Hydrothermal Carbonization and Pyrolysis of Sewage Sludge: Effects on Lolium perenne Germination and Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Sample Material
2.2. Greenhouse Experiment
2.3. Statistical Analysis
3. Results
3.1. Germination and Survival Rates
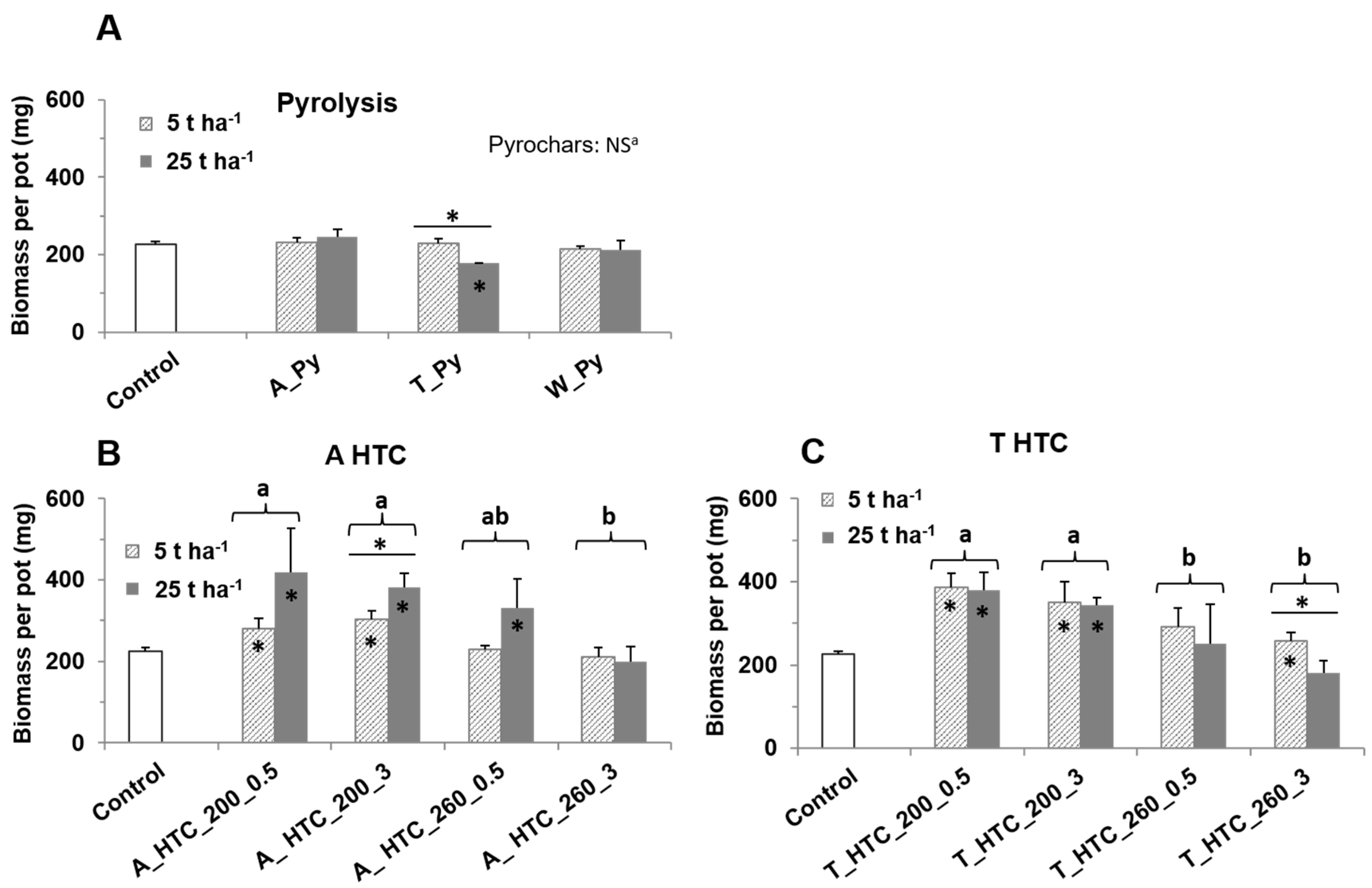
3.2. Biomass Production
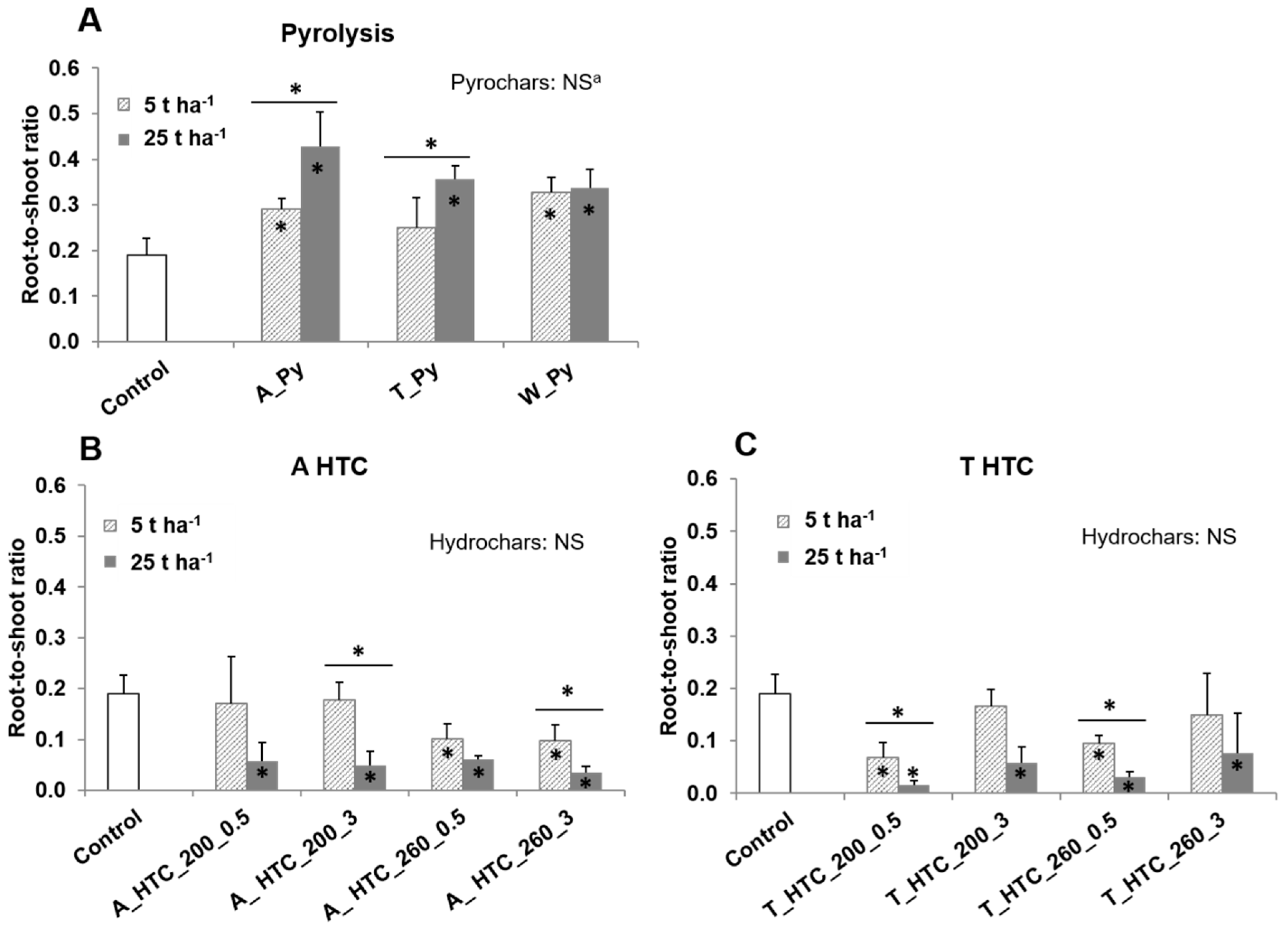
3.3. Root-to-Shoot Ratios
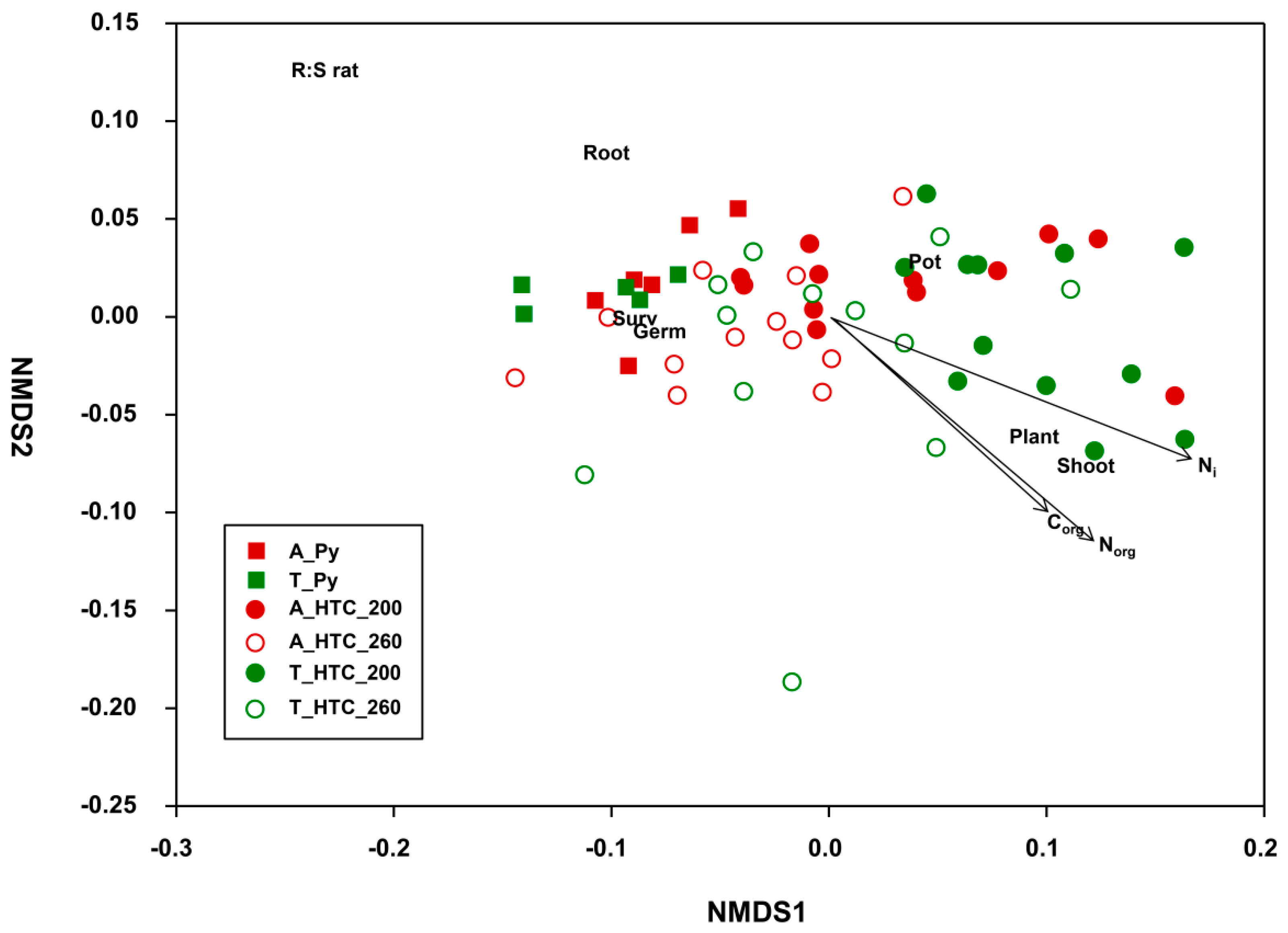
3.4. Relationship between Plant Response and Chars’ Properties
4. Discussion
4.1. Germination and Survival Rates
4.2. Biomass Production and Chars Properties
4.3. Root-to-Shoot Ratios and Char Properties
4.4. Fertilization Potential of Thermally Treated SS
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milieu Ltd.; WRc and Risk; Policy Analysts Ltd. (RPA). Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land. Final Report, Part I: Overview Report. Available online: http://ec.europa.eu/environment/archives/waste/sludge/pdf/part_i_report.pdf (accessed on 1 April 2019).
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 2nd ed.; Earthscan: London, UK, 2015. [Google Scholar]
- Libra, J.; Ro, K.; Kammann, C.; Funke, A.; Berge, N.; Neubauer, Y.; Titirici, M.; Fuhner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 89–124. [Google Scholar] [CrossRef]
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar that is Used in Soil: Version Number 2.1. 2015. Available online: https://www.biochar-international.org/wp-content/uploads/2018/04/IBI_Biochar_Standards_V2.1_Final.pdf (accessed on 1 April 2019).
- European Biochar Foundation. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar: Version 8E 2019. Available online: http://www.european-biochar.org/biochar/media/doc/ebc-guidelines.pdf (accessed on 1 April 2019).
- Bachmann, H.J.; Bucheli, T.D.; Dieguez-Alonso, A.; Fabbri, D.; Knicker, H.; Schmidt, H.P.; Ulbricht, A.; Becker, R.; Buscaroli, A.; Buerge, D.; et al. Toward the standardization of biochar analysis: The COST Action TD1107 interlaboratory comparison. J. Agric. Food Chem. 2016, 64, 513–527. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79 days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of green waste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Chan, K.Y.; van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Paneque, M.; De la Rosa, J.M.; Kern, J.; Reza, M.T.; Knicker, H. Hydrothermal carbonization and pyrolysis of sewage sludges: What happen to carbon and nitrogen? J. Anal. Appl. Pyrolysis 2017, 128, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Frišták, V.; Pipíška, M.; Soja, G. Pyrolysis treatment of sewage sludge: A promising way to produce phosphorus fertilizer. J. Clean. Prod. 2018, 172, 1772–1778. [Google Scholar] [CrossRef]
- Knicker, H. “Black nitrogen”—An important fraction in determining the recalcitrance of charcoal. Org. Geochem. 2010, 41, 947–950. [Google Scholar] [CrossRef]
- Huang, R.; Tang, Y. Evolution of phosphorus complexation and mineralogy during (hydro)thermal treatments of activated and anaerobically digested sludge: Insights from sequential extraction and P K-edge XANES. Water Res. 2016, 100, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandl, G.; Eckhardt, K.U.; Bargmann, I.; Kücke, M.; Greef, J.M.; Knicker, H.; Leinweber, P. Hydrothermal carbonization of biomass residues: Mass spectrometric characterization for ecological effects in the soil–plant system. J. Environ. Qual. 2013, 42, 199–207. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.; Grünhage, L.; Müller, C. Simple biotoxicity tests for evaluation of carbonaceous soil additives: Establishment and reproducibility of four test procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and biochar effects on germination of spring barley. J. Agron. Crop Sci. 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Thuille, A.; Laufer, J.; Höhl, C.; Gleixner, G. Carbon quality affects the nitrogen partitioning between plants and soil microorganisms. Soil Biol. Biochem. 2015, 81, 266–274. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source of contamination – Phytotoxic effects on cress seed (Lepidium sativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Working Document on Sludge. 3rd Draft; ENV.E.3/ LM; Directorate-General for the Environment: Brussels, Belgium, 2000. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106. FAO: Rome. Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 1 April 2019).
- Veihmeyer, F.J.; Hendrickson, A.H. Methods of measuring field capacity and wilting percentages of soils. Soil Sci. 1949, 68, 75–94. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package Version 2.4-4. 2015. Available online: http://cran.r-project.org/ (accessed on 27 February 2019).
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Stang, A.; Kern, J.; Wirth, S.; Gessler, A. Degradability of raw and post-processed chars in a two-year field experiment. Sci. Total Environ. 2018, 628–629, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, J.M.; Paneque, M.; Hilber, I.; Blum, F.; Knicker, H.; Bucheli, T.D. Assessment of polycyclic aromatic hydrocarbons in biochar and biochar-amended agricultural soil from Southern Spain. J. Soils Sediments 2016, 16, 557–565. [Google Scholar] [CrossRef]
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The effect of biochars on maize (Zea mays) germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Chen, J.; Zimmerman, A.R. Hydrochars derived from plant biomass under various conditions: Characterization and potential applications and impacts. Chem. Eng. J. 2015, 267, 253–259. [Google Scholar] [CrossRef]
- Andrews, M.; Sprent, J.I.; Raven, J.A.; Eady, P.E. Relationships between shoot to root ratio, growth and leaf soluble protein concentration of Pisum sativum, Phaseolus vulgaris and Triticum aestivum under different nutrient deficiencies. Plant Cell Environ. 1999, 22, 949–958. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s role as an alternative N fertilizer: Ammonia capture. Plant Soil 2012, 350, 35–42. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Ågren, G.I.; Franklin, O. Root:shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, J.M.; Knicker, H. Bioavailability of N released from N-rich pyrogenic organic matter: An incubation study. Soil Biol. Biochem. 2011, 43, 2368–2373. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001. [Google Scholar] [CrossRef]
- Paneque, M.; De la Rosa, J.M.; Franco-Navarro, J.D.; Colmenero-Flores, J.M.; Knicker, H. Effect of biochar amendment on morphology, productivity and water relations of sunflower plants under non-irrigation conditions. Catena 2016, 147, 280–287. [Google Scholar] [CrossRef] [Green Version]


| pH | Corg | NT | Norg | Nia of NT | Corg/NT | PT | KT | Cd | Cu | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 | g kg−1 | % | (w/w) | g kg−1 | g kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | ||
| A_SS | 7.4 | 228 | 19.2 | 18.9 | 0.5 | 11.9 | 12.3 | 6.8 | 3.2 | 401 | 1329 |
| A_HTC_200_0.5 | 6.5 | 224 | 16.0 | 15.6 | 2.6 | 14.0 | 13.9 | 7.3 | 3.5 | 423 | 1399 |
| A_ HTC_200_3 | 6.5 | 214 | 13.9 | 13.7 | 2.5 | 15.4 | 14.5 | 7.6 | 3.7 | 446 | 1459 |
| A_ HTC_260_0.5 | 6.4 | 213 | 11.2 | 10.8 | 1.5 | 19.1 | 15.4 | 8.0 | 4.0 | 477 | 1585 |
| A_ HTC_260_3 | 6.6 | 221 | 12.1 | 11.8 | 1.9 | 18.2 | 14.9 | 7.9 | 4.0 | 464 | 1554 |
| A_Py | 9.3 | 134 | 8.6 | 9.0 | 0.0 | 15.5 | 17.0 | 9.5 | 4.6 | 533 | 1766 |
| T_SS | 7.5 | 245 | 32.3 | 31.9 | 0.4 | 7.6 | 16.7 | 6.1 | 3.5 | 418 | 1821 |
| T_ HTC_200_0.5 | 6.7 | 233 | 25.0 | 24.5 | 2.2 | 9.3 | 20.0 | 6.8 | 4.2 | 493 | 1983 |
| T_ HTC_200_3 | 6.2 | 233 | 23.9 | 23.7 | 1.4 | 9.7 | 20.8 | 7.1 | 4.3 | 506 | 2017 |
| T_ HTC_260_0.5 | 6.3 | 225 | 19.7 | 19.8 | 1.2 | 11.4 | 21.6 | 7.3 | 4.6 | 528 | 2128 |
| T_ HTC_260_3 | 6.4 | 224 | 19.0 | 18.7 | 1.4 | 11.8 | 21.6 | 7.6 | 4.8 | 530 | 2162 |
| T_Py | 10.0 | 168 | 16.3 | 16.0 | 0.0 | 10.3 | 25.2 | 9.2 | 5.3 | 618 | 2432 |
| W_Py | 9.3 | 829 | 1.80 | n.a. b | n.a. | 922 | 0.7 | 4.5 | n.a. | n.a. | n.a. |
| Soil | 8.4 | 10 | 1 | n.a. | n.a. | 7 | 0.4 | 1.7 | n.a. | n.a. | n.a. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paneque, M.; Knicker, H.; Kern, J.; De la Rosa, J.M. Hydrothermal Carbonization and Pyrolysis of Sewage Sludge: Effects on Lolium perenne Germination and Growth. Agronomy 2019, 9, 363. https://doi.org/10.3390/agronomy9070363
Paneque M, Knicker H, Kern J, De la Rosa JM. Hydrothermal Carbonization and Pyrolysis of Sewage Sludge: Effects on Lolium perenne Germination and Growth. Agronomy. 2019; 9(7):363. https://doi.org/10.3390/agronomy9070363
Chicago/Turabian StylePaneque, Marina, Heike Knicker, Jürgen Kern, and José María De la Rosa. 2019. "Hydrothermal Carbonization and Pyrolysis of Sewage Sludge: Effects on Lolium perenne Germination and Growth" Agronomy 9, no. 7: 363. https://doi.org/10.3390/agronomy9070363






