Generation of CSF1-Independent Ramified Microglia-Like Cells from Leptomeninges In Vitro
Abstract
:1. Introduction
2. Materials and Methods
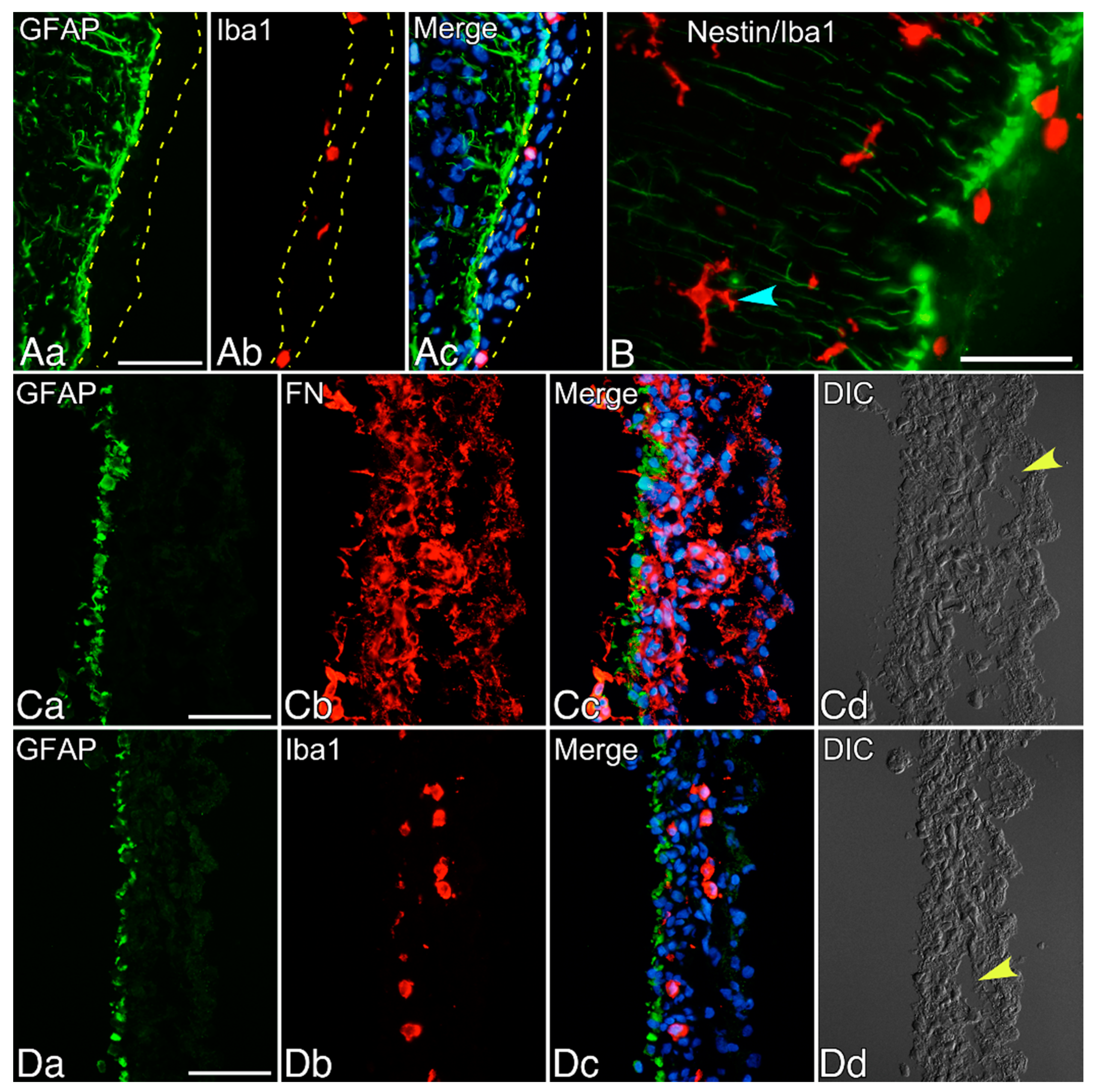
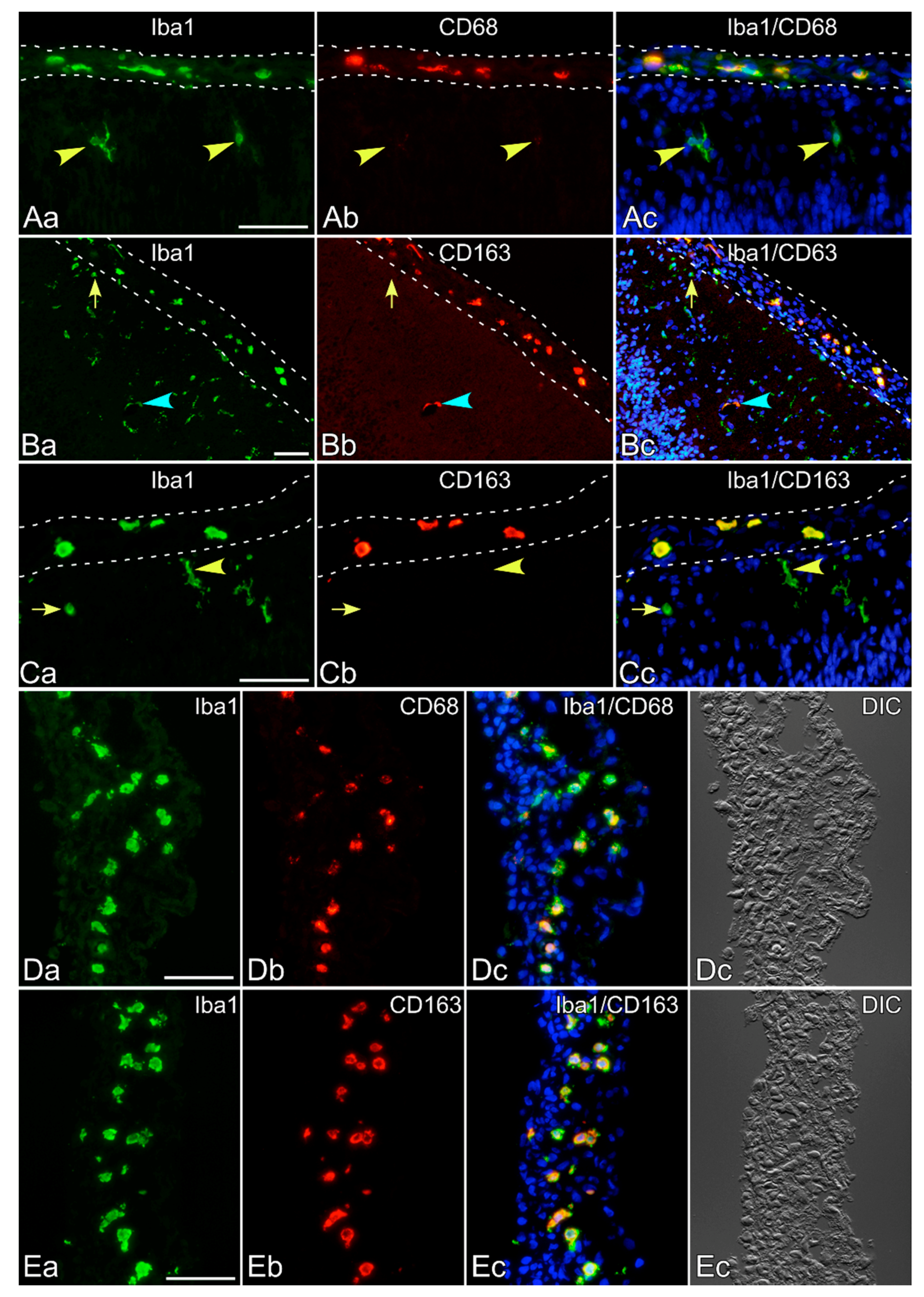
2.1. Immunohistochemical Staining of Neonatal Rat Brains
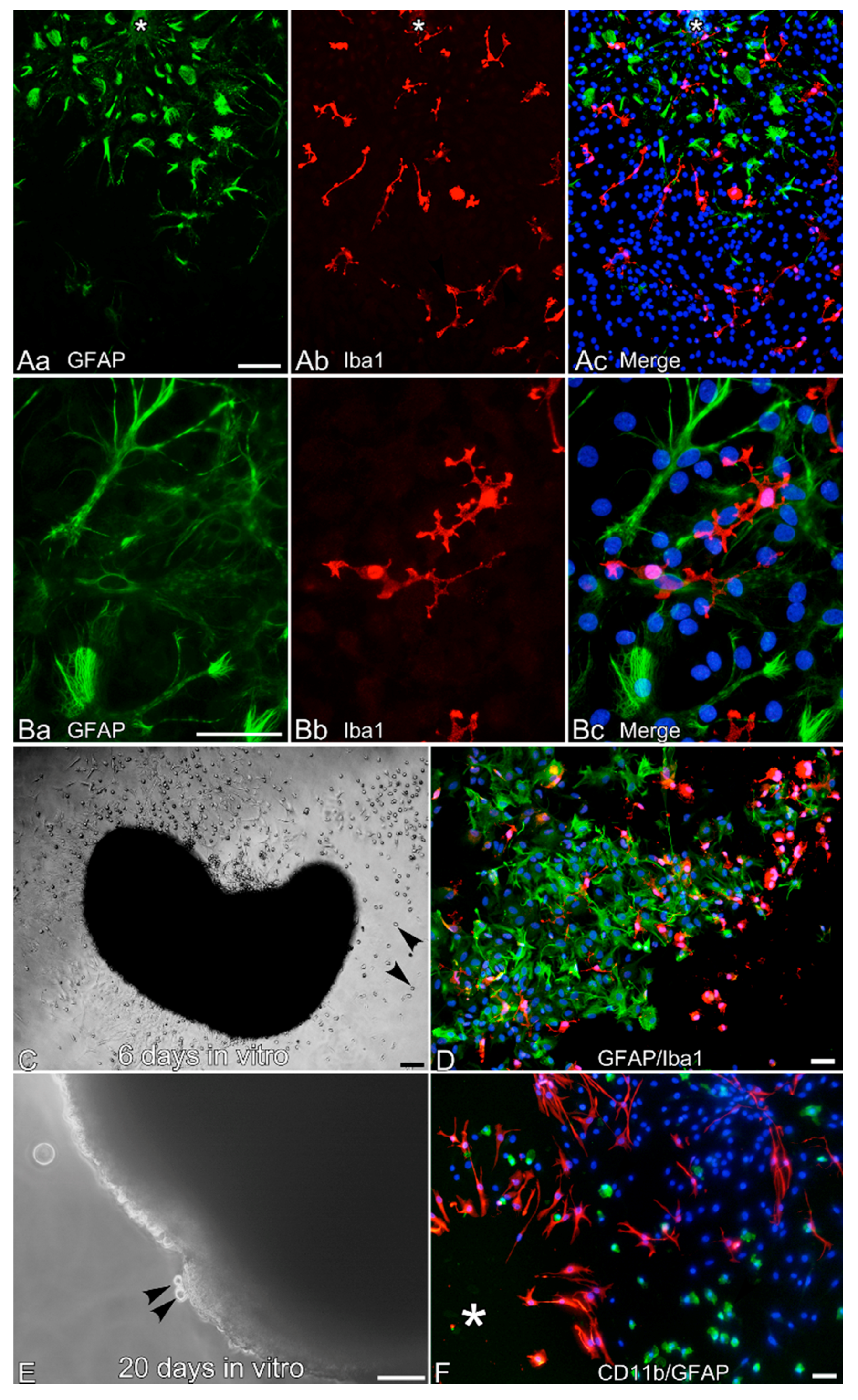
2.2. Culture of Leptomeningeal Cells
2.3. Immunocytochemistry of Microglia-Like Cells from Leptomeningeal Cultures
2.4. Preparation of Primary Microglia and Astrocytes from Mixed Glial Culture
2.5. Immunoblot Analysis of the Generation of GFAP+ or Iba1+ Cells
2.6. RT-PCR Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Río-Hortega, P. The microglia. Lancet 1939, 233, 1023–1026. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Schulz, C.; Gomez Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, H.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Kierdorf, K.; Erny, D.; Goldmann, T. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Perdiguero, E.G.; Klapproth, K.; Schulz, C. Tissue-resident macrophages orignate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada Gonzalez, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353. [Google Scholar] [CrossRef]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef]
- Mildner, A.; Schmidt, H.; Nitsche, M.; Merkler, D.; Hanisch, U.K.; Mack, M.; Heikenwalder, M.; Bruck, W.; Priller, J.; Prinz, M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007, 10, 1544–1553. [Google Scholar] [CrossRef]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 39, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef]
- Goldmann, T.; Wieghofer, P.; Jordao, M.J.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Staszewski, O.; Kierdorf, K.; Amann, L.; Krueger, M.; et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef]
- Suzumura, A.; Mezitis, S.G.; Gonatas, N.K.; Silberberg, D.H. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: Induction of Ia antigen expression by γ-interferon. J. Neuroimmunol. 1987, 15, 263–278. [Google Scholar] [CrossRef]
- Tanaka, J.; Maeda, N. Microglial ramification requires nondiffusible factors derived from astrocytes. Exp. Neurol. 1996, 137, 367–375. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kumon, Y.; Watanabe, H.; Ohnishi, T.; Shudou, M.; Chuai, M.; Imai, Y.; Takahashi, H.; Tanaka, J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2008, 28, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, J.; Fujita, H.; Matsuda, S.; Toku, K.; Sakanaka, M.; Maeda, N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids. Glia 1997, 20, 23–37. [Google Scholar] [CrossRef]
- Mori, K.; Ozaki, E.; Zhang, B.; Yang, L.; Yokoyama, A.; Takeda, I.; Maeda, N.; Sakanaka, M.; Tanaka, J. Effects of norepinephrine on rat cultured microglial cells that express α1, α2, β1 and β2 adrenergic receptors. Neuropharmacology 2002, 43, 1026–1034. [Google Scholar] [CrossRef]
- Yokoyama, A.; Yang, L.; Itoh, S.; Mori, K.; Tanaka, J. Microglia, a potential source of neurons, astrocytes, and oligodendrocytes. Glia 2004, 45, 96–104. [Google Scholar] [CrossRef]
- Lazar, L.M.; Blum, M. Regional distribution and developmental expression of epidermal growth factor and transforming growth factor-alpha mRNA in mouse brain by a quantitative nuclease protection assay. J. Neurosci. 1992, 12, 1688–1697. [Google Scholar] [CrossRef] [Green Version]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef] [Green Version]
- Eggen, B.J.L.; Raj, D.; Hanisch, U.K.; Boddeke, H.W. Microglial phenotype and adaptation. J. Neuroimmune Pharmacol. 2013, 8, 807–823. [Google Scholar] [CrossRef]
- Dijkstra, C.D.; Döpp, E.A.; Joling, P.; Kraal, G. The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 1985, 54, 589–599. [Google Scholar] [PubMed]
- Serrats, J.; Schiltz, J.C.; Garcïa-Bueno, B.; van Rooijen, N.; Reyes, T.M.; Sawchenko, P.E. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 2010, 65, 94–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelov, D.N.; Krebs, C.; Walther, M.; Martinez-Portillo, F.J.; Gunkel, A.; Lay, C.H.; Streppel, M.; Guntinas-Lichius, O.; Stennert, E.; Neiss, W.F. Altered expression of immune-related antigens by neuronophages does not improve neuronal survival after severe lesion of the facial nerve in rats. Glia 1998, 24, 155–171. [Google Scholar] [CrossRef]
- Carlson, S.L.; Parrish, M.E.; Springer, J.E.; Doty, K.; Dossett, L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998, 151, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Kumon, Y.; Watanabe, H.; Ohnishi, T.; Shudou, M.; Ii, C.; Takahashi, H.; Imai, Y.; Tanaka, J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J. Neurosci. Res. 2007, 85, 994–1009. [Google Scholar] [CrossRef]
- Graeber, M.B.; Streit, W.J.; Kreutzberg, G.W. Identity of ED2-positive perivascular cells in rat brain. J. Neurosci. Res. 1989, 22, 103–106. [Google Scholar] [CrossRef]
- Tanaka, J.; Toku, K.; Sakanaka, M.; Maeda, N. Morphological differentiation of microglial cells in culture: Involvement of insoluble factors derived from astrocytes. Neurosci. Res. 1999, 34, 207–215. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Yokoyama, A.; Yang, L.; Toku, K.; Mori, K.; Takeda, I.; Shigekawa, T.; Zhang, B.; Maeda, N.; Sakanaka, M.; et al. Two populations of microglial cells isolated from rat primary mixed glial cultures. J. Neurosci. Res. 2003, 73, 22–30. [Google Scholar] [CrossRef]
- Aloisi, F. Immune function of microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef]
- Perry, V.H.; Cunningham, C.; Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007, 7, 161–167. [Google Scholar] [CrossRef]
- Hart, A.D.; Wyttenbach, A.; Perry, V.H.; Teeling, J.L. Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain Behav. Immun. 2012, 26, 754–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamak, B.; Mallat, M. Fibronectin and laminin regulate the in vitro differentiation of microglial cells. Neuroscience 1991, 45, 513–527. [Google Scholar] [CrossRef]
- Ferrer, I.; Alcantara, S.; Ballabriga, J.; Olive, M.; Blanco, R.; Rivera, R.; Carmona, M.; Berruezo, M.; Pitarch, S.; Planas, A.M. Transforming growth factor-α (TGF-α) and epidermal growth factor-receptor (EGF-R) immunoreactivity in normal and pathologic brain. Prog. Neurobiol. 1996, 49, 99–123. [Google Scholar] [CrossRef]
- Planas, A.M.; Justicia, C.; Soriano, M.A.; Ferrer, I. Epidermal growth factor receptor in proliferating reactive glia following transient focal ischemia in the rat brain. Glia 1998, 23, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Witmer-Pack, M.D.; Hughes, D.A.; Schuler, G.; Lawson, L.; McWilliam, A.; Inaba, K.; Steinman, R.M.; Gordon, S. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J. Cell. Sci. 1993, 104, 1021–1029. [Google Scholar]
- Wegiel, J.; Wisniewski, H.M.; Dziewiatkowski, J.; Tarnawski, M.; Kozielski, R.; Trenkner, E.; Wiktor-Jedrzejczak, W. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res. 1998, 804, 135–139. [Google Scholar] [CrossRef]
- Sasaki, A.; Yokoo, H.; Naito, M.; Kaizu, C.; Shultz, L.D.; Nakazato, Y. Effects of macrophage-colony-stimulating factor deficiency on the maturation of microglia and brain macrophages and on their expression of scavenger receptor. Neuropathology 2000, 20, 134–142. [Google Scholar] [CrossRef]
- Sawada, M.; Itoh, Y.; Suzumura, A.; Marunouchi, T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci. Lett. 1993, 160, 131–134. [Google Scholar] [CrossRef]
- Giulian, D.; Ingeman, J.E. Colony-stimulating factors as promoters of ameboid microglia. J. Neurosci. 1988, 8, 4707–4717. [Google Scholar] [CrossRef]
- Lee, S.C.; Liu, W.; Brosnan, C.F.; Dickson, D.W. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia 1994, 12, 309–318. [Google Scholar] [CrossRef]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmau, I.; Finsen, B.; Tonder, N.; Zimmer, J.; Gonzalez, B.; Castellano, B. Development of microglia in the prenatal rat hippocampus. J. Comp. Neurol. 1997, 377, 70–84. [Google Scholar] [CrossRef]
- Marin-Teva, J.L.; Almendros, A.; Calvente, R.; Cuadros, M.A.; Navascues, J. Tangential migration of ameboid microglia in the developing quail retina: Mechanism of migration and migratory behavior. Glia 1998, 22, 31–52. [Google Scholar] [CrossRef]
- Tassi, M.; Calvente, R.; Marin-Teva, J.L.; Cuadros, M.A.; Santos, A.M.; Carrasco, M.C.; Sanchez-Lopez, A.M.; Navascues, J. Behavior of in vitro cultured ameboid microglial cells migrating on Müller cell end-feet in the quail embryo retina. Glia 2006, 54, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.P.; Lu, J.; Cao, Q.; Kaur, C.; Ling, E.A. Nestin expression in Müller glial cells in postnatal rat retina and its upregulation following optic nerve transection. Neuroscience 2006, 143, 117–127. [Google Scholar] [CrossRef] [PubMed]



| Antigen | Antibody | Dilution | Source |
|---|---|---|---|
| CD11b | Mouse monoclonal (MRC OX-42) | 200 | Serotec (Oxford, UK) |
| CD68 | Mouse monoclonal (ED1) | 200 | Serotec (Oxford, UK) |
| CD163 | Mouse monoclonal (ED2) | 200 | Serotec (Oxford, UK) |
| EGFR | Mouse monoclonal | 200 | BD Biosciences (Tokyo, Japan) |
| pEGFR | Mouse monoclonal (9H2) | 200 | Santa Cruz (Santa Cruz, CA, USA) |
| FN | Rabbit polyclonal | 500 | Sigma-Aldrich (St. Louis, MO, USA) |
| GFAP | Rabbit polyclonal | 500 | Shima (Tokyo, Japan) |
| GFAP | Mouse monoclonal | 500 | Chemicon (Temecula, CA, USA) |
| Iba1 | Rabbit polyclonal | 5000 | Wako (Osaka, Japan) |
| Ki67 | Rabbit monoclonal (SP6) | 500 | NeoMakers (Fremont, CA, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, J.; Takahashi, H.; Yano, H.; Nakanishi, H. Generation of CSF1-Independent Ramified Microglia-Like Cells from Leptomeninges In Vitro. Cells 2021, 10, 24. https://doi.org/10.3390/cells10010024
Tanaka J, Takahashi H, Yano H, Nakanishi H. Generation of CSF1-Independent Ramified Microglia-Like Cells from Leptomeninges In Vitro. Cells. 2021; 10(1):24. https://doi.org/10.3390/cells10010024
Chicago/Turabian StyleTanaka, Junya, Hisaaki Takahashi, Hajime Yano, and Hiroshi Nakanishi. 2021. "Generation of CSF1-Independent Ramified Microglia-Like Cells from Leptomeninges In Vitro" Cells 10, no. 1: 24. https://doi.org/10.3390/cells10010024
APA StyleTanaka, J., Takahashi, H., Yano, H., & Nakanishi, H. (2021). Generation of CSF1-Independent Ramified Microglia-Like Cells from Leptomeninges In Vitro. Cells, 10(1), 24. https://doi.org/10.3390/cells10010024






