Abstract
Complaints about the lack of control of Rapistrum rugosum with tribenuron-methyl and iodosulfuron-methyl-sodium in winter cereals in Northeastern Spain motivated this study. During 2015–2018, greenhouse trials were conducted to test the responses of two possibly resistant (R1 and R2) and two susceptible populations to both active ingredients to determine the response of these populations to alternative herbicides. In the first trial that was repeated twice, populations were treated with both active ingredients (three rates, six replicates), and the lack of control confirmed resistance both times. The second trial was conducted on the self-pollinated progeny of the initial populations (13 rates, 6 replicates) to confirm the heritable character of resistance and to determine the resistance factors related to survival and biomass. Resistance factors based on biomass were 188 and 253 for tribenuron-methyl and 42 and 26 for iodosulfuron-methyl-sodium for R1 and R2, respectively, confirming the strong resistance of the progeny. In the third trial, nine active ingredients (a.i.) registered for broadleaved weed control in winter cereals were tested on the four populations (two rates, four replicates). All the alternative herbicides, except florasulam, results in important phytotoxicity to all tested populations, with 100% efficacy for several a.i. This work is the first report of R. rugosum that is resistant to iodosulfuron-methyl-sodium and the first report in Europe of R. rugosum that is resistant to tribenuron-methyl.
1. Introduction
Chemical weed control is, at present, the most widespread weed control method in the developed countries, especially for main crops such as cereals and fruit trees. This control method has revolutionized agriculture due to its high efficacy, low cost, and fast weed control. Yields have increased substantially, and, in comparison with tillage, herbicide use reduces erosion, fuel use, greenhouse gas emissions, and nutrient run-off, and conserves water [1]. In 1982, an exhaustive review was published [2] describing how integrated weed management could solve the emerging problems of relying too much on chemical weed control.
After more than 60 years of herbicide usage, weed resistance to herbicides stands is one of the main challenges associated with their use [3]. This problem may become even more serious in the near future, mainly because farmers have increasingly fewer possibilities for rotating herbicides because steadily fewer active ingredients (a.i.) are available. The authorization of new active ingredients for use in agriculture has not grown as fast as the number of a.i. that are no longer sold because they do not satisfy environmental requirements or because the companies are not interested in applying for registration. Generally, newly authorized herbicides do not possess new modes of action [4] because no herbicides with new sites of action have been developed in the last 30 years [5,6].
Herbicide resistance is the naturally occurring and inheritable ability of some weed biotypes within a given weed population to survive a herbicide treatment that should, under normal use conditions, effectively control that weed population [7]. In the case of suspected resistance, it is necessary to confirm the lack of activity of an active ingredient able to control individuals of the same species and that this ability is inherited by the progeny [8]. Generally, two resistance mechanisms are used, target-site and non-target-site mechanisms, based on the reduced or lost ability of the herbicide to bind to its target protein. In the first case, a mutation at the site of action of the herbicide occurs, impeding its activity. Non-target-site mechanisms can arise for several reasons: changes in the plant that reduce or impede herbicide retention, alterations in absorption or transport, and increased metabolism degrading the herbicide previous to its action [9,10].
At present, 495 confirmed cases of herbicide resistance worldwide affect 255 weed species [11]. If this problem continues and increases, weed control using herbicides could be seriously compromised; integrated weed management provides the only possibility to achieve acceptable efficacy [12]. The acetolactate synthase (ALS) inhibitors mode of action group has the highest number of cases of herbicide resistance (161 cases), far ahead of the second group, the photosystem II inhibitors, with 74 cases [11]. The ALS inhibitor tribenuron-methyl has been broadly applied in winter cereals in Spain since its authorization in 1986 for broadleaved weed control. ALS-inhibiting herbicides have been widely used in many crops, including rainfed winter cereals, given their many advantages (broad spectrum, low mammalian toxicity, flexible timing of application, etc.) and are still considered an important alternative to auxin-based herbicides for weed control. However, they are prone to the development of resistant weed biotypes. In the nearby region of Catalonia, populations of Papaver rhoeas L.that are resistant to tribenuron-methyl have been described since 1998 [13], and in a survey conducted in the 1990s testing 134 populations in Northeastern Spain, 72% of the analyzed poppy populations were resistant to this active ingredient [14], describing the generalized problem. Other weeds that are resistant to herbicides with this mode of action that have been found in winter cereals include Sinapis alba L. in Spain and Stellaria media (L.) Vill. and Centaurea cyanus L. in other European countries [11].
Rapistrum rugosum (L.) All. is a weed in Spanish rainfed cereal fields and in many other countries with a Mediterranean climate. In this species, a single seed is located inside each silique (fruit). In nature, seeds are not released from the fruits at maturity, but fruits containing the seeds fall off the plants. Once in the soil, the fruits rot slowly, thus conferring seeds a physical dormancy status until they are able to emerge from the hard fruit. In spring 2015, the first complaints of a lack of control were received at the Plant Protection Service (CSCV de Aragón) after using herbicides from the sulfonylurea family, i.e., tribenuron-methyl and iodosulfuron-methyl-sodium, in barley and wheat fields in the Aragon region (Northeastern Spain). In the sampled fields, tribenuron-methyl had been applied for at least five years, and iodosulfuron-methyl-sodium was applied in 2014 and 2015, always at one application per year (JA Cambra, pers. comm.). At present, only two cases of herbicide resistance of this weed have been confirmed worldwide: one in Australia, resistant to chlorsulfuron (1996), and one in Iran, resistant to bispyribac-Na, florasulam, flucarbazone-Na, and tribenuron-methyl [11]. In this second case, target-site and non-target-site mechanisms are involved [15]. Concerning resistance to ALS-inhibiting herbicides, the most common cause of resistance is single point mutations that alter the structure of the ALS, making it less sensitive to certain herbicides. In this context, the mutation Pro 197/Ser has been described as a frequent mutation in resistant weeds of the Brassicaceae family, but it can occur at other sites of the ALS gene [15].
Herbicide resistance to ALS inhibitors in other species of the Brassicaceae family has been reported: Raphanum raphanistrum [16], tribenuron-methyl in Sinapis alba [17,18], Descurainia sophia [19,20], and ethametsulfuron-methyl in Sinapis arvensis [21]. The possibility that the complaints in the Spanish fields correspond to herbicide resistance is high because (1) the Brassicaceae family is ranked third in the frequency of resistance appearance (22 cases), (2) the ALS-inhibiting herbicides are those with the most cases of resistance (160: 92 to tribenuron-methyl and 98 to iodosulfuron and with many cases of cross-resistance between both active ingredients), and (3) the winter cereals are the group of crops with the most resistance cases worldwide (137 cases in wheat and barley) [11].
The objectives of this work were as follows: (1) to confirm the resistance of two R. rugosum populations and their progeny to the active ingredients tribenuron-methyl and iodosulfuron-methyl-sodium following Heap’s criteria [8], (2) to determine the response of these populations to the use of alternative labelled herbicides in winter cereals, and (3) to determine whether the mutation of Proline 197 may be involved in the resistance mechanism of the resistant populations.
2. Materials and Methods
The response to herbicides was tested on two resistant and two susceptible R. rugosum populations in three experiments conducted between 2015 and 2019.
2.1. Plant Material
Seed pods of the two suspected resistant populations were collected in June 2015 from plants that survived the sequential application of both herbicides. Populations are referred to as R1 (42°10′52″ N, 1°2′11″ W, Luna, Zaragoza, Spain) and R2 (41°54′22″ N, 0°29′9″ W, Senés de Alcubierre, Huesca, Spain). Populations serving as susceptible comparisons growing in non-treated areas were collected in Valareña (Zaragoza, Spain, 42°5′6″ N, 1°18′43″ W) and in Zaragoza (41°39′7″ N, 0°51′52″ W) and named S1 and S2, respectively. Seed pods were collected at maturity a few days prior to cereal harvest. Siliques were kept at room temperature in trays in the laboratory to ensure drying and then kept in glass pots containing silica gel at 4 °C until use. The dose-response experiment (trial 2) was conducted with the progeny of each population. To obtain descendants, additional surplus plants of trial 1 of each population were confined separately in different greenhouses to impede crossings between individuals of the different populations. Pollination was forced inside each single plant using brushes. The collected fruits were treated the same as those collected from the fields. To overcome physical dormancy, seeds were extracted from the siliques using a scalpel immediately prior to conducting the trials. Preliminary tests demonstrated high and homogenous emergence after this procedure. One seed was placed per pot (measuring 7 × 7 × 8 cm) containing horticultural substrate (90% organic matter, 10% ash, 0.2% N, and 0.1% P). The trials were installed in a greenhouse belonging to the CITA facilities at Montañana (Zaragoza, Spain) with minimum temperatures above 5 °C and maximum temperatures under 30 °C. No artificial light was used so that the photoperiod was the natural day length. Watering was conducted manually when needed.
At the end of the trials, which were concluded between 28 and 30 days after treatment (DAT), survival and above-ground dry plant biomass were recorded. Survival was assessed in each pot as 100% corresponding to alive and 0% corresponding to dead plants, and dry above-ground biomass was determined for each plant after drying the plants at 60 °C until constant weight (PSelecta, Digitheat, Barcelona, Spain). The percentage biomass reduction of each plant was calculated compared to the untreated mean as follows: % Biomass = bi × 100/mean biomass of untreated plants, where bi is the biomass of each treated plant.
The details of each trial are described below.
2.2. Verification of Herbicide Resistance (Trial 1) and Quantification of the Resistance (Trial 2)
Trial 1 was repeated twice, in December 2015 and February 2016, and both trials were identical in treatment and structure. Both active ingredients were tested at 0, x/2, x, and 2x, with x being the highest recommended rate for each product. These rates were 10 g a.i. ha−1 and 18.75 g a.i. ha−1 for iodosulfuron-methyl-sodium and tribenuron-methyl, respectively. Commercial formulations were Hussar® (Bayer AG, Leverkusen, Germany) and Granstar 50 SX® (FMC International Switzerland Sàrl, Genève, Switzerland). The adjuvants used were alkylethersulfate-sodium (Biopower®, Bayer CropScience, Frankfurt am Main, Germany) at 405 g a.i. ha−1 for iodosulfuron-methyl-sodium and isodecyl alcohol ethoxylate (Trend® Du Pont Ibérica, Silla, Valencia, Spain) at 270 g a.i. ha−1 for tribenuron-methyl.
Application was performed at the 4–6-leaf stage with a fixed constant-pressure sprayer at 2 bar using Teejet® XR 110 nozzles (Wheaton, IL, USA) at a 300 L ha−1 spray volume. The lowest dosages were applied first with increasing concentrations thereafter. The four populations, S1, S2, R1, and R2, were tested, and six replicates per treatment were distributed in the greenhouse randomly after treatment.
Trial 2 was conducted in May–June 2017 and again in April–May 2019 using the progeny of the initial seeds obtained as explained previously with two aims: (1) to verify that the resistance is a heritable trait and (2) to calculate LD50 (dose causing death of 50% of the individuals) and WG50 (dose reducing dry weight 50% compared to the untreated control) values as well as the resistance factor of the resistant populations toward both active ingredients. In the first trial, 7 herbicide rates were tested and were insufficient to obtain the desired mortality of the resistant populations (at dose 32x), and mortality of the S1 and S2 populations occurred at the lowest tested herbicide rate (dose x/2). Therefore, in the second trial, 13 herbicide rates were tested: 0, x/64, x/32, x/16, x/8, x/4 x/2, x, 2x, 4x, 8x, 16x, 32x, and 64x, with x being the highest recommended rate for each product, i.e., 18.75 and 10 g a.i. for tribenuron-methyl and iodosulfuron-methyl, respectively. Only data from the second trial are shown. Populations S1, S2, R1, and R2 were used, and treatments were applied as described above. The highest recommended rates (x) were the same as in trial 1, and the same adjuvants were used.
2.3. Alternative Herbicides to Tribenuron-Methyl and to Iodosulfuron-Methyl (Trial 3)
This trial was conducted in February–March 2018. A total of 9 different active ingredients were tested in this trial on the initial seeds of the same four populations, applying, in this case, rates x and 2x, with x being the highest recommended rate for each product (Table 1). All the selected herbicides are registered for use in winter cereals in Europe and are thus candidates as alternative herbicides for R. rugosum control. The 4 populations, 19 treatments (including one untreated control), and 4 replicates per treatment were randomly distributed in the same greenhouse.

Table 1.
Active ingredients tested in the trial searching for alternative herbicides for Rapistrum rugosum control.
2.4. Proline 197 for Resistance Mechanism Identification; Process of ALS Gene Amplification and Sequencing
Rapistrum rugosum seeds from S (susceptible) and R (resistant) populations were germinated and grown in suitable pots in a greenhouse. DNA was extracted from young leaves, taking two disks of 1.5 cm in diameter for each sample. This material was ground and homogenized with a plastic pestle, and DNA extraction was subsequently performed by using an EZNA Plant DNA kit (Omega Bio-tek, Norcross, GA, USA) following the manufacturer’s protocol. Finally, the DNA was quantified spectrophotometrically. At the time these studies were started, no R. rugosum ALS sequence was available in any international sequence database. For this reason, two strategies were considered. First, primers already published for Sinapis arvensis [23] were used for PCR amplification (named RaprugALSF1deg and RaprugALSR1deg), and simultaneously, a sequence alignment was launched by including DNA and amino acid information of other related Brassicaceae species: Capsella bursa-pastoris (L.) Medik. (GenBank: HQ880661.1), Raphanus raphanistrum (AJ344984.1), and Sinapis arvensis (FJ655877.1). Conserved homologous regions near the Proline 197 residue were targeted for designing another pair of primers (named RaprugALSF1 and RaprugALSR1) that could be more specific for the plant species analyzed (primer sequences listed in Table 2). With the primers designed for S. arvensis, the amplification did not work properly, and the bands obtained were extremely faint. The PCR was improved using primers designed by aligning different Brassicaceae ALS sequences and obtaining a predicted 590-bp DNA fragment, which was sequenced and analyzed. Each PCR was conducted in a final volume of 25 µL with 75 ng of purified DNA as a template. Each reaction included 0.75 µL of 10 µM forward and reverse oligonucleotides, 10 µL of 2.5× Mastermix (5 Prime, Gaithersburg, MD, USA) and 2.5 µL of 10× load dye (5 Prime, Gaithersburg, MD, USA). The cycling conditions were performed as follows: denaturation at 94 °C for 2 min; 32 cycles of 94 °C for 30 s, 50 °C for 45 s, and 72 °C for 1 min; and finally, an extension step of 72 °C for 5 min. Subsequently, PCR products were visualized by running 1.5% agarose gels, extracted using a QIAquick Gel Extraction Kit (Qiagen, Crawley, West Sussex, UK) and sequenced by capillary sequencing outsourced to Secugen (Madrid, Spain).

Table 2.
List of primers used to amplify a fragment of the ALS gene of R. rugosum.
2.5. Data Processing
Survival and biomass data were tested for normality and homoscedasticity, and the data that did not fulfil these criteria were transformed following arcsin before applying the ANOVA test using R version 2.15.0 (R Foundation for Statistical Computing, Vienna, Austria) [24]. The results from both replicates of trial 1 were identical from a statistical point of view; thus, the data could be pooled together and presented as a single experiment with 12 replicates per treatment. The interaction of populations × treatment was significant, so each product was analyzed separately to determine if the response of populations R and S were significantly different for each herbicide dose. Data from trial 3 were analyzed similarly to determine the effect of each treatment on the four tested populations. The Tukey mean separation test was applied in trials 1 and 3 (p < 0.05). All these analyses were performed using the Agricolae package in R (R Foundation for Statistical Computing, Vienna, Austria).
The dry biomass and survival data from trial 2 were adjusted to 10 dose-response models proposed by Ritz et al. [25]. The best adjustments were achieved using the log-logistic model with four parameters:
where c is the lower curve limit; d is the upper limit; e is the EC50 value, which is the herbicide rate that halves the value of the parameter under consideration; and b is the slope at the inflection point of the curve. Adjustments were completed with the same R program (R Foundation for Statistical Computing, Vienna, Austria). In addition, the resistance factor was calculated by dividing the e values of the R1 and R2 populations by that of S1 or S2, choosing the lowest value in each case. Following Heap [8], resistance is confirmed when the resistance factor is higher than 10 and when this response is inherited.
Sequence alignments in the molecular analysis were performed using ClustalW and BLAST (Basic Local Alignment Search Tool) from the NCBI webpage.
3. Results and Discussion
3.1. Confirmation and Quantification of the Resistance to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium
3.1.1. Response of the Initial Population: Trial 1
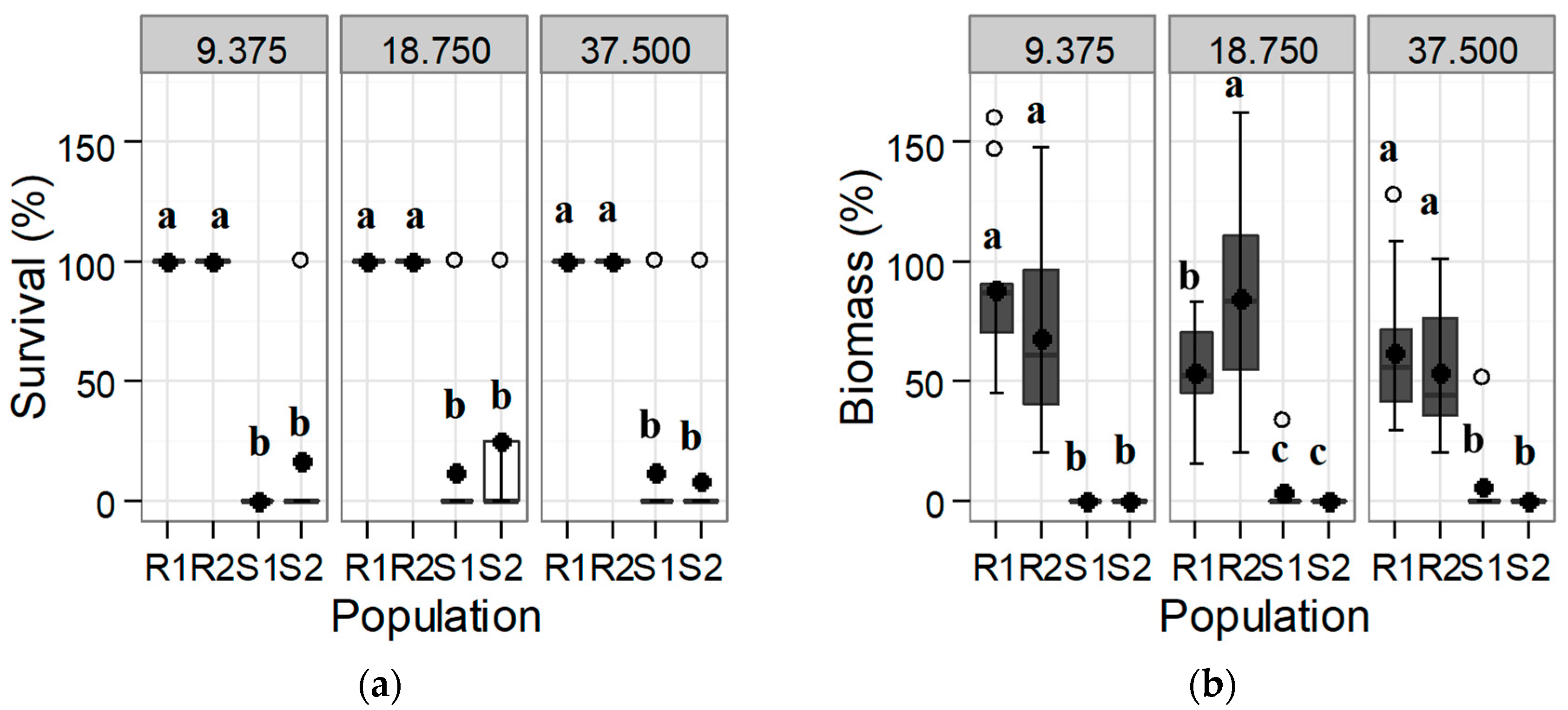
A significantly different response was found in the S and R populations both for survival and dry biomass at 30 DAT at the rate of 9.375 g a.i. ha−1 of tribenuron-methyl and upwards (Figure 1). All individuals of both R populations were capable of remaining alive at double the maximum recommended field rate (37.5 g a.i. ha−1), maintaining more than half the dry biomass of the untreated control (Figure 1). Although some individuals of the S populations were still alive at 30 DAT at rates of 9.375 g a.i. ha−1 and upward, their biomass was negligible, and plants most likely died a few days later (Figure 1).
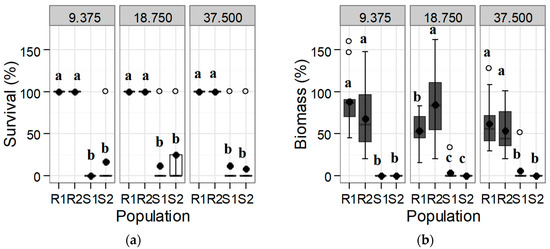
Figure 1.
Box-plot showing (a) survival and (b) biomass for each of the tested Rapistrum rugosum populations compared with those of the untreated control depending on the tribenuron-methyl herbicide rate shown in the upper part of the graphs (in g a.i. ha−1) 30 days after treatment (DAT). Bold points denote means, and white points denote outliers. Populations with different letters for each herbicide rate and for each parameter correspond to statistically significant differences following Tukey’s test (p < 0.05).
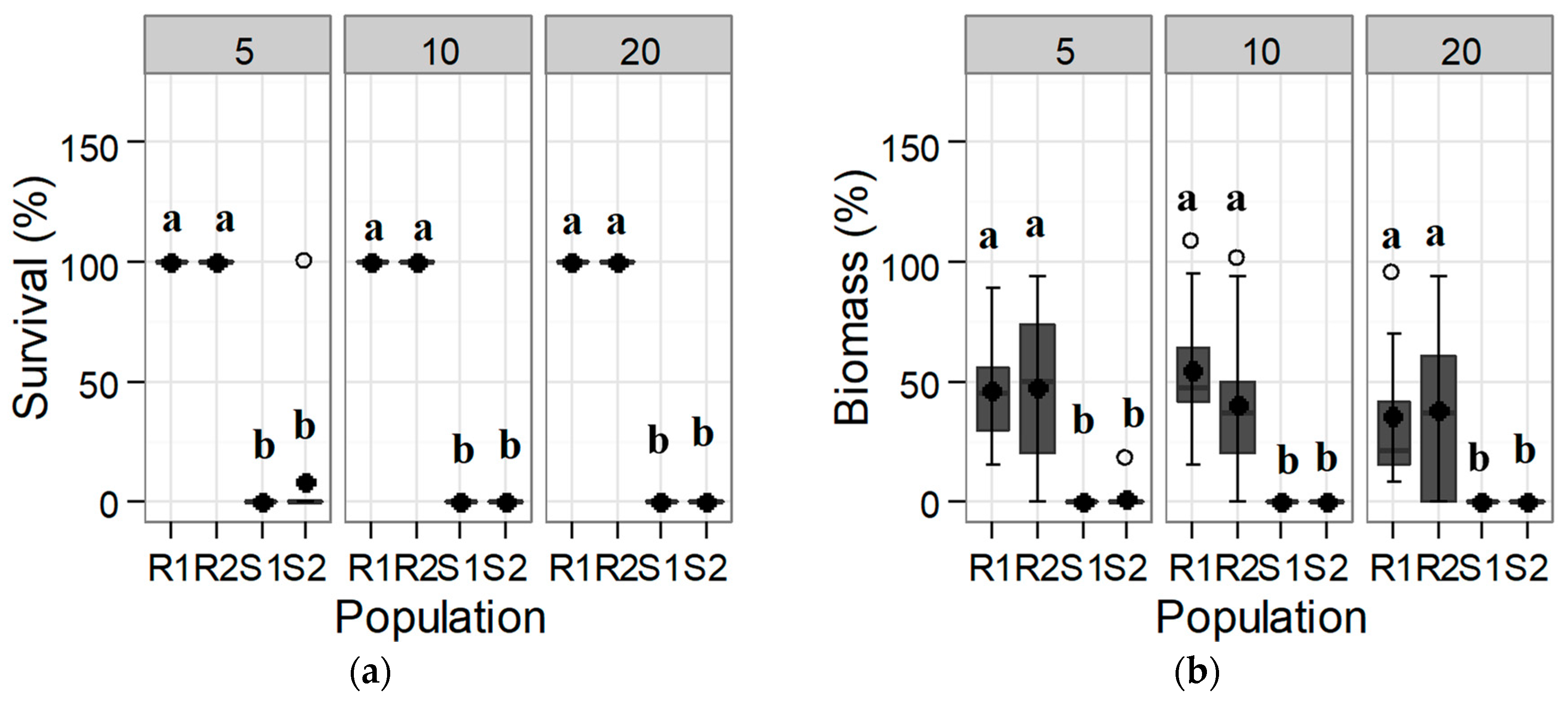
The responses of the populations to iodosulfuron-methyl-sodium were similar to those found for tribenuron-methyl, but the differences between populations were even more significant. Mortality for the S populations was virtually complete at half of the recommended field dose (5 g a.i. ha−1); however, the R populations showed a higher susceptibility to this active ingredient (Figure 2). These trials thus confirm a distinct response to tribenuron-methyl and to iodosulfuron-methyl for the tested S and R populations, meeting the first requirement for herbicide resistance following Heap [8].
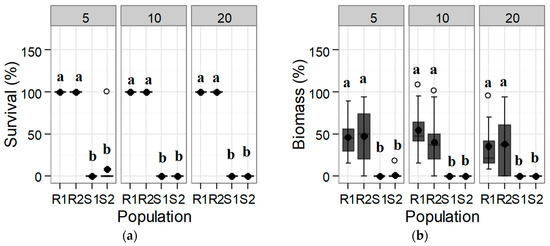
Figure 2.
Box-plot showing (a) survival and (b) biomass for each of the tested Rapistrum rugosum populations compared with those of the untreated control depending on the iodosulfuron-methyl-sodium herbicide rate shown in the upper part of the graphs (in g a.i. ha−1) 30 DAT. Bold points denote means, and white points denote outliers. Populations with different letters for each herbicide rate and for each parameter correspond to statistically significant differences following Tukey’s test (p < 0.05).
3.1.2. Dose-Response of the Progeny to Tribenuron-Methyl and Iodosulfuron-Methyl-Sodium: Trial 2
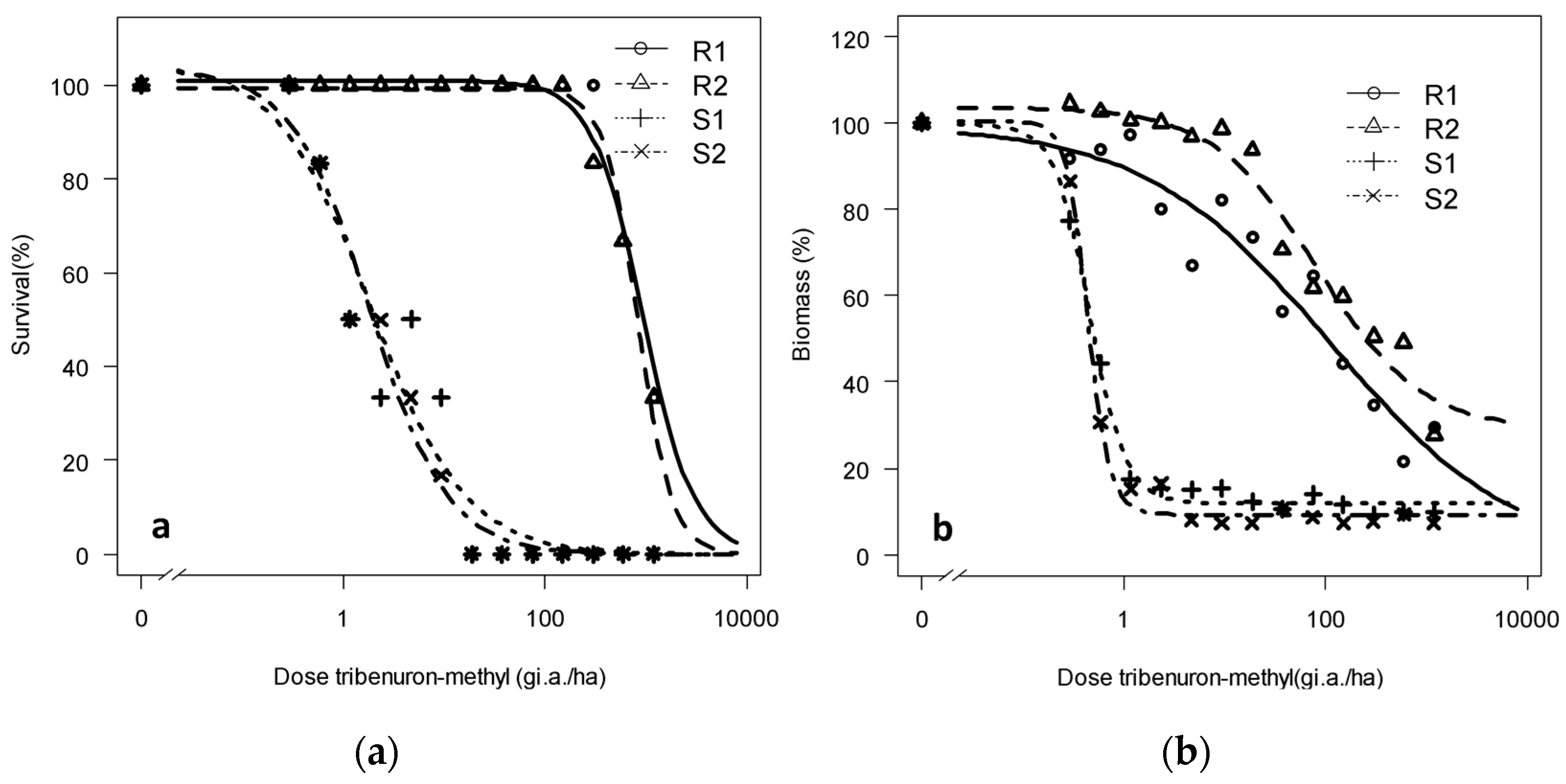
A lack of R. rugosum control was also visible in the progeny from both the survival and the biomass reduction points of view (Figure 3). Thus, R1 and R2 meet the second requirement, confirming the herbicide resistance of both populations toward tribenuron-methyl [8]. R1 and R2 maintained 20%–30% biomass at the highest tested rate (1200 g a.i. ha−1) with a resistance factor of 188 and 253 for the R1 and R populations 2, respectively, calculated based on the S1 value.
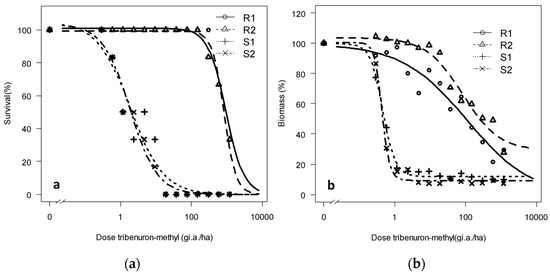
Figure 3.
(a) Survival and (b) dry biomass of Rapistrum rugosum calculated based on those of the untreated control depending on the population and on the applied tribenuron-methyl dose 28 DAT.
Resistance was even more evident when focusing on survival data. Some R individuals survived 28 DAT (Figure 3) at the highest tested dose, 64x (1200 g a.i. ha−1), whereas the first S individuals started to die at the second-lowest dose upward, x/32 (0.59 g a.i. ha−1), and all S plants died at x/2 (9.36 g a.i. ha−1). The resistance factors (RFs) calculated based on survival data were 511 and 450 for R1 and R2, respectively (Table 3). Thus, for both measured parameters of survival and biomass reduction, an RF >10, which is the requirement to confirm resistance, is surpassed by a wide margin [8].

Table 3.
Estimated log-logistic regression parameters for resistant (R) and susceptible (S) Rapistrum rugosum populations to tribenuron-methyl.
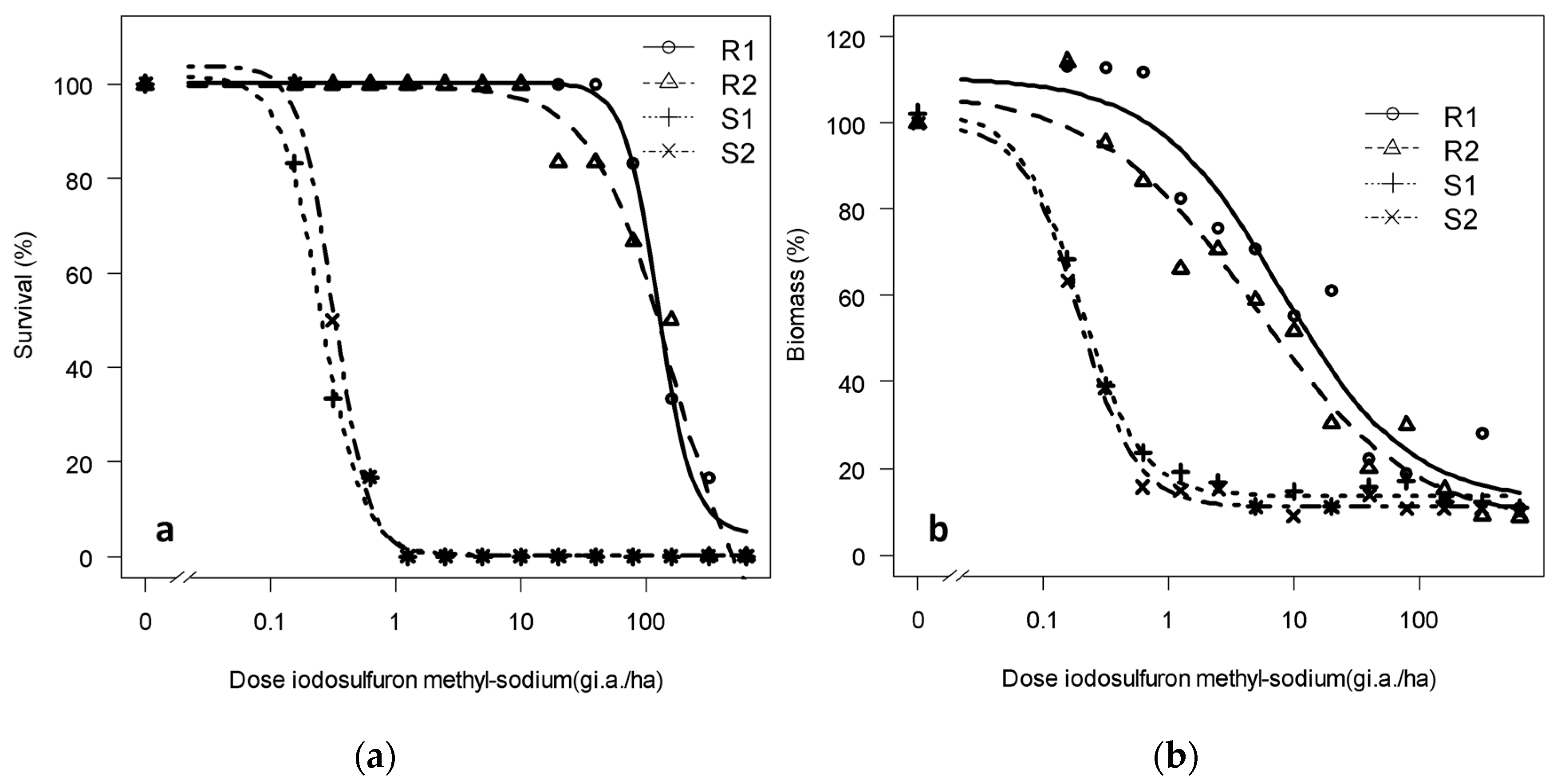
The regression graphs of the adjustment to this active ingredient also show that the results are clear, and the S and R populations have different responses to iodosulfuron-methyl-sodium in both the tested parameters, especially for survival (Figure 4).
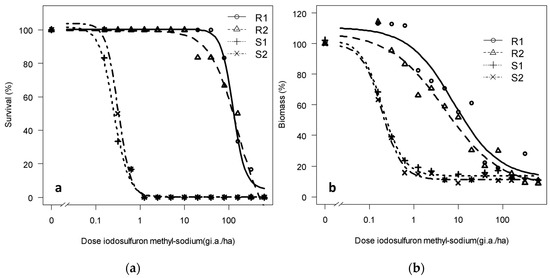
Figure 4.
(a) Survival and (b) dry biomass of Rapistrum rugosum calculated based on those of the untreated control depending on the population and on the applied iodosulfuron-methyl-sodium dose 28 DAT.
The resistance factors calculated from the biomass of populations R1 and R2 were 41.6 and 26.1, respectively, but when calculated based on survival, they increased to 633 and 487 (Table 4), respectively. Therefore, the two tested R populations are also resistant to iodosulfuron-methyl-sodium according to the third resistance criterion [8].

Table 4.
Estimated log-logistic regression parameters for Rapistrum rugosum populations resistant (R) and susceptible (S) to iodosulfuron-methyl-sodium.
According to the International Survey of Herbicide Resistant Weeds [11], 16 out of 92 cases of resistance to tribenuron-methyl and 8 out of 98 cases of iodosulfuron-methyl-sodium resistance have been reported in Brassicaceae, and five Brassicaceae species are known to be cross-resistant to both active ingredients, confirming that this family is prone to developing resistance toward ALS-inhibiting herbicides. The most similar case found in the literature is the R. rugosum population in Iran, which is resistant to the ALS-inhibiting herbicides bispyribac-Na, florasulam, flucarbazone-Na, and tribenuron-methyl, despite iodosulfuron-methyl-sodium not being tested in that case [15]. In Spain, a different Brassicaceae species, Sinapis alba, was found to be resistant to several ALS inhibitors [18]. In both cases, several mutations in the ALS enzyme (at Pro197, Ser653, etc.) are involved in the resistance to these ALS-inhibiting active ingredients of different chemical families. In the Iranian R. rugosum population, other non-target-site mechanisms based on enhanced metabolism have also been identified, with the lack of efficacy originating in plants capable of detoxifying tribenuron-methyl metabolites. Other mechanisms, such as a lack of retention, absorption, or translocation in the leaves, stems, and roots of the plant, were discarded because they were the same for the R and S populations [15]. The very high resistance factors found in the present populations suggest that the target-site mechanism might confer resistance. In several other Brassicaceae species, this mechanism has been confirmed to ALS-inhibiting herbicides in Sinapis alba [17,18], Sinapis arvensis [5,26,27,28], Raphanus raphanistrum [29], Sisymbrium orientale and Brassica tournefortii [30], and Descurainia sophia [19,20,31]. However, despite being rarer, metabolism-based resistance to ALS-inhibiting herbicides has also been found in some Brassicaceae, such as R. raphanistrum [15] and Sinapis arvensis [21].
Finally, it is important to highlight the extent of the resistance of the tested populations, as the only other published case, in Iran [15], had much lower resistance factors. Whereas the resistance factors in the Spanish populations R1 and R2 to tribenuron-methyl based on biomass were 250 and 180, respectively, the Iranian populations showed a resistance factor of only 2.5 to 6.6. The confirmed resistance of a R. rugosum population to iodosulfuron-methyl-sodium is the first instance worldwide.
3.2. Alternative Herbicides to Tribenuron-Methyl and to Iodosulfuron-Methyl: Trial 3
3.2.1. ALS Inhibitors
Resistance to tribenuron-methyl and to iodosulfuron-methyl was again confirmed in this trial (Table 5). Florasulam, which belongs to a different chemical family within the ALS inhibitors (triazopyrimidines vs. sulfonylureas), did not control the resistant populations, which is not surprising because cross-resistance to different chemical families is well described in other weed species [18,28]. The efficacy on the R populations was 50%–75% at the double field dose (15 g a.i. ha−1) (Table 4). However, the phytotoxic effect was high, as shown with the biomass data, which were statistically the same for the R and S populations. Thus, the R plants would likely die at this dose when competing in the field with cereal plants, but this active ingredient should not be recommended because this lack of complete susceptibility indicates a first step in developing more serious cross-resistance. In contrast, the R. rugosum populations from Iran that were resistant to bispyribac-Na, florasulam, flucarbazone-Na and tribenuron-methyl were susceptible to imazamox, with all of these active ingredients being ALS inhibitors belonging to different chemical families [15].

Table 5.
Survival and dry biomass calculated based on those of the untreated control depending on the Rapistrum rugosum population and on the applied herbicide doses (active ingredient in g ha–1) for available herbicides in winter cereals. Rows with different letters for each dose and for each parameter correspond to statistically significant differences following Tukey’s test (p < 0.05). Survival and biomass were determined 30 days after herbicide application.
3.2.2. Auxinic Herbicides
The three auxinic herbicides tested, 2,4-D, MCPA, and fluroxypyr, controlled all four populations at 100% starting with the lowest tested dose (Table 4). No green biomass was available at 30 DAT for any of the tested populations.
These results show that some herbicides are still effective in controlling this species despite being commercialized for almost seven decades and confirm that the auxinic mode of action is less prone to generating resistance than the sulfonylureas commercialized in Spain since the mid-1980s. Resistance to auxinic herbicides is still not common; at present, there are only 39 recorded cases [11]. However, some Brassicaceae species are resistant to auxinic herbicides, including R. rugosum and four weed species resistant to fluroxypyr worldwide [11], so none of these herbicides should be used in large quantities in the coming years.
3.2.3. Other Post-Emergence Herbicides
All three tested herbicides (bentazone, bromoxynil, and carfentrazone) also had high efficacy at both tested rates. Even though a few individuals of different populations survived, their biomass was low, and the plants were not capable of finishing the biological cycle or producing seeds (Table 4). In Spain, Sinapis alba populations resistant to sulfonylureas were well controlled with bromoxynil and MCPA [17]. However, the existence of three weed species resistant to bentazone and/or to bromoxynil and three other species resistant to carfentrazone worldwide [11] indicates that the risk of developing multiple resistances to these herbicides also exists.
3.3. Sequencing of Rapistrum Rugosum ALS Point Mutations Associated with Herbicide Resistance
Sequence analysis revealed that none of the samples studied presented the Pro197/Ser mutation. Based on these data, we conclude that the resistance mechanism may be due to other point mutations at other genome sites, as has been already reported in other species [18]. The metabolic cause is, in our opinion, less probable due to the high resistance factors found. Thus, to completely characterize the resistance mechanism of R. rugosum, a deeper molecular analysis might be performed. This would probably also explain why the resistance factor of R. rugosum to iodosulfuron-methyl-sodium was higher than that to tribenuron-methyl.
4. Conclusions
This work confirms the first case of resistance of R. rugosum to tribenuron-methyl in Europe and to iodosulfuron-methyl-sodium worldwide. Fortunately, several alternative herbicides are still available to control the R. rugosum populations that have confirmed herbicide resistance to tribenuron-methyl and to iodosulfuron-methyl-sodium. Among the tested active ingredients, sex alternative herbicides caused plant death in all four tested populations at a high dose (2x), and four caused plant death at the field rate. The tested populations exhibit incipient cross-resistance to the ALS-inhibiting herbicide florasulam but do not yet exhibit multiple resistance. As resistance toward all the still-effective herbicides has been confirmed for other weed species, it is thus recommended to rotate these active ingredients and to combine them with non-chemical weed control methods to delay the appearance of multiple resistance.
Author Contributions
G.P. and A.C. designed the experiments; G.P., A.I.M. and J.A. carried out the trials with plants and L.V. the molecular analysis; G.P., A.C. and L.V. analysed the data and wrote the manuscript.
Funding
This research was partially funded by the Research Group of Comunidad Autónoma de Aragón (Group A11-17R, PROVESOS).
Acknowledgments
We thank J.A. Cambra for facilitating seed collection, to J. Pueyo and E. Armero for their help in the greenhouse trials, and F. Arrieta and J.A. Alins for completing the herbicide treatments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.H.; Buchanan, G.A. Crop manipulation in integrated weed management systems. Weed Sci. 1982, 30, 17–24. [Google Scholar] [CrossRef]
- Duke, S.O.; Heap, I. Evolution of weed resistance to herbicides: What have we learned after 70 years? In Biology, Physiology and Molecular Biology of Weeds; Jugulam, M., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 63–86. [Google Scholar]
- Ruegg, W.T.; Quadranti, M.; Zoschke, A. Herbicide research and development: Challenges and opportunities. Weed Res. 2007, 47, 271–275. [Google Scholar] [CrossRef]
- Heap, I.M. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- HRAC. Guideline to the Management of Herbicide Resistance. Available online: https://hracglobal.com/files/Management-of-Herbicide-Resistance.pdf (accessed on 24 January 2019).
- Heap, I. Criteria for Confirmation of Herbicide-Resistant Weeds—With Specific Emphasis on Confirming Low Level Resistance. Available online: http://www.weedscience.org/Documents/ResistanceCriterion.pdf (accessed on 15 December 2018).
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Bio. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Menne, H.; Köcher, H. HRAC classification of herbicides and resistance development. In Modern Crop Protection Compounds; Krämer, W., Schirmer, U., Jeschke, P., Witschel, M., Eds.; Wiley-VCH Publishing: Weingeim, Germany, 2007; pp. 5–26. [Google Scholar]
- Heap, I. International Survey of Herbicide Resistant Weeds. Annual Report Internet. Available online: http://www.weedscience.com (accessed on 15 December 2018).
- Drobny, H.G. Weed control in distress—Can all weeds still be controlled with herbicides in future? Julius Kühn Archiv 2016, 452, 19–23. [Google Scholar] [CrossRef]
- Claude, J.P.; Gabard, J.; De Prado, R.; Taberner, A. AnALS-resistantpopulation of Papaver rhoeas in Spain. In Proceedings of the 6th Mediterranean Symposium EWRS (EWRS 1998), Montpellier, France, 13–15 May 1998; pp. 181–187. [Google Scholar]
- Cirujeda, A. Integrated Management of Herbicide Resistant Papaver rhoeas L. Populations. Ph.D. Thesis, Universitat de Lleida, Lleida, Spain, 2001; p. 274. Available online: http://www.tdx.cat/TDX-0829103-105707 (accessed on 20 December 2019).
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcántara, R.; Fernández, P.; Sadeghipour, H.R.; De Prado, R. Multiple mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, X.Q.; Hashem, A.; Walsh, M.J.; Powles, S.B. ALS gene proline (197) mutations confer ALS herbicide resistance in eight separated wild radish (Raphanus raphanistrum) populations. Weed Sci. 2003, 51, 831–838. [Google Scholar] [CrossRef]
- Rosario, J.M.; Cruz-Hipolito, H.; Smeda, R.; De Prado, R. White mustard (Sinapis alba) resistance to ALS-inhibiting herbicides and alternative herbicides for control in Spain. Eur. J. Agron. 2011, 35, 57–62. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Rosario, J.; Ioli, G.; Osuna, M.D.; Smeda, R.J.; González-Torralva, F.; De Prado, R. Resistance mechanism to tribenuron-methyl in white mustard (Sinapis alba) from Southern Spain. Weed Sci. 2013, 61, 341–347. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.Q.; Chen, S.L.; Fan, C.Q.; Li, B.H. Confirmation of flixweed (Descurainia sophia) resistance to tribenuron-methyl, using three different assay methods. Weed Sci. 2010, 58, 56–60. [Google Scholar] [CrossRef]
- Deng, W.; Liu, M.J.; Yang, Q.; Mei, Y.; Li, X.F.; Zheng, M.Q. Tribenuron-methyl resistance and mutation diversity of Pro197 in flixweed (Descurainia sophia L.) accessions from China. Pestic. Biochem. Physiol. 2015, 117, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, L.J.; Hall, L.M.; O’Donovan, J.T.; Dyer, W.; Hall, J.C. Metabolism-based resistance of a wild mustard (Sinapis arvensis L.) biotype to ethametsulfuron-methyl. J. Agric. Food Chem. 2000, 48, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- HRAC. Classification of Herbicides According to Site of Action. Available online: http://www.weedscience.org/Documents/ShowDocuments.aspx?DocumentID=1193 (accessed on 15 October 2018).
- Topuz, M.; Nemli, Y.; Fatima, T.; Mattoo, A.K. Seed dormancy is modulated in recently evolved chlorsulfuron-resistant Turkish biotypes of wild mustard (Sinapis arvensis). Front.Chem. 2015, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.R-project.org/ (accessed on 20 August 2018).
- Ritz, C.; Streibig, J.C. Bioassay Analysis using R. J. Stat. Soft. 2005, 12, 1–22. Available online: http://bioassay.dk/ (accessed on 15 December 2016). [CrossRef]
- Warwick, S.I.; Sauder, C.; Beckie, H. Resistance in Canadian biotypes of wild mustard (Sinapis arvensis) to acetolactate synthase inhibiting herbicides. Weed Sci. 2005, 53, 631–639. [Google Scholar] [CrossRef]
- Christoffers, J.M.; Nandula, V.K.; Howatt, K.A.; Wehking, T.R. Target-site resistance to acetolactate synthase inhibitors in wild mustard (Sinapis arvensis). Weed Sci. 2006, 54, 191–197. [Google Scholar] [CrossRef]
- Ntoanidou, S.; Madesis, P.; Diamantidis, G.; Eleftherohorinos, I. Trp574 substitution in the acetolactate synthase of Sinapis arvensis confers cross-resistance to tribenuron and imazamox. Pestic. Bioch. Phys. 2017, 142, 9–14. [Google Scholar] [CrossRef]
- Tan, M.K.; Medd, R.W. Characterization of the acetolactate synthase (ALS) gene of Raphanus raphanistrum L. and the molecular assay of mutations associated with herbicide resistance. Plant Sci. 2002, 163, 195–200. [Google Scholar] [CrossRef]
- Boutsalis, P.; Karotam, J.; Powles, S.B. Molecular basis of resistance to acetolactate synthase–inhibiting herbicides in Sisymbrium orientale and Brassica tournefortii. Pestic. Sci. 1999, 55, 507–516. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.X.; Wei, S.H.; Zhang, H.J.; Li, X.J.; Zhang, Y.Q.; Wang, G.Q. Acetolactate synthase gene proline (197) mutations confer tribenuron-methyl resistance in flixweed (Descurainia sophia) populations from China. Weed Sci. 2011, 59, 376–379. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).