Planting Locations with Higher Temperature Produce More Bioactive Compounds and Antioxidant Capacities of Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Field Trials
2.3. Soil Properties
2.4. Determination of Bioactive Compounds Contents and Antioxidant Capacities
2.5. q-PCR Validation of Phytic Acid and Total Phenolics Genes
2.6. Statistical Analysis
3. Results
3.1. Environmental Effect on Grain Bioactive Compounds Contents and Antioxidant Capacities
3.2. Relationship Between Bioactive Compounds Contents, Antioxidant Capacities, Soil Factors, and Meteorological Conditions
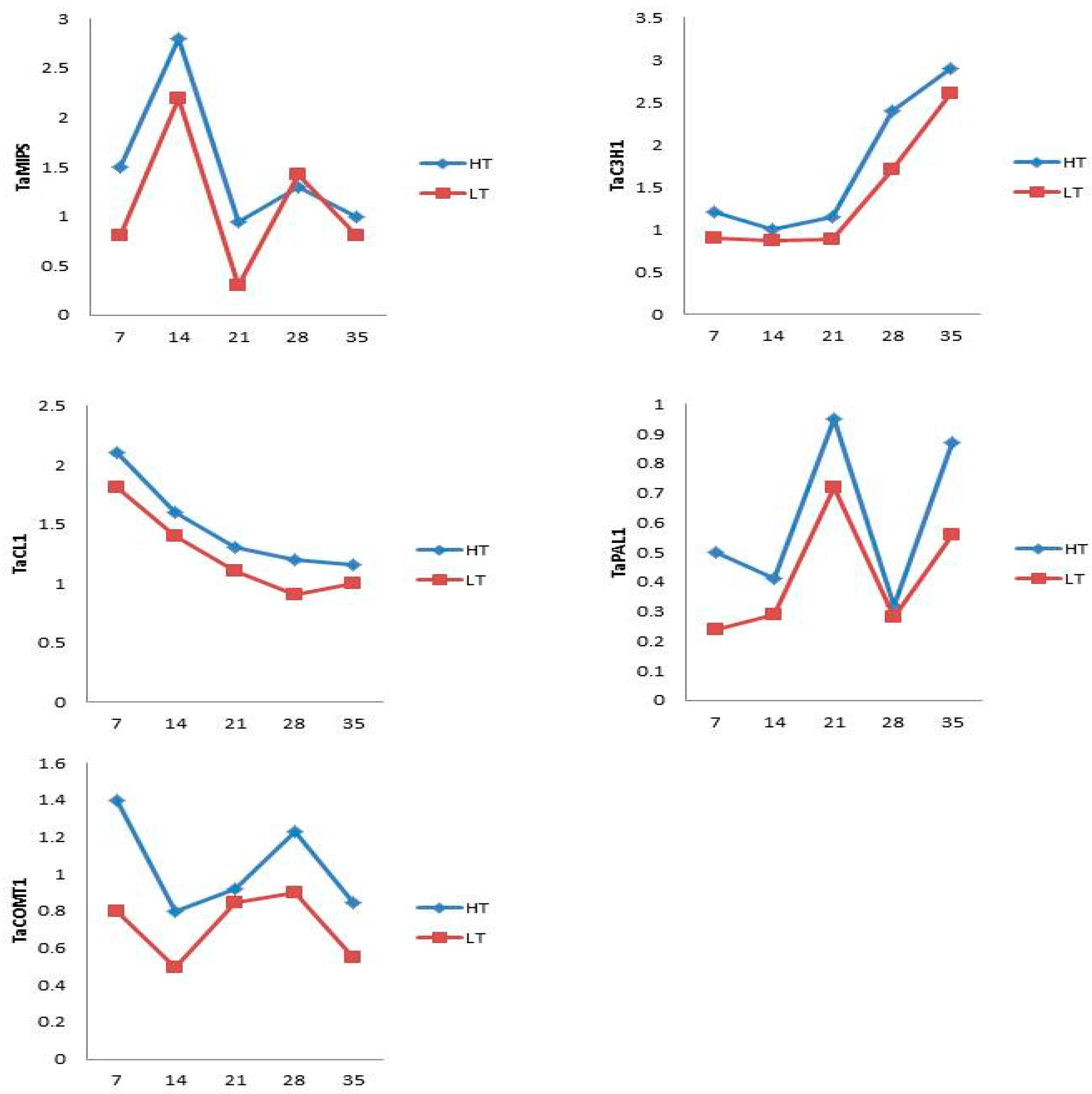
3.3. q-PCR Validation of Phytic Acid and Total Phenolics Genes
4. Discussion
4.1. Genotypic Variation Effect on Grain Bioactive Compounds Contents and Antioxidant Capacities
4.2. Temperature Effect On Grain Bioactive Compounds Contents and Antioxidant Capacities
4.3. Other Factors Effect on Grain Bioactive Compounds Contents and Antioxidant Capacities
4.4. Gene Expression and Bioactive Compounds Accumulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lampi, A.M.; Nurmi, T.; Ollilainen, V.; Piironen, V. Tocopherols and tocotrienols in wheat genotypes in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9716–9721. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, S. Bioactive components and functional properties of biologically activated cereal grains: A bibliographic review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3051–3071. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Belton, P.S.; Beta, T.; Duodu, K.G. Increasing the utilisation of sorghum, millets and pseudocereals: Developments in the science of their phenolic phytochemicals, biofortification and protein functionality. J. Cereal Sci. 2014, 59, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Shivappa, N.; Hebert, J.R.; Lee, A.H.; Xu, F.; Binns, C.W. Dietary inflammatory index and risk of oesophageal cancer in Xinjiang Uyghur autonomous region, China. Br. J. Nutr. 2018, 119, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Eaton, J.W. Suppression of colonic cancer by dietary phytic acid. Nutr. Cancer 1993, 19, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Res. Int. 2018, 107, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Guan, J.P.; Yang, X.H.; Tang, R.C.; Yao, F. A bio-resourced phytic acid/chitosan polyelectrolyte complex for the flame retardant treatment of wool fabric. J. Clean. Prod. 2019, 223, 342–349. [Google Scholar] [CrossRef]
- Özkaya, B.; Turksoy, S.; Özkaya, H.; Baumgartner, B.; Özkeser, İ.; Köksel, H. Changes in the functional constituents and phytic acid contents of firiks produced from wheats at different maturation stages. Food Chem. 2018, 246, 150–155. [Google Scholar] [CrossRef]
- Hegeman, C.E.; Good, L.L.; Grabau, E.A. Expression of D-myo-inositol-3-phosphate synthase in soybean. Implications for phytic acid biosynthesis. Plant Physiol. 2001, 125, 1941–1948. [Google Scholar] [CrossRef]
- Moheb, A.; Grondin, M.; Ibrahim, R.K.; Roy, R.; Sarhan, F. Winter wheat hull (husk) is a valuable source for tricin, a potential selective cytotoxic agent. Food Chem. 2013, 138, 931–937. [Google Scholar] [CrossRef]
- Mateo Anson, N.; Aura, A.M.; Selinheimo, E.; Mattila, I.; Poutanen, K.; van den Berg, R.; Haenen, G.R. Bioprocessing of Wheat Bran in Whole Wheat Bread Increases the Bioavailability of Phenolic Acids in Men and Exerts Antiinflammatory Effects ex Vivo–3. J. Nutr. 2010, 141, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Liu, J.G.; Zhou, K.; Yu, L. Effects of genotype and environment on the antioxidant properties of hard winter wheat bran. J. Agric. Food Chem. 2006, 54, 5313–5322. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, K. Antioxidant properties of bran extracts from Platte’wheat grown at different locations. Food Chem. 2005, 90, 311–316. [Google Scholar] [CrossRef]
- Nurmi, T.; Lampi, A.M.; Nystroöm, L.; Turunen, M.; Piironen, V. Effects of Genotype and Environment on Steryl Ferulates in Wheat and Rye in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2010, 58, 9332–9340. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Piironen, V.; Lampi, A.M.; Nystroöm, L.; Li, L.; Rakszegi, M.; Delcour, J.A. Phytochemical and Fiber Components in Oat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.A.; Lampi, A.M.; Nystroöm, L.; Piironen, V.; Li, L.; Ward, J.L.; Gebruers, K.; Courtin, C.M.; Delcour, J.A.; Boros, D.; et al. Phytochemical and Dietary Fiber Components in Barley Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9767–9776. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, A.; Paulhe-Massol, A.; Mouloungui, Z.; Merah, O. Effects of technical management on fatty acid composition of high oleic and linoleic cultivars of sunflower. Oilseeds Fats Crops Lipids (OCL) 2016, 23, 1–5. [Google Scholar] [CrossRef]
- GB 7173-1987. Method for Total Nitrogen the Determination of Soil, National standard. China. 1987.
- Turner, B.L.; Driessen, J.P.; Haygarth, P.M.; Mckelvie, I.D. Potential contribution of lysed bacterial cells to phosphorus solubilisation in two rewetted Australian pasture soils. Soil Biol. Biochem. 2003, 35, 187–189. [Google Scholar] [CrossRef]
- NY/T 1377-2007. Determination of pH in Soil, Professional standard. China. 2007.
- Guttieri, M.; Bowen, D.; Dorsch, J.A.; Raboy, V.; Souza, E. Identification and characterization of a low phytic acid wheat. Crop. Sci. 2004, 44, 418–424. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, J.; Wang, C.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. Accumulation of phenolic compounds and expression profiles of phenolic acid biosynthesis-related genes in developing grains of white, purple, and red wheat. Front. Plant Sci. 2016, 7, 528. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. Vegan, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, K.; Singh, R.P. Study of coefficient of variation, heritability and genetic advance in hybrid rice. ORYZA Int. J. Rice 2007, 44, 160–162. [Google Scholar]
- Megyeri, M.; Mikó, P.; Molnár, I.; Kovács, G. Development of synthetic amphiploids based on Triticum turgidum× T. monococcum crosses to improve the adaptability of cereals. Acta Agron. Hung. 2011, 59, 267–274. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Roche, J.; Mouloungui, Z.; Cerny, M.; Merah, O. Fatty acid and phytosterol accumulation during seed ripening in three oilseed species. Int. J. Food Sci. Technol. 2016, 51, 1820–1826. [Google Scholar] [CrossRef]
- Brankovic, G.; Dragičević, V.; Dodig, D.; Zoric, M.; Denčić, S. Genotype x environment interaction for antioxidants and phytic acid contents in bread and durum wheat as influenced by climate. Chil. J. Agric. Res. 2015, 75, 139–146. [Google Scholar] [CrossRef]
- Couceiro, M.A.; Afreen, F.; Zobayed, S.M.A.; Kozai, T. Variation in concentrations of major bioactive compounds of St. John’s wort: Effects of harvesting time, temperature and germplasm. Plant Sci. 2006, 170, 128–134. [Google Scholar] [CrossRef]
- Roche, J.; Mouloungui, Z.; Cerny, M.; Merah, O. Effect of sowing date on fatty acid and phytosterols patterns of carthamus tinctoria L. Appl. Sci. 2019, 9, 2839. [Google Scholar] [CrossRef]
- Merah, O.; Mouloungui, Z. Tetraploid wheats: Valuable source of phytosterols and phytostanols. Agronomy 2019, 9, 201. [Google Scholar] [CrossRef]
- Su, D.; Lei, B.T.; Li, Z.W.; Cao, Z.Z.; Huang, F.D.; Pan, G.; Cheng, F.M. Influence of high temperature during filling period on grain phytic acid and its relation to spikelet sterility and grain weight in non-lethal low phytic acid mutations in rice. J. Cereal Sci. 2014, 60, 331–338. [Google Scholar] [CrossRef]
- Ma, D.; Zuo, Y.; Sun, D.; Wang, C.; Guo, T. Characterization of the TaMIPS gene from winter wheat (Triticum aestivum L.) and changes in its expression pattern with phytic acid accumulation in seeds during grain filling. J. Cereal Sci. 2013, 57, 437–443. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Neugart, S.; Toutounchi, P.M.; Brestic, M. Precultivation of young seedlings under different color shades modifies the accumulation of phenolic compounds in Cichorium leaves in later growth phases. Environ. Exp. Bot. 2019, 165, 30–38. [Google Scholar] [CrossRef]
- Nunes, A.C.S.; Vianna, G.R.; Cuneo, F.; Amaya-Farfán, J.; Capdeville, G.; Rech, E.L.; Aragão, F.J.L. RNAi-mediated silencing of the myo-inositol-1-phosphate synthase gene (GmMIPS1) in transgenic soybean inhibited seed development and reduced phytate content. Planta 2006, 224, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Name | Pedigree | Name | Pedigree |
|---|---|---|---|
| Shumai969 | SHW-L1/SW8188//ChuanYu18/3/Chuanmai42 | Shumai51 | ((10-A/88-1843)/Chuanyu12)//Miannong4/N2401 |
| Chuan27 | Chuannong19/R3301 | L09-110 | SHW-L1/R59//Chuanyu18///Chuanmai42 |
| Chuanmai104 | Chuamai42/Chuannong16 | Xichang18 | 9Pin3/Zhengyumai9987 |
| 11N21 | LB0218/N711//Chuanyu18 | Yumai1 | Xing’aibai/Deguodunmai |
| Zhengmai9023 | {(Xiaoyan6//Xinong65)/[83(2)3-3/84(14)43]}F3/Shan213 | Mianmai37 | 96EW37/Mianyang90-100 |
| Chuannong16 | Chuanyu12//Jing′e57/((Alondra /783-5918)/(983-Caucasus/Fan7)) | Liangmai2 | Mianyang26//(10-A/88-1643)/Chuanyu12 |
| L11-6706 | SHW-L1/SY95-71//700-011689///MY68942 | Chuanmai55 | SW3243/SW8688 |
| Xichang19 | Xichang15(2043-5957)/7739-3-1712//(Qianhuan5/Alangda)/4676-1761 | Shumai375 | (Mianyang93-7/92R141)F2/Mianyang96-324 |
| Rongmai4 | 96-2547/133-3 | Neimai9 | Mianyang26/92R178 |
| Mianmai227 | No details | Liangmai3 | ((10-A/88-1843)/Chuanyu12)/Miannong4//(10-A/Mianyang20)/Chuanyu12 |
| Fan 9048 | No details | Fan07217 | No details |
| Chuanmai44 | 96xia440/guinong21 | Changmai26 | 3461/xichang16 |
| Shumai482 | (Mianyang93-7/92R141)F2/Mianyang96-324 | Liangmai4 | N1491/N1071 |
| Nei3416 | No details | Chuanyu20 | SW3243//35050/21530 |
| Locations | Climate Type | Landform | Longitude and Latitude | Accumulated Daylight (h) | Average Temperature (°C) | Annual Precipitation (mm) |
|---|---|---|---|---|---|---|
| Renshou | subtropical monsoon | hilly | 104°21′ E, 30°06′ N | 1196.6 | 17.4 °C | 1009.4 |
| Xichang | tropical plateau monsoon | Plain hinterland | 101°46′ E, 27°32′ N | 2423.1 | 17.0 °C | 1013.1 |
| Hanyuan | subtropical monsoon | Panxi valley | 102°16′ E, 29°05′ N | 1475.8 | 17.9 °C | 741.8 |
| Mianyang | subtropical humid monsoon | basin | 104°82′ E, 31°38′ N | 898.0 | 16.5 °C | 859.9 |
| Ya’an | subtropical monsoon | hilly | 102°99′ E, 29°98′ N | 1005.0 | 16.2 °C | 2000.0 |
| Wenjiang | subtropical humid monsoon | plain | 103°87′ E, 30°74′ N | 1104.5 | 15.9 °C | 966.1 |
| Chongzhou | subtropical humid monsoon | plain | 103°65′ E, 30°55′ N | 1164.5 | 15.9 °C | 1012.4 |
| Year | Location | PA (μg/g) | TPC (mg of GAE/g) | ABTS•+ (%) | DPPH (%) | O2– (%) | –OH (%) |
|---|---|---|---|---|---|---|---|
| 2013–2014 | WenJiang | 20.77cC | 2.59cC | 63.05cC | 57.21aA | 9.22aA | 4.18cC |
| MianYang | 27.66bB | 2.88bB | 72.56aA | 45.45dCD | 5.05cC | 5.65bAB | |
| Ya’An | 29.43aA | 2.62cC | 68.05bB | 47.71cBC | 5.85cC | 4.46cC | |
| XiChang | 30.11aA | 2.09eE | 44.35dD | 48.14cB | 3.90dD | 3.54dD | |
| RenShou | 21.27cC | 2.32dD | 64.18cC | 39.6fE | 3.83dD | 5.46bB | |
| ChongZhou | 16.98dD | 2.05eE | 45.93dD | 42.93eD | 5.50cC | 2.76eE | |
| HanYuan | 27.85bB | 3.31aA | 63.33cC | 55.2bA | 7.72bB | 6.15aA | |
| 2014–2015 | WenJiang | 30.50bcA | 2.00eD | 45.17eD | 41.68dC | 7.23aA | 3.73Ff |
| MianYang | 29.75cA | 2.29cC | 45.64eD | 47.01bcB | 4.46Cc | 4.69dD | |
| Ya’An | 29.97cAB | 3.02aA | 72.1abA | 50.77aA | 4.02cBC | 7.64aA | |
| XiChang | 31.16abB | 2.16dC | 49.57dC | 47.34bB | 4.92abAB | 4.51cC | |
| RenShou | 24.60eB | 2.73bB | 60.95cB | 46.3bcB | 5.20aA | 4.86cC | |
| ChongZhou | 22.92fC | 2.18dC | 73.04aA | 45.17cB | 4.19bcBC | 5.99bB | |
| HanYuan | 26.83dD | 2.92aA | 70.42bA | 46.11bcB | 3.59cC | 7.57aA |
| Characteristics | Y | L | G | L × Y | G × Y | L × G | G × Y × L | G (%) | E (%) | G × E (%) | PCV (%) | GCV (%) | H (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | 1329.51 ** | 321.252 ** | 6.20 ** | 182.67 ** | 1.61 * | 1.31 ** | 1.17 | 0.34 | 89.53 | 10.07 | 18.18 | 3.23 | 3.45 |
| TPC | 18.29 ** | 2.06 | 2.67 ** | 102.44 ** | 1.45 | 2.06 ** | 1.64 ** | 2.04 | 15.58 | 81.12 | 18.46 | 2.81 | 2.33 |
| ABTS•+ | 2.35 | 190.58 ** | 1.54 * | 248.28 ** | 1.34 | 1.26 * | 1.25 * | 0.34 | 43.20 | 56.18 | 63.02 | 0.81 | 0.02 |
| DPPH | 18.92 ** | 31.60 ** | 3.86 ** | 57.36 ** | 1.68 ** | 1.61 ** | 1.36 ** | 3.32 | 43.41 | 52.11 | 29.88 | 8.13 | 7.63 |
| O2– | 88.10 ** | 24.95 ** | 2.56 ** | 28.39 ** | 1.55 * | 1.26 * | 1.32 ** | 1.73 | 76.32 | 21.06 | 20.88 | 6.13 | 6.30 |
| –OH | 83.01 ** | 100.48 ** | 2.06 ** | 80.13 ** | 1.41 | 1.51 ** | 1.40 ** | 0.76 | 67.96 | 30.76 | 15.77 | 3.29 | 4.42 |
| PA | TPC | ABTS | O2– | DPPH | –OH | |
|---|---|---|---|---|---|---|
| Soil Factor | ||||||
| P | 43.65 | −181.87 | −454.18 | −7443.13 | −2557.33 | 337.46 |
| pH | −0.77 | 167.01 | −153.06 | 1110.29 | 51.48 | −118.83 |
| Ah.N | 3.88 | −275.72 | −349.72 | −5523.01 | −1708.46 | −1055.98 |
| N | 0.30 | −73.74 | 168.84 | 426.41 | −214.60 | 87.35 |
| TOC | 3.40 | −186.26 | −576.16 | −5341.95 | −1523.98 | 279.79 |
| AP | −1.89 | 54.29 | 20.48 | 3530.10 | 1428.73 | −83.55 |
| Meteorological Factor | ||||||
| Rainfall_V | −3.18 | 215.14 | 293.44 | 4891.25 | 1617.25 | −1065.75 |
| Rainfall_R | 2.73 | −174.39 | −190.19 | −5379.44 | −1394.87 | 998.47 |
| Rainfall_W | 0.33 | 35.92 | −128.60 | −608.35 | −323.65 | −103.87 |
| AT_V | −18.54 | 979.05 | 1585.92 | 31,715.09 | 10,028.24 | 82.47 |
| AT_R | −6.82 | 364.97 | 680.74 | 11,504.74 | 3449.10 | 982.09 |
| AT_W | −24.39 | 1200.00 | 2175.60 | 42,263.00 | 13,442.78 | 550.47 |
| ST_V | −19.17 | 1013.20 | 1773.40 | 31,681.54 | 10,276.53 | 393.38 |
| ST_R | −9.05 | 509.79 | 568.84 | 16,263.09 | 4881.08 | 158.90 |
| ST_W | −27.26 | 1454.48 | 1853.07 | 47,697.09 | 15,078.42 | 779.52 |
| MinT_V | −14.54 | 894.78 | 1129.46 | 22,651.30 | 7562.76 | 174.65 |
| MinT_R | −6.65 | 300.09 | 456.87 | 11,855.97 | 4008.37 | −465.74 |
| MinT_W | −21.38 | 1214.15 | 1745.38 | 36,046.72 | 11,269.89 | 124.97 |
| MaxT_V | −24.69 | 1265.95 | 2147.93 | 42,482.20 | 13,441.79 | 468.24 |
| MaxT_R | −4.81 | 264.03 | 511.33 | 8523.91 | 2338.24 | 517.03 |
| MaxT_W | −29.23 | 1525.45 | 2385.17 | 51,043.80 | 15,858.50 | 824.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Z.; Liu, Q.; Li, Z.; Chen, S.; Liu, Y.; Qi, P.; Wei, Y.; Zheng, Y. Planting Locations with Higher Temperature Produce More Bioactive Compounds and Antioxidant Capacities of Wheat. Agronomy 2019, 9, 538. https://doi.org/10.3390/agronomy9090538
Pu Z, Liu Q, Li Z, Chen S, Liu Y, Qi P, Wei Y, Zheng Y. Planting Locations with Higher Temperature Produce More Bioactive Compounds and Antioxidant Capacities of Wheat. Agronomy. 2019; 9(9):538. https://doi.org/10.3390/agronomy9090538
Chicago/Turabian StylePu, Zhien, Qianqian Liu, Zhengyang Li, Shihao Chen, Yongjian Liu, Pengfei Qi, Yuming Wei, and Youliang Zheng. 2019. "Planting Locations with Higher Temperature Produce More Bioactive Compounds and Antioxidant Capacities of Wheat" Agronomy 9, no. 9: 538. https://doi.org/10.3390/agronomy9090538
APA StylePu, Z., Liu, Q., Li, Z., Chen, S., Liu, Y., Qi, P., Wei, Y., & Zheng, Y. (2019). Planting Locations with Higher Temperature Produce More Bioactive Compounds and Antioxidant Capacities of Wheat. Agronomy, 9(9), 538. https://doi.org/10.3390/agronomy9090538





