Caffeine: The Allelochemical Responsible for the Plant Growth Inhibitory Activity of Vietnamese Tea (Camellia sinensis L. Kuntze)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Screening of Phytotoxic Potential of Tea Samples by the Sandwich Method
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Specific and Total Inhibitory Activity Bioassay
2.5. Effect of Aqueous Tea Extracts of Vinatea-Green Tea (V2) on the Germination and Growth of Different Species of Plants
2.5.1. Extraction Procedure
2.5.2. Germination Bioassay
2.5.3. Seedling Growth Bioassay
2.5.4. Soil Sampling for Rhizosphere Soil Method
2.5.5. Analysis of Caffeine Residue in the Soil
2.6. Statistical Analysis
3. Results and Discussion
3.1. Screening of Phytotoxic Potential of Tea Samples by the Sandwich Method
3.2. Determination of Caffeine Concentration in Tea Samples
3.3. Specific and Total Inhibitory Activity
3.3.1. Specific Activity
3.3.2. Total Inhibitory Activity
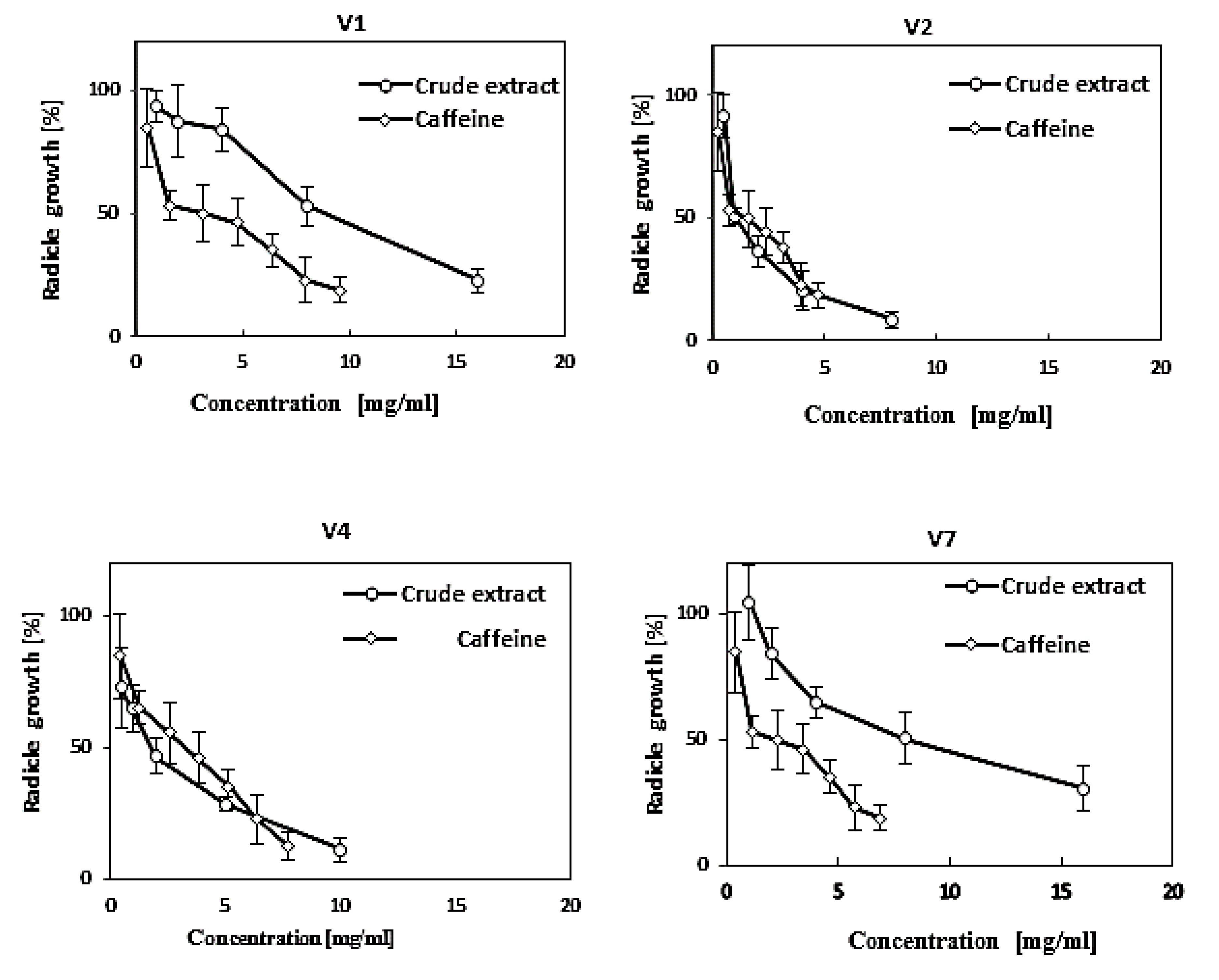
3.4. Effect of Vinatea-Green Tea Extracts and Caffeine on the Germination and Growth of Some Crop and Weed Species
3.5. Phytotoxic Potential of Caffeine from Vinatea-Green Tea in Soil
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nishioka, M.G.; Lewis, R.G.; Brinkman, M.C.; Burkholder, H.M.; Hines, C.E.; Menkedick, J.R. Distribution of 2,4-D in Air and on Surfaces inside Residences after Lawn Applications: Comparing Exposure Estimates from Various Media for Young Children. Environ. Health Perspect. 2001, 109, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B. Toxicity of 2, 4-Dichlorophenoxyacetic Acid - Molecular Mechanisms. Pol. J. Environ. Stud. 2006, 15, 365–374. [Google Scholar]
- Ayoola, S.O. Toxicity of Glyphosate Herbicide on Nile Tilapia (Oreochromis niloticus) Juvenile. Afr. J. Agric. Res. 2008, 3, 825–834. [Google Scholar]
- Uddin, M.R.; Won, O.J.; Pyon, J.Y. Herbicidal Effects and Crop Selectivity of Sorgoleone, a Sorghum Root Exudate under Greenhouse and Field Conditions. Korean J. Weed Sci. 2012, 30, 412–420. [Google Scholar] [CrossRef]
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.M.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A New Selective Herbicide for Use in Maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Watson, S.B.; Asolkar, R.N.; Boddy, L.G. Sarmentine, a Natural Herbicide from Piper Species with Multiple Herbicide Mechanisms of Action. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic Interactions and Allelochemicals: New Possibilities for Sustainable Weed Management. CRC Crit. Rev. Plant Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and Physiological Mechanisms Mediated by Allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichi, T.; Khanh, T.D.; Chung, I.M. Biological Control of Weeds and Plant Pathogens in Paddy Rice by Exploiting Plant Allelopathy: An Overview. Crop Prot. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pedrol, N.; González, L. (Eds.) Allelopathy: A Physiological Process with Ecological Implications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Rizvi, S.J.H.; Mukerji, D.; Mathur, S.N. Selective Phyto-Toxicity of 1,3,7-Trimethylxanthine between Phaseolus mungo and Some Weeds. Agric. Biol. Chem. 1981, 45, 1255–1256. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sano, H. Pathogen Resistance of Transgenic Tobacco Plants Producing Caffeine. Phytochemistry 2008, 69, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates—Nature, Occurrence, Dietary Burden, Absorption and Metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Takeda, Y. Differences in Caffeine and Tannin Contents between Tea Cultivars, and Application to Tea Breeding. JARQ 1994, 28, 117–123. [Google Scholar]
- Rezaeinodehi, A.; Khangholi, S.; Aminidehaghi, M.; Kazemi, H. Allelopathic Potential of Tea (Camellia sinensis (L.) Kuntze) on Germination and Growth of Amaranthus retroflexus L. and Setaria glauca (L.) P. Beauv. J. Plant Dis. Prot. Suppl. 2006, 454, 447–454. [Google Scholar]
- Dibah, H.; Majd, A.; Nejadsattari, T.; Ghanati, F. Allelopathic potential of Camellia sinensis L.(Kuntze) on seed germination and seedling growth of Vicia sp. Adv. Environ. Biol. 2012, 6, 2846–2853. [Google Scholar]
- Waris, A.; Waris, L.; Khan, M.A.; Shad, A.A. Allelopathic Effect of Methanol and Water Extracts of Camellia sinensis L. on Seed Germination and Growth of Triticum aestivum L. and Zea mays L. JBM 2016, 3, 5. [Google Scholar]
- Hiradate, S. Isolation Strategies for Finding Bioactive Compounds: Specific Activity vs. Total Activity. Am. Chem. Soc. Symp. Ser. 2006, 927, 113–126. [Google Scholar]
- Fujii, Y.; Hiradate, S. A Critical Survey of Allelochemicals in Action—The Importance of Total Activity and the Weed Suppression Equation. In Proceedings of the 4th World Congress on Allelopathy, Establishing the Scientific Base, Wagga, Australia, 21–26 August 2005; pp. 73–76. [Google Scholar]
- Fujii, Y.; Shibuya, T.; Yasuda, T. L-3, 4-Dihydroxyphenylalanine as an Allelochemical Candidate From Mucuna Pruriens (L.) Dc. Var. Utilis. Agric. Biol. Chem. 1991, 55, 617–618. [Google Scholar] [CrossRef]
- Kamo, T.; Hiradate, S.; Fujii, Y. First Isolation of Natural Cyanamide as a Possible Allelochemical from Hairy Vetch Vicia villosa. J. Chem. Ecol. 2003, 29, 275–283. [Google Scholar] [CrossRef]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and Total Activities of the Allelochemicals Identified in Buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Elakovich, S.D.; Stevens, K.L. Phytotoxic Properties of Nordihydroguaiaretic Acid, a Lignan from Larrea tridentata (Creosote Bush). J. Chem. Ecol. 1985, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.N.; Cheema, Z.A.; Khaliq, A. Effects of Mixture of Allelopathic Plant Aqueous Extracts on Trianthema portulacastrum L. Weed. Allelopath. J. 2010, 25, 205–212. [Google Scholar]
- Vuong, Q.V.; Nguyen, V.; Golding, J.B.; Roach, P.D. The Content of Bioactive Constituents as a Quality Index for Vietnamese Teas. Int. Food Res. J. 2011, 18, 329–336. [Google Scholar]
- Smit, A.W. What Is the Potential of Marine Algae, in Combination with Sewage Sludge, as a Composite of Bio-Fertilizer? Report No. 5895; Norwegian Institute for Water Research: Oslo, Norway, 2009; p. 30. [Google Scholar]
- Ito, I.; Kobayashi, K.; Yoneyama, T. Fate of Dehydromatricaria Ester Added to Soil and Its Implications for the Allelopathic Effect of Solidago Altissima L. Ann. Bot. 1998, 82, 625–630. [Google Scholar] [CrossRef]
- Ponder, F.; Tadros, S.H. Juglone concentration in the soil beneath black walnut interplanted with nitrogen-fixing species. J. Chem. Ecol. 1985, 11, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Wang, H.B.; Yang, X.Y.; Zhang, Q.; Li, J.Y.; Jia, X.L.; Kong, X.H.; He, H.B. Autotoxicity of the Soil of Consecutively Cultured Tea Plantations on Tea (Camellia Sinensis) Seedlings. Acta Physiol. Plant. 2016, 38, 195. [Google Scholar] [CrossRef]
- Fujii, Y.; Parvez, S.S.; Parvez, M.M.; Ohmae, Y.; Uda, O. Screening of 239 Medicinal Plant Species for Allelopathic Activity Using the Sandwich Method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Salam, A.; Kato-Noguchi, H. Evaluation of Allelopathic Potential of Neem (Azadirachta Indica. A. Juss) Against Seed Germination and Seedling Growth of Different Test Plant Species. J. Sustain. Agric. 2010, 2, 20–25. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowsk, A. Allelochemicals as Bioherbicides—Present and Perspectives. Herbic. Curr. Res. Case Stud. Use 2013. [Google Scholar] [CrossRef]
- Wink, M. Evolution of Secondary Metabolites in Legumes (Fabaceae). South Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.G.; Oliveros-Bastidas, A.; Marín, D.; Chinchilla, D. Allelopathy. A Natural Strategy for Weed Control. Commun. Agric. Appl. Biol. Sci. 2004, 69, 13–23. [Google Scholar] [PubMed]
- Chatterjee, P.; Chandra, S.; Dey, P.; Bhattacharya, S. Comparative Study of Allelopathic Effects of Green Tea and Black Tea. Curr. Trends Biotechnol. Pharm. 2013, 7, 644–649. [Google Scholar]
- Komes, D.; Horžić, D.; Belščak, A.; Ganič, K.K.; Baljak, A. Determination of Caffeine Content in Tea and Maté Tea by Using Different Methods. Czech J. Food Sci. 2009, 27. [Google Scholar] [CrossRef]
- Khoa, G.T.; Hai, N.T.; Manh, N.X.M.; Thuy, N.T.B.; Nghia, P.D.; Oanh, N.T.; Huong, P.T.; Duez, P. Effects of Raw Material Types on the Chemical Composition of Trung Du Tea Variety (Camellia sinensis Var. Sinensis). J. Sci. Dev. 2013, 11, 373–379. [Google Scholar]
- Ashihara, H.; Kubota, H. Patterns of Adenine Metabolism and Caffeine Biosynthesis in Different Parts of Tea Seedlings. Physiol. Plant. 1986, 68, 275–281. [Google Scholar] [CrossRef]
- Thi, H.L.; Toshiaki, T.; Kiyotake, S.; Van Chin, D.; Kato-Noguchi, H. Allelopathy and the Allelopathic Activity of a Phenylpropanol from Cucumber Plants. Plant Growth Regul. 2008, 56, 1–5. [Google Scholar] [CrossRef]
- Stach, D.; Schmitz, O.J. Decrease in Concentration of Free Catechins in Tea over Time Determined by Micellar Electrokinetic Chromatography. J. Chromatogr. A 2001, 924, 519–522. [Google Scholar] [CrossRef]
- Takemura, T.; Sakuno, E.; Kamo, T.; Hiradate, S.; Fujii, Y. Screening of the Growth-Inhibitory Effects of 168 Plant Species against Lettuce Seedlings. Am. J. Plant Sci. 2013, 4, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Kato-Noguchi, H. Effects of Lemon Balm (Melissa Officinalis, L.) Extract on Germination and Seedling Growth of Six Plants. Acta Physiol. Plant. 2001, 23, 49–53. [Google Scholar] [CrossRef]
- Bogatek, R.; Gniazdowska, A.; Zakrzewska, W.; Oracz, K.; Gawroński, S.W. Allelopathic Effects of Sunflower Extracts on Mustard Seed Germination and Seedling Growth. Biol. Plant. 2006, 50, 156–158. [Google Scholar] [CrossRef]
- Khursheed, T.; Ansari, M.Y.K.; Shahab, D. Studies on the Effect of Caffeine on Growth and Yield Parameters in Helianthus annuus, L. Variety Modern. Biol. Med. 2009, 1, 56–60. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic Effects of Volatile Monoterpenoids Produced by Salvia Leucophylla: Inhibition of Cell Proliferation and DNA Synthesis in the Root Apical Meristem of Brassica campestris Seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Daffalla, H.M.; Yagoub, S.O.; Osman, M.G.; Gani, M.E.A.; Babiker, A.E.G. Allelopathic Effects of Some Botanical Extracts on Germination and Seedling Growth of Sorghum bicolor L. J. Agric. Technol. 2012, 8, 1459–1469. [Google Scholar]


| The Concentration of Tea Samples (mg of dried leaves/ mL of agar) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | Tea type | 0.05 | 0.1 | 0.2 | 0.5 | 1 | 5 | ||
| Percentage of radicle growth of lettuce seedling compared to control | *EC50 | ||||||||
| V1 | Fresh tea | R | 98.21 ± 13.78 | 94.64 ± 10.5 | 71.55 ± 14.78 | 41.42 ± 7.95 | 28.02 ± 8.46 | 10.15 ± 4.36 | 0.40a |
| H | 103.41± 1 2.23 | 110 ± 17.82 | 101.02 ± 15.7 | 103.67 ± 12.6 | 88.23 ± 16.81 | 52.94 ± 14.03 | 5.10a | ||
| V2 | Green tea | R | 87.66 ± 10.21 | 52.19 ± 17.56 | 35.14 ± 14.96 | 22.94 ± 8.90 | 17.05 ± 10.61 | 4.46 ± 1.55 | 0.12c |
| H | 100.11 ± 8.46 | 92.27 ± 18.55 | 76.32 ± 17.12 | 66.17 ± 25.46 | 54.26 ± 25.4 | 23.38 ± 9.42 | 1.00c | ||
| V3 | Oolong tea | R | 90.42 ± 9.84 | 76.37 ± 10.03 | 40.86 ± 8.44 | 34.92 ± 10.02 | 18.27 ± 4.83 | 5.07 ± 2.18 | 0.23c |
| H | 99.28 ± 13.53 | 93.63 ± 19.70 | 94.85 ± 13.15 | 92.20 ± 18.29 | 65.29 ± 23.43 | 18.97 ± 12.17 | 2.20bc | ||
| V4 | Oolong tea | R | 85.47 ± 11.41 | 53.09 ± 16.23 | 35.94 ± 9.22 | 31.47 ± 7.69 | 20.50 ± 5.63 | 5.27 ± 1.37 | 0.17c |
| H | 98.46 ± 14.1 | 101.81 ± 14.45 | 95.73 ± 21.78 | 87.35 ± 18.75 | 67.94 ± 15.82 | 13.67 ± 7.47 | 1.40bc | ||
| V5 | Green tea | R | 89.75 ± 7.23 | 56.36 ± 12.78 | 31.37 ± 8.25 | 20.50 ± 5.57 | 21.31 ± 8.78 | 3.65 ± 1.24 | 0.16c |
| H | 100.57 ± 12.06 | 99.54 ± 14.06 | 90.88 ± 17.05 | 63.97 ± 21.46 | 65.73 ± 23.12 | 12.79 ± 3.50 | 2.10bc | ||
| V6 | Green tea | R | 88.65 ± 8.24 | 68.46 ± 11.13 | 35.98 ± 18.02 | 22.94 ± 5.38 | 17.055 ± 3.38 | 3.45 ± 1.06 | 0.21c |
| H | 104.66 ± 10.26 | 92.72 ± 15.62 | 84.26 ± 21.44 | 84.70 ± 12.80 | 51.61 ± 13.63 | 11.91 ± 2.81 | 2.20b | ||
| V7 | Black tea | R | 90.55 ± 13.27 | 80.44 ± 14.18 | 72.28 ± 12.58 | 57.46 ± 13.17 | 59.69 ± 16.14 | 13.40 ± 3.74 | 1.90b |
| H | 106.24 ± 15.72 | 112.05 ± 20.66 | 100.58 ± 17.41 | 113.82 ± 16.24 | 107.72 ± 20.08 | 50.05 ± 14.65 | 5.00a | ||
| Samples ID | Type of Tea | EC50 (mg D.W. per mL of water) | Concentration of Caffeine (µg/mL) (Camellia sinensis) | Total Activity (no unit) |
|---|---|---|---|---|
| V1 | Fresh tea | 10.2 a | 20.7 f (±0.02) | 0.27 |
| V2 | Green tea | 1.22 b | 38.2 a (±0.06) | 0.51 |
| V3 | Oolong tea | 1.31 b | 21.4 e (±0.06) | 0.29 |
| V4 | Oolong tea | 1.98 b | 23.3 d (±0.24) | 0.31 |
| V5 | Green tea | 1.56 b | 35.5 b (±0.23) | 0.47 |
| V6 | Green tea | 2.10 b | 26.0 c (±0.15) | 0.35 |
| V7 | Black tea | 10.2 a | 26.1 c (±0.02) | 0.35 |
| Scientific Name (English Name) (family a) | EC50 (mg/mL) b | |
|---|---|---|
| Crude Extract | Pure Caffeine | |
| Phleum pratensis (Timothy) (po) | 1.24 a | 0.15 ab |
| Trifolium repens (White clover) (fa) | 1.12 a | 0.10 a |
| Trifolium pretense (Red clover) (fa) | 3.01 ab | 0.10 a |
| Hordeum vulgare (Barley) (po) | 4.34 bc | 0.15 ab |
| Lotus corniculatus (Birdsfoot trefoil) (fa) | 5.06 c | 0.10 a |
| Lolium perenne (Perennial ryegrass) (po) | 5.62 c | 2.50 b |
| Lolium multiflorum (Italian ryegrass) (po) | 5.17 c | 2.50 b |
| Dactylis glomerata (Orchard grass) (po) | 5.91 c | 2.50 b |
| Avena sativa (Oat) (po) | 6.02 c | 2.50 b |
| Vicia villosa (Hairy vetch) (fa) | 8.32 d | >2.50 c |
| Daucus carota (Carrot) (ap) | 8.64 de | >2.50 c |
| Oryza sativa, cv. Jhona (Rice) (po) | 8.82 de | >2.50 c |
| Oryza sativa, cv. shinshu (Rice) (po) | 10.4 e | >2.50 c |
| Soil Samples | Concentration of Caffeine in Soil (μg/g) |
|---|---|
| 1 | 0.137 (±0.004) |
| 2 | 0.142 (±0.002) |
| 3 | 0.145 (±0.005) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
PHAM, V.T.T.; ISMAIL, T.; MISHYNA, M.; APPIAH, K.S.; OIKAWA, Y.; FUJII, Y. Caffeine: The Allelochemical Responsible for the Plant Growth Inhibitory Activity of Vietnamese Tea (Camellia sinensis L. Kuntze). Agronomy 2019, 9, 396. https://doi.org/10.3390/agronomy9070396
PHAM VTT, ISMAIL T, MISHYNA M, APPIAH KS, OIKAWA Y, FUJII Y. Caffeine: The Allelochemical Responsible for the Plant Growth Inhibitory Activity of Vietnamese Tea (Camellia sinensis L. Kuntze). Agronomy. 2019; 9(7):396. https://doi.org/10.3390/agronomy9070396
Chicago/Turabian StylePHAM, Van Thi Thanh, Tamer ISMAIL, Maryia MISHYNA, Kwame Sarpong APPIAH, Yosei OIKAWA, and Yoshiharu FUJII. 2019. "Caffeine: The Allelochemical Responsible for the Plant Growth Inhibitory Activity of Vietnamese Tea (Camellia sinensis L. Kuntze)" Agronomy 9, no. 7: 396. https://doi.org/10.3390/agronomy9070396
APA StylePHAM, V. T. T., ISMAIL, T., MISHYNA, M., APPIAH, K. S., OIKAWA, Y., & FUJII, Y. (2019). Caffeine: The Allelochemical Responsible for the Plant Growth Inhibitory Activity of Vietnamese Tea (Camellia sinensis L. Kuntze). Agronomy, 9(7), 396. https://doi.org/10.3390/agronomy9070396






