochem. Explor. 2013, 129, 40–44. [Google Scholar] [CrossRef]
- Crews, T.E.; Carton, W.; Olsson, L. Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Glob. Sustain. 2018, 1. [Google Scholar] [CrossRef]
- Davies, F.; Potter, J.; Linderman, R. Drought resistance of mycorrhizal pepper plants independent of leaf P concentration-response in gas exchange and water relations. Physiol. Plant. 1993, 87, 45–53. [Google Scholar] [CrossRef]
- Duchicela, J.; Vogelsang, K.M.; Schultz, P.A.; Kaonongbua, W.; Middleton, E.L.; Bever, J.D. Non-native plants and soil microbes: Potential contributors to the consistent reduction in soil aggregate stability caused by the disturbance of North American grasslands. New Phytol. 2012, 196, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Emam, T. Local soil, but not commercial AMF inoculum, increases native and non-native grass growth at a mine restoration site. Restor. Ecol. 2016, 24, 35–44. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- House, G.L.; Bever, J.D. Disturbance reduces the differentiation of mycorrhizal fungal communities in grasslands along a precipitation gradient. Ecol. Appl. 2018, 28, 748. [Google Scholar] [CrossRef] [PubMed]
- Jannoura, R.; Joergensen, R.G.; Bruns, C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur. J. Agron. 2014, 52, 259–270. [Google Scholar] [CrossRef]
- Jasper, D.; Abbott, L.; Robson, A. The effect of soil disturbance on vesicular—Arbuscular mycorrhizal fungi in soils from different vegetation types. New Phytol. 1991, 118, 471–476. [Google Scholar] [CrossRef]
- Jastrow, J. Changes in soil aggregation associated with tallgrass prairie restoration. Am. J. Bot. 1987, 74, 1656–1664. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093–2098. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Hart, M.M. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 2002, 12, 181–184. [Google Scholar]
- Koziol, L.; Bever, J.D. Mycorrhizal response trades off with plant growth rate and increases with plant successional status. Ecology 2015, 96, 1768–1774. [Google Scholar] [CrossRef]
- Koziol, L.; Bever, J.D. The missing link in grassland restoration: Arbuscular mycorrhizal fungi inoculation increases plant diversity and accelerates succession. J. Appl. Ecol. 2016, 54, 1301–1309. [Google Scholar] [CrossRef]
- Koziol, L.; Bever, J.D. Mycorrhizal feedbacks generate positive frequency dependence accelerating grassland succession. J. Ecol. 2019, 107, 622–632. [Google Scholar] [CrossRef]
- Koziol, L.; Rieseberg, L.H.; Kane, N.; Bever, J.D. Reduced drought tolerance during domestication and the evolution of weediness results from tolerance-growth trade-offs. Evolution 2012, 66, 3803–3814. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.; Schultz, P.A.; House, G.L.; Bauer, J.T.; Middleton, E.L.; Bever, J.D. The plant microbiome and native plant restoration: The example of native mycorrhizal fungi. Bioscience 2018, 68, 996–1006. [Google Scholar] [CrossRef]
- Larimer, A.L.; Clay, K.; Bever, J.D. Synergism and context dependency of interactions between arbuscular mycorrhizal fungi and rhizobia with a prairie legume. Ecology 2013, 95, 1045–1054. [Google Scholar] [CrossRef]
- Leake, J.; Johnson, D.; Donnelly, D.; Muckle, G.; Boddy, L.; Read, D. Networks of power and influence: The role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can. J. Bot. 2004, 82, 1016–1045. [Google Scholar] [CrossRef]
- Li, L.; Yang, S.; Li, X.; Zhang, F.; Christie, P. Interspecific complementary and competitive interactions between intercropped maize and faba bean. Plant Soil 1999, 212, 105–114. [Google Scholar] [CrossRef]
- Maltz, M.R.; Treseder, K.K. Sources of inocula influence mycorrhizal colonization of plants in restoration projects: A meta-analysis. Restor. Ecol. 2015, 23, 625–634. [Google Scholar] [CrossRef]
- Middleton, E.L.; Bever, J.D. Inoculation with a native soil community advances succession in a grassland restoration. Restor. Ecol. 2012, 20, 218–226. [Google Scholar] [CrossRef]
- Middleton, E.L.; Richardson, S.; Koziol, L.; Palmer, C.E.; Yermakov, Z.; Henning, J.A.; Schultz, P.A.; Bever, J.D. Locally adapted arbuscular mycorrhizal fungi improve vigor and resistance to herbivory of native prairie plant species. Ecosphere 2015, 6, 276. [Google Scholar] [CrossRef]
- Mills, K.E.; Bever, J.D. Maintenance of diversity within plant communities: Soil pathogens as agents of negative feedback. Ecology 1998, 79, 1595–1601. [Google Scholar] [CrossRef]
- Neuenkamp, L.; Prober, S.M.; Price, J.N.; Zobel, M.; Standish, R.J. Benefits of mycorrhizal inoculation to ecological restoration depend on plant functional type, restoration context and time. Fungal Ecol. 2018, 40, 140–149. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Ohsowski, B.M.; Klironomos, J.N.; Dunfield, K.E.; Hart, M.M. The potential of soil amendments for restoring severely disturbed grasslands. Appl. Soil Ecol. 2012, 60, 77–83. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Olasantan, F.; Lucas, E.; Ezumah, H.C. Effects of intercropping and fertilizer application on weed control and performance of cassava and maize. Field Crop. Res. 1994, 39, 63–69. [Google Scholar] [CrossRef][Green Version]
- Pringle, A.; Bever, J.D. Analogous effects of arbuscular mycorrhizal fungi in the laboratory and a North Carolina field. New Phytol. 2008, 180, 162–175. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol. Lett. 2004, 7, 740–754. [Google Scholar] [CrossRef]
- Rúa, M.A.; Antoninka, A.; Antunes, P.M.; Chaudhary, V.B.; Gehring, C.; Lamit, L.J.; Piculell, B.J.; Bever, J.D.; Zabinski, C.; Meadow, J.F. Home-field advantage? evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol. Biol. 2016, 16, 122. [Google Scholar] [CrossRef]
- Ryan, M.; Chilvers, G.; Dumaresq, D. Colonisation of wheat by VA-mycorrhizal fungi was found to be higher on a farm managed in an organic manner than on a conventional neighbour. Plant Soil 1994, 160, 33–40. [Google Scholar] [CrossRef]
- SAS. SAS 9.4 User’s Guide: Survey Data Analysis; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Sikes, B.A.; Cottenie, K.; Klironomos, J.N. Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J. Ecol. 2009, 97, 1274–1280. [Google Scholar] [CrossRef]
- Skene, K.R. Cluster roots: Some ecological considerations. J. Ecol. 1998, 86, 1060–1064. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. A review. Agron. Sustain. Dev. 2016, 36, 58. [Google Scholar] [CrossRef]
- Vejsadova, H.; Siblikova, D.; Gryndler, M.; Simon, T.; Miksik, I. Influence of inoculation with Bradyrhizobium japonicum and Glomus claroideum on seed yield of soybean under greenhouse and field conditions. J. Plant Nutr. 1993, 16, 619–629. [Google Scholar] [CrossRef]
- Wilson, G.W.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Wubs, E.; van der Putten, W.; Bosch, M.; Bezemer, T.B. Soil inoculation steers restoration of terrestrial ecosystems. Nat. Plants 2016, 2, 16107. [Google Scholar] [CrossRef] [PubMed]



| Experimental Density | kg/ha | Products/Scientific Studies Using Similar Densities |
|---|---|---|
| 0 | 0 | |
| 1 | 2 | MycoApply (Mycorrhizal Applications) Endo ~2 kg/ha |
| 2 | 8 | Sustainable Agricultural Technologies, INC ~11 kg/ha |
| 3 | 32 | Root Naturally Granular EndoMycorrhize ~24 kg/ha |
| 4 | 128 | MycoBloom Mycorrhizae~168 kg/ha |
| 5 | 512 | Emam 2016 (772 kg/ha whole soil) |
| 6 | 2048 | Koziol and Bever 2017 (1790 kg/ha mycorrhizae) |
| 7 | 8192 | Bever et al. 2003 (10,000+ kg/ha whole soil) |
| Kura Clover | Alfalfa | Lupine | Kernza™ 5 | Heritage Kernza | Perennial Sorghum | Rosinweed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p | |
| Block | 7 | 3.68 | 0.002 | 18.01 | <0.0001 | 1.02 | 0.43 | 10.35 | <0.0001 | 6.15 | <0.0001 | 7.79 | <0.0001 | 110.95 | <0.0001 |
| Inoculation Rate | 7 | 1.29 | 0.27 | 1.94 | 0.08 | 1.16 | 0.34 | 0.7 | 0.67 | 1.38 | 0.23 | 1.11 | 0.37 | 2.71 | 0.02 |
| Linear Contrast of the Slope of Response to Inocula Densities | 1 | 4 | 0.05 | 9.85 | 0.00 | 0.57 | 0.45 | 0.86 | 0.36 | 0.85 | 0.36 | 3.86 | 0.05 | 3.72 | 0.06 |
| Inoculated vs. Non-Inoculated | 1 | 2.67 | 0.11 | 1.22 | 0.27 | 0.29 | 0.59 | 2.21 | 0.14 | 2.59 | 0.11 | 4.07 | 0.05 | 0.68 | 0.41 |
| Scientific Densities vs. Others | 1 | 4.23 | 0.04 | 5.37 | 0.02 | 1.22 | 0.27 | 0.26 | 0.61 | 0.3 | 0.58 | 2.53 | 0.12 | 2.85 | 0.10 |
| Commercial vs. Scientific Densities | 1 | 2.48 | 0.12 | 5.7 | 0.02 | 1.53 | 0.22 | 0.93 | 0.34 | 3.79 | 0.06 | 0.13 | 0.72 | 0 | 1.00 |
| Commercial vs. Non-Inoculated | 1 | 1.04 | 0.31 | 0 | 0.97 | 0 | 1.00 | 1.18 | 0.28 | 0.89 | 0.35 | 2.28 | 0.14 | 1.78 | 0.19 |
| Scientific Densities vs. Non-Inoculated | 1 | 4.23 | 0.04 | 5.37 | 0.02 | 1.22 | 0.27 | 2.81 | 0.10 | 4.44 | 0.04 | 4.96 | 0.03 | 0 | 1.00 |
| Differences Among Commercial Rates | 3 | 1.47 | 0.23 | 0.2 | 0.90 | 0 | 1.00 | 0.38 | 0.77 | 1.32 | 0.28 | 0.76 | 0.52 | 5.34 | 0.00 |
| Differences Among Scientific Rates | 2 | 0.14 | 0.87 | 1.92 | 0.16 | 3.05 | 0.06 | 0.48 | 0.62 | 0.52 | 0.59 | 0.25 | 0.78 | 0 | 1.00 |
| Kura Clover | Alfalfa | Lupine | Kernza™ 5 | Heritage Kernza | Perennial Sorghum | Rosinweed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p | F-value | p | |
| Block | 7 | 2.95 | 0.01 | 12.24 | <0.0001 | 1.02 | 0.43 | 12.02 | <0.0001 | 6.79 | <0.0001 | 14.74 | <0.0001 | 7.85 | <0.0001 |
| Inoculation Rate | 7 | 1.36 | 0.24 | 4.37 | 0.0006 | 1.16 | 0.34 | 0.68 | 0.69 | 1.67 | 0.13 | 0.92 | 0.49 | 1.14 | 0.35 |
| Linear Contrast of the Slope of Response to Inocula Densities | 1 | 5.31 | 0.02 | 18.6 | <0.0001 | 0.57 | 0.45 | 1.23 | 0.27 | 0.44 | 0.51 | 2.89 | 0.09 | 4.99 | 0.03 |
| Inoculated vs. Non-Inoculated | 1 | 2.24 | 0.14 | 0.91 | 0.34 | 0.29 | 0.59 | 2.58 | 0.11 | 4.68 | 0.03 | 1.77 | 0.19 | 0.99 | 0.32 |
| Scientific Densities vs. Others | 1 | 5.07 | 0.03 | 8.04 | 0.006 | 1.22 | 0.27 | 0.24 | 0.62 | 0.04 | 0.84 | 2.11 | 0.15 | 2.03 | 0.16 |
| Commercial vs. Scientific Densities | 1 | 4.38 | 0.04 | 9.0 | 0.004 | 1.53 | 0.22 | 1.01 | 0.32 | 1.54 | 0.22 | 0.26 | 0.61 | 1.89 | 0.17 |
| Commercial vs. Non-Inoculated | 1 | 0.39 | 0.54 | 0.29 | 0.59 | 0.0 | 1.00 | 1.47 | 0.23 | 3.03 | 0.09 | 0.96 | 0.33 | 0.05 | 0.83 |
| Scientific Densities vs. Non-Inoculated | 1 | 5.19 | 0.03 | 7.04 | 0.01 | 1.22 | 0.27 | 3.1 | 0.08 | 4.99 | 0.03 | 2.21 | 0.14 | 3.13 | 0.08 |
| Differences Among Commercial Rates | 3 | 1.0 | 0.40 | 1.58 | 0.20 | 0.0 | 1.00 | 0.4 | 0.76 | 2.12 | 0.11 | 1.14 | 0.34 | 0.69 | 0.56 |
| Differences Among Scientific Rates | 2 | 0.12 | 0.89 | 4.98 | 0.01 | 3.05 | 0.06 | 0.22 | 0.81 | 0.1 | 0.91 | 0.42 | 0.66 | 0.7 | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koziol, L.; Crews, T.E.; Bever, J.D. Benefits of Native Mycorrhizal Amendments to Perennial Agroecosystems Increases with Field Inoculation Density. Agronomy 2019, 9, 353. https://doi.org/10.3390/agronomy9070353
Koziol L, Crews TE, Bever JD. Benefits of Native Mycorrhizal Amendments to Perennial Agroecosystems Increases with Field Inoculation Density. Agronomy. 2019; 9(7):353. https://doi.org/10.3390/agronomy9070353
Chicago/Turabian StyleKoziol, Liz, Timothy E. Crews, and James D. Bever. 2019. "Benefits of Native Mycorrhizal Amendments to Perennial Agroecosystems Increases with Field Inoculation Density" Agronomy 9, no. 7: 353. https://doi.org/10.3390/agronomy9070353
APA StyleKoziol, L., Crews, T. E., & Bever, J. D. (2019). Benefits of Native Mycorrhizal Amendments to Perennial Agroecosystems Increases with Field Inoculation Density. Agronomy, 9(7), 353. https://doi.org/10.3390/agronomy9070353




