1. Introduction
Culinary herbs are widely used to enhance the flavors of foods during cooking [
1]. While they are grown in fields for large-scale production and processing, fresh-cut culinary herbs are also increasingly grown under protection for year-round and value-added crop production [
2]. Culinary herb production in protected culture, including greenhouses, has been increasing concomitantly with the increased popularity in fresh-cut culinary herbs [
3,
4,
5]. Of the several environmental factors affecting hydroponic culinary herb production, temperature and humidity are generally more favorable and easily managed in controlled environments compared to outdoor field environments; however, mineral nutrition and photosynthetic light must be more actively managed to stimulate plant growth and development [
6].
Nutrient solutions serve several functions in hydroponic crop production. In water-culture systems such as nutrient-film (NFT) and deep-flow (DFT) technique systems, nutrient solutions act as a primary substrate for roots, but also provide essential mineral nutrients to plants [
7]. In recirculating hydroponic systems, nutrient solutions are recirculated and the pH and electrical conductivity (EC) are adjusted periodically [
8]. The target EC values to which fertilizer solutions are formulated, mixed to, and subsequently maintained at as they recirculate reflect crop requirements for promoting growth and healthy-appearing foliage [
6,
7]. Low EC values may be used for species with diminished nutrient requirements, while higher solution EC is used for crops with greater nutrient requirements [
9]. Subsequently, crop growth may not be promoted and/or plants may be unmarketable due to visual nutrient deficiency symptoms if the EC is too low; or, if the EC is too high, salt stress may suppress growth or excess fertilizer may be poorly utilized [
10,
11].
In addition to nutrient solution, photosynthetic light has a pronounced effect on culinary herb yields, especially for greenhouse-produced crops. Ambient photosynthetic light varies throughout the course of the year [
12], and this variability can affect crop production. During late fall, winter, and early spring, DLI is at seasonally low levels and can be sub-optimal. This can result in longer production schedules to achieve growth targets, or necessitate the use of supplemental lighting to increase DLI [
13]. Alternatively, high light intensities can create other challenges, such as managing air temperatures inside the greenhouse with additional thermal energy from high levels of solar radiation. The ambient temperature can affect plant nutrient uptake [
6], and nutrient solutions may need be adjusted during different times of the year [
14].
We only found one report quantifying the effects of varying nutrient solution EC under different DLI for hydroponically grown culinary herbs [
15], though the research only used basil (
Ocimum) species. While basil is the most popular culinary herb grown, commercial herb producers usually grow a wide variety of genera and cultivars. In contrast to many hydroponic food crops (i.e., tomato, cucumber, pepper, lettuce, and strawberry), culinary herbs encompass a broad diversity of plant taxa, and broader studies on nutrient solution EC and DLI including more genera is warranted. Therefore, the objectives of our study were to quantify the growth responses and tissue nutrient concentrations of cilantro, dill, and parsley grown hydroponically under low or high DLI at a range of nutrient solution EC treatments.
2. Materials and Methods
Sheets of 162-cell phenolic foam propagation cubes (Oasis® Horticubes® XL Multi-seed; Smithers-Oasis, Kent, OH, USA) were placed in a plastic flat (25 cm-wide × 50 cm-long), hydrated with deionized water (DI), and allowed to drain. Two seeds each of cilantro, dill, or parsley (Johnny’s Selected Seeds, Albion, ME) were sown per cell, after which flats were immediately placed in an environmental growth chamber (E-41L; Percival Scientific, Perry, IA, USA) with a 24-h target air temperature of 23.0 °C and photosynthetic photon flux density (PPFD) of 390 µmol·m−2·s−1 provided by fluorescent lamps from 0600 to 2200 h The temperature and light were measured every 15 s with a naturally-aspirated temperature sensor (TMC1-HD; Onset Computer Corporation, Bourne, MA, USA) in a solar radiation shield (RS3; Onset Computer Corporation, Bourne, MA, USA) and an amplified quantum sensor (SQ-222; Apogee Instruments, Logan, UT, USA), respectively, and averages were logged every 15 min by a data logger (HOBO U12; Onset Computer Corporation, Bourne, MA, USA). Changes to air temperature and light intensity were made, based on logged averages, to maintain target setpoint. Seeds were irrigated daily with deionized water until radical emergence, after which seedlings were provided with deionized water supplemented with 100 mg∙L−1 nitrogen (N) from a complete, balanced, water-soluble fertilizer (Jack’s Hydro FeED 16N−1.8P−14.3K; JR Peters, Allentown, PA, USA).
Three weeks after sowing seeds, each cell was thinned to a single seedling and cells were individually transplanted into nutrient-film technique (NFT) hydroponic systems. Each NFT system consisted of troughs that were 10-cm-wide, 5-cm-tall, and 203-cm-long (GT50-612; FarmTek, Dyersville, IA, USA) with a 3% slope. A 151-L reservoir (Premium Reservoir; Botanicare, Chandler, AZ, USA) held nutrient solution, which was delivered to troughs using a submersible water pump (Active Aqua 33-Watt pump; Hydrofarm, Grand Prairie, TX, USA) adjusted to provide ~1 L∙min−1 per trough. Transplants were placed in 3.5-cm-diameter holes cut into the top of the NFT troughs with the base of the phenolic foam cell resting on the trough bottom on 20-cm centers for a final density of 25 plants per square meter.
Two independent, identical glass-glazed greenhouses (Ames, Iowa, USA; Lat. 42.0° N; elev. 280 m) for the low and high DLI treatments held five NFT systems each. The target DLI for low and high DLI treatments were 7.0 or 18.0 mol∙m
−2∙d
−1, respectively (
Table 1). For both DLI treatments, a supplemental
PPFD of ~180 µmol∙m
−2∙s
−1 from 1000-W high-pressure sodium lamps (PL 3000; P.L. Light Systems, Beamsville, Ontario, Canada) was provided beginning at 0600 and ending at 2200 hr. However, between these times, HPS lamps turned off when outdoor instantaneous light intensity rose above or turned back on when below DLI-dependent set points for more than 15 min. In order to maintain target DLI values within and across replications in time, set points changed throughout each experiment as outdoor ambient
PPFD and day length changed, but maintained the same 16-h photoperiod between treatments. Aluminized shade cloth (XLS 15 Revolux; Ludvig Svensson, Kinna, Sweden) was also utilized in the low DLI house to decrease ambient light intensity mid-day to maintain the target DLI between replications when ambient light levels were higher. A 24-hr constant air temperature of 22 °C was maintained with radiant hot-water heating and fog cooling with actual temperatures reported in
Table 1. Shade curtains, HPS lamps, heating, and cooling were controlled with an environmental control system (ARGUS Titan; ARGUS Control Systems LTD., Surrey, British Columbia, Canada). The air temperature was measured every 15 s by four temperature probes (41342; R.M. Young Company, Traverse City, MI, USA) in an actively aspirated radiation shield (43502; R.M. Young Company, Traverse City, MI, USA), while the PPFD was measured every 15 s by eight quantum sensors (LI-190SL; LI-COR Biosciences, Lincoln, NE, USA) per greenhouse. Temperature probes and quantum sensors were connected to a datalogger (CR1000 Measurement and Control System; Campbell Scientific, Logan, UT, USA) with means logged every 15 min. Environmental data for three replications of the experiment are presented in
Table 1.
The nutrient solutions consisted of DI water, MgSO
4∙7H
2O, and 16N−1.8P−14.3K fertilizer (Jack’s Hydro FeED; JR Peters;
Table 2) with a 5:1 target N: magnesium (Mg) ratio. The pH was measured daily with a pH probe (HI 981504 pH/TDS/Temperature Monitor; Hanna Instruments, Woonsocket, RI, USA) and adjusted to 6.0 using an alkali (K
2CO
3, pH Up; General Hydroponics, Sebastopol, CA, USA) to raise pH or a combination of phosphoric and citric acid (H
3PO
4 and C
6H
8O
7, pH Down; General Hydroponics) to decrease pH. Electrical conductivity (EC) was measured with a handheld meter (HI 9813-6 Portable pH/EC/TDS Meter; Hanna Instruments) and adjusted to 0.5, 1.0, 2.0, 3.0, or 4.0 dS∙m
−1 daily using DI water or concentrated fertilizer (16N−1.8P−14.3K Hydro FeED; JR Peters). The solution was constantly aerated with four 15-cm long air stones (Active Aqua air stone; Hydrofarm) per system attached to a 110-L air pump (Active Aqua commercial air pump; Hydrofarm). Oxygen concentrations (8.3 ± 0.2 mg·L
−1) in the nutrient solutions were measured daily with a dissolved oxygen meter (HI 9147; Hanna Instruments). Nutrient solutions were continuously circulated through a heater/chiller unit (SeaChill TR-10; TECO, Terrell, TX, USA) to maintain a water temperature of 22.0 °C.
Nutrient solution samples were collected from each system before transplanting and after harvesting to determine initial and final nutrient concentrations. Nitrogen was measured with a flow-injection analyzer (QuickChem 8500; Lachat Instruments, Loveland, CO, USA). Phosphorus (P), K, Mg, calcium (Ca), sulfur (S), zinc (Zn), Mn, copper (Cu), iron (Fe), and boron (B) were analyzed by inductively coupled plasma−optical emission spectroscopy (Optima 4300 DV; Perkin Elmer, Waltham, MA, USA). Four weeks after transplanting, the number of nodes with unfolded leaves was recorded, plants were severed at the surface of the substrate, and fresh mass was immediately recorded. Shoots were then triple-rinsed in DI water, placed into paper bags, then dried for 3 d in a forced-air oven maintained at 67 °C, after which dry mass was recorded. Dried shoot tissue was analyzed at a commercial laboratory (AgSource, Lincoln, NE, USA) to determine nutrient concentrations. Determination of Kjeldahl nitrogen for all tissue samples began with standard digestion in concentrated sulfuric acid at 360 °C for ~1.5 h using a Tecator 40 block digestor. The resultant ammonium fraction was measured with a flow-injection analyzer (QuickChem 8500; Lachat Instruments) using a buffered salicylate-hypochlorite solution for color development. Determination of P, K, Ca, Mg, S, Zn, Mn, Cu, Fe, and B in all tissue samples began with initial digestion in concentrated nitric acid at 90 °C followed by three small additions of 30% hydrogen peroxide with a total time for digestion being ~1 h. Digested samples were filtered and analyzed by inductively coupled plasma−optical emission spectroscopy (Optima 4300 DV; Perkin Elmer).
The experiment was organized in a randomized complete block design with a factorial arrangement for each species. Factors included DLI (two levels) and nutrient solution concentration (five levels). There were 10 samples (individual plants) of each species per individual NFT system per replication. The experiment was repeated two additional times for a total of three replications. Analyses of variance and t-tests with an α = 0.05 were performed using SPSS 21.0 (IBM Corp., Armonk, NY, USA), and regression analyses were performed using Sigma Plot 13.0 (Systat Software, San Jose, CA, USA). For parameters without an interaction between EC and DLI, main effects were analyzed. To analyze the main effect of EC, data were pooled across DLI within EC for each species and regression analyses were performed. To analyze the main effect of DLI, data were pooled across EC within DLI for each species and t-tests were performed across DLI.
4. Discussion
The effects of DLI on fresh and dry mass of cilantro, dill, and parsley were clear in the present study, and our results align with existing literature describing the effects of photosynthetic light on hydroponic herb growth. For example, Hälvä et al. [
16] report dill fresh and dry shoot mass increases from 1.2 and 0.03 g, respectfully, under 70% shade (~325 µmol·m
−2·s
−1 on a sunny day) to 13.0 and 0.45 g, respectively, under no shade (~1,383 µmol·m
−2·s
−1 on a sunny day). Similarly, Litvin and Currey [
17] report shoot fresh and dry mass of cilantro, dill, and parsley increased by 86, 175, and 71 g, respectively, as DLI increased from 2.0 to 20.0 mol·m
−2·d
−1. Based on their growth responses to DLI, parsley is classified as a medium-light plant (optimum DLI between 10 and 20 mol·m
−2·d
−1), whereas cilantro and dill are categorized as high- or very high-light crops (optimum DLI between 20 and 30 mol·m
−2·d
−1 or >30 mol·m
−2·d
−1, respectively) [
17]. When our data are taken together with other research on culinary herbs [
15,
17,
18], they reinforce the impact of
PPFD on culinary herb shoot mass, having direct implications for the profitability of commercial operations, as herbs are sold on a per-unit-mass basis. Light is less- or not limiting in the late spring, summer, and early fall for culinary herbs when ambient and greenhouse DLI are high [
12]. However, under the seasonally low DLI of late fall, winter, and early spring, producers will need to decide whether to: (1) accept smaller yields, (2) increase production time to allow plants to reach a target mass; or (3) employ practices to maintain target yields such as providing supplemental lighting to enhance individual plant mass or increase planting density to maintain target mass per unit area.
While DLI affected growth and development, nutrient solution EC did not affect fresh or dry mass of cilantro, dill, or parsley. There are conflicting reports on the effect of nutrient solution concentration on hydroponic culinary herb growth. For example, Udagawa [
11] reports as nutrient solution concentration increases from 1.2 to 3.6 dS∙m
−1, shoot fresh and dry mass of hydroponically grown dill increases from 1.87 g and 196 mg, respectively, to 7.88 g and 645 mg, respectively; thyme (
Thymus vulgaris) follows a similar trend. On the other hand, nutrient solution EC does not affect hydroponically grown sweet basil (
Ocimum basilicum), lemon basil (
O. ×
citriodorum ‘Lime’), and holy basil (
O.
tenuiflorum ‘Holy’) fresh or dry mass [
15]. In the present study, and in Walters and Currey [
15], nutrient solution EC was monitored and adjusted daily and nutrient solution flow in channels was maintained at 2 L·min
−1. Alternatively, Ugadawa [
11] adjusted nutrient solution concentrations twice per week and no nutrient solution flow rate was reported. With adequate nutrient solution flow, low nutrient concentrations do not limit growth due to a lack of gradient at the root [
19], so inadequate flow may suppress growth. Furthermore, nutrients may be less- or not limiting for culinary herb growth under frequent replenishment and, therefore, have no growth-promoting effect when provided in higher concentrations.
Leaf number was affected differently by EC and DLI among cilantro, parsley, and dill. Whereas increasing DLI increased the leaf number of cilantro and dill, and EC had no effect, nutrient solution EC interacted with DLI to affect the unfolded leaf number for parsley. Temperature increases with increased radiation [
20], especially with high-pressure sodium lamps [
21,
22], which were used in this experiment. Though plant temperatures were not measured in the present study, the minimal increase in leaf number with increasing DLI for dill and parsley could likely be attributed to this effect. However, the larger increase in leaf number with increasing DLI for cilantro seems to be a more pronounced response and one, we believe, which may be attributed to limited assimilate availability in plants under the low DLI as a result of less photosynthesis.
Tissue nutrient concentrations were generally either affected by DLI or nutrient solution EC. As DLI increased, the concentration of nutrients affected by DLI increased, except for K (cilantro, dill, and parsley) and Mn (dill), which decreased (
Table 4,
Table 5 and
Table 6). Increasing light intensity can promote nutrient uptake, thereby enhancing tissue nutrient concentrations. For example, elements including Ca and B are taken up slowly by plants [
23], and increasing light can promote transpiration and, therefore, their uptake. We saw tissue Ca for cilantro and B for cilantro and parsley increase as DLI increased in this study. This agrees with previous research reporting enhanced tissue Ca and/or B from enhancing transpiration through increasing light and/or reducing humidity. For example, Frantz et al. [
24] reports enhancing light and transpiration promotes Ca uptake in lettuce (
Lactuca sativa). Similarly, increasing transpiration promotes Ca and B uptake for pansy (
Viola ×
wittrockiana ‘Dynamite Yellow’), petunia (
Petunia ×
hybrida ‘White Storm’), and gerbera (
Gerbera jamesonii ‘Festival Apricot’) [
25]. For those nutrients whose concentration was lower under the high DLI compared to the low DLI, uptake increased for plants when grown under the higher DLI but was not proportional to increasing dry mass. In our study, the dry mass increased with DLI for all species (
Table 4,
Table 5 and
Table 6) as did tissue nutrient content for all micro- and macronutrients (data not shown). We attribute the diminished tissue K and Mn concentrations at higher light intensities to the “dilution effect”, whereby nutrient content and growth may both be increasing, but not proportionally to one another [
26].
All tissue nutrient concentrations, except for Ca and Mg, were either unaffected by EC or increased with increasing nutrient solution EC. Increases in tissue nutrient concentrations with increasing solution strength generally agrees with previous reports on hydroponic herb nutrient solution concentrations [
11,
15]. In our present study, EC did not affect fresh or dry mass for cilantro, dill, or parsley. Therefore, we do not believe the diminished Ca and Mg concentrations at increasing EC is from a dilution effect accompanying enhanced growth. Rather, we attribute it to competition with K. In all nutrient solutions, K increased with increasing nutrient solution EC, as well as over the course of the experiment from using alkali containing K to increase solution pH throughout the experiment (
Table 2). Within the antagonistic relationship between K, Ca, and Mg [
26,
27], K is a particularly competitive cation [
28] and likely suppressed uptake of Ca and Mg in our study.
In addition to maximizing shoot mass, hydroponic fresh-cut herb products must be healthy-appearing and blemish-free from mineral nutrient deficiency symptoms. Tissue nutrient concentrations are a good indicator of nutrient status and, in comparing our data to published species-specific sufficiency ranges [
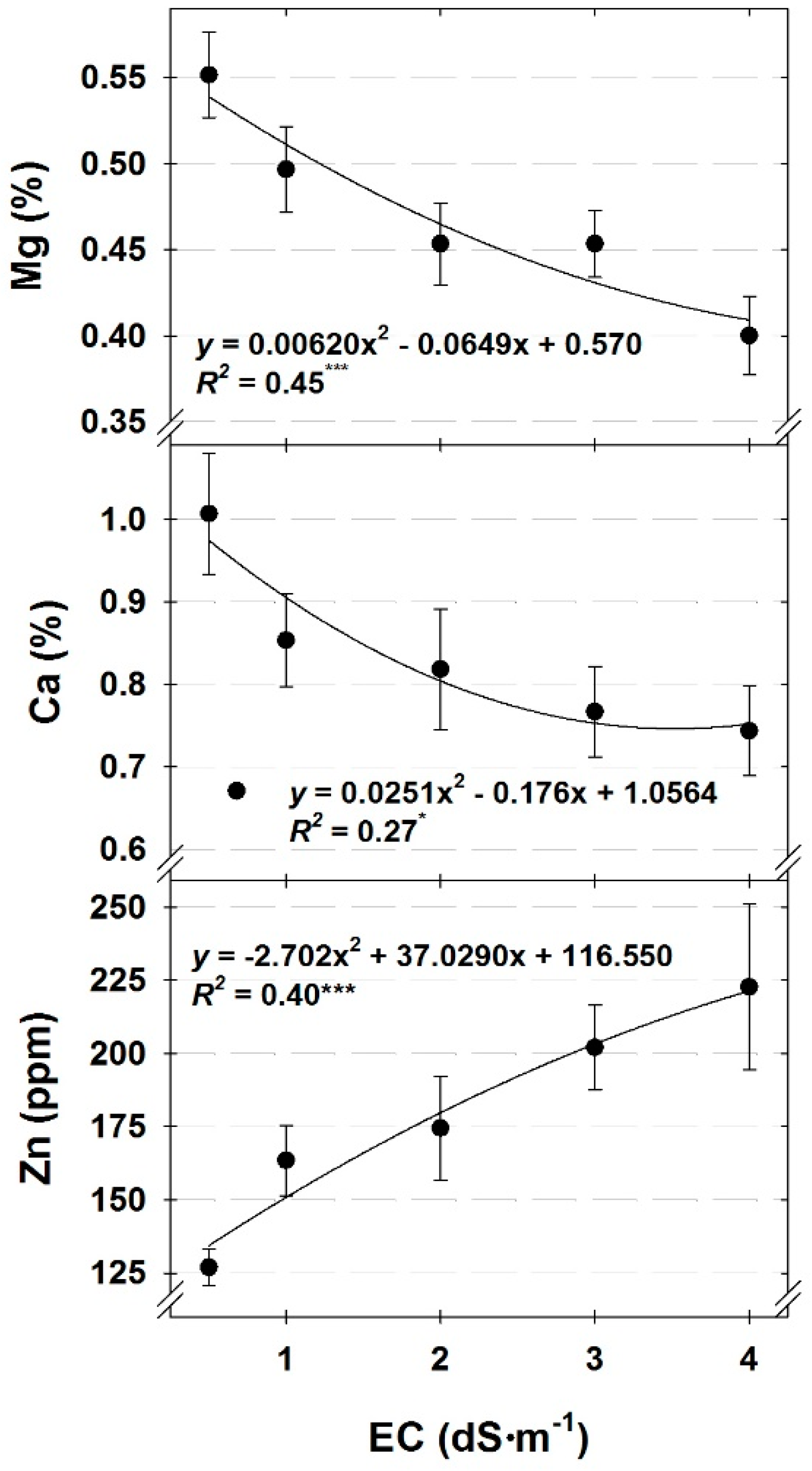
29], the only nutrient with tissue concentrations below the minimum acceptable concentrations was Ca in cilantro under a low DLI or for cilantro and dill grown with nutrient solution EC ranging from 2.0 to 4.0 dS∙m
−1 (
Figure 1 and
Figure 3). For all other macro- and micro-nutrients, tissue concentrations were either within recommended sufficiency ranges or exceeded them. We believe comparing our results from this study to published sufficiency ranges from field-grown plants [
29], not hydroponically grown plants in controlled environments, is still useful. Nonetheless, development and refinement of tissue sufficiency ranges specifically for hydroponically grown culinary herbs would be useful, especially given the increasing popularity of this production method.
As previously stated, it is a common practice for hydroponic crop producers to reduce the strength of their nutrient solution during summer conditions, when air temperatures are warm and light intensity is high to minimize excessive nutrient uptake and to avoid accumulation of nutrients in the root environment [
14]. Walters and Currey [
15] postulate it is warmer air temperatures, not high light intensities, which cause excessive nutrient uptake and, thus, the need to reduce nutrient solution strength during the summer months. The results we present in this paper provide further support for this supposition for culinary herbs, as there was a minimal effect of DLI on tissue nutrient concentrations for cilantro, dill, and parsley. This information is especially useful for greenhouse producers who are manipulating light independently of temperature, such as utilizing supplemental light during the late fall, winter, and early spring when air temperatures are more easily controlled.