Pulsed Electrical Stimulation Enhances Consistency of Directional Migration of Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
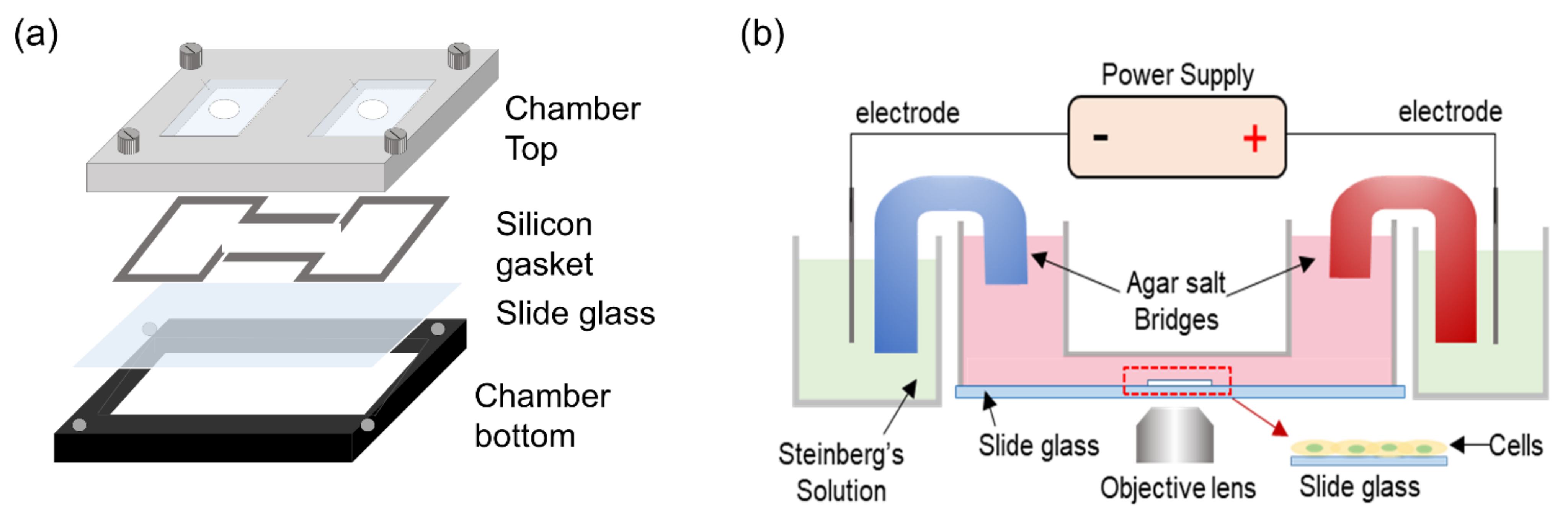
2.1. Electrical Stimulation of ADSCs to Induce Electrotaxis
2.2. Measurement of ADSC Migration
2.3. Cell Viability Assay
2.4. Immunofluorescence Staining and Golgi Polarization Analysis
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, Y.; Son, M.; Jeong, H.; Shin, J.H. Electric field–induced migration and intercellular stress alignment in a collective epithelial monolayer. Mol. Biol. Cell 2018, 29, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuzzo, P.; Van Troys, M.; Ampe, C.; Martens, L. Taking aim at moving targets in computational cell migration. Trends Cell Biol. 2016, 26, 88–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Wang, J.; Fu, D.; Yan, Q.; Pang, D.; Zhang, Z. Topography guiding the accelerated and persistently directional cell migration induced by vaccinia virus. Chin. Chem. Lett. 2020, 31, 167–171. [Google Scholar] [CrossRef]
- De Lucas, B.; Pérez, L.M.; Gálvez, B.G. Importance and regulation of adult stem cell migration. J. Cell. Mol. Med. 2018, 22, 746–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzhor, E.; Leshansky, L.; Blumenthal, J.; Barash, H.; Warshawsky, D.; Mazor, Y.; Shtrichman, R. Cell-based therapy approaches: The hope for incurable diseases. Regen. Med. 2014, 9, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forcales, S.V. Potential of adipose-derived stem cells in muscular regenerative therapies. Front. Aging Neurosci. 2015, 7, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freiman, A.; Shandalov, Y.; Rozenfeld, D.; Shor, E.; Segal, S.; Ben-David, D.; Meretzki, S.; Egozi, D.; Levenberg, S. Adipose-derived endothelial and mesenchymal stem cells enhance vascular network formation on three-dimensional constructs in vitro. Stem Cell Res. Ther. 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.I.; Kim, K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, J.P.; Fuchs, A.F.; Rubel, E.W. Hair cell regeneration and recovery of the vestibuloocular reflex in the avian vestibular system. J. Neurophysiol. 1996, 76, 3301–3312. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, R. A role for endogenous electric fields in wound healing. Curr. Top. Dev. Biol. 2003, 58, 1–26. [Google Scholar] [CrossRef]
- Nuccitelli, R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat. Prot. Dosim. 2003, 106, 375–383. [Google Scholar] [CrossRef]
- Creed, M.C.; Hamani, C.; Bridgman, A.; Fletcher, P.J.; Nobrega, J.N. Contribution of decreased serotonin release to the antidyskinetic effects of deep brain stimulation in a rodent model of tardive dyskinesia: Comparison of the subthalamic and entopeduncular nuclei. J. Neurosci. 2012, 32, 9574–9581. [Google Scholar] [CrossRef] [Green Version]
- Illingworth, C.M.; Barker, A.T. Measurement of electrical currents emerging during the regeneration of amputated finger tips in children. Clin. Phys. Physiol. Meas. 1980, 1, 87–89. [Google Scholar] [CrossRef]
- Huang, C.P.; Chen, X.M.; Chen, Z.Q. Osteocyte: The impresario in the electrical stimulation for bone fracture healing. Med. Hypotheses 2008, 70, 287–290. [Google Scholar] [CrossRef]
- Macginitie, L.A.; Wu, D.D.; Cochran, G.V.B. Streaming potentials in healing, remodeling, and intact cortical bone. J. Bone Miner. Res. 1993, 8, 1323–1325. [Google Scholar] [CrossRef]
- McLeod, K.J.; Rubin, C.T. The effect of low-frequency electrical fields on osteogenesis. J. Bone Jt. Surg. Am. Vol. 1992, 74, 920–929. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, W.; Clark, C.C.; Brighton, C.T. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthr. Cartil. 2009, 17, 297–405. [Google Scholar] [CrossRef] [Green Version]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behaviour electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Watt, C.; Karystinou, A.; Roelofs, A.J.; McCaig, C.D.; Gibson, I.R.; Bari, C.D. Directed migration of human bone marrow mesenchymal stem cells in a physiological direct current electric field. Ecells Mater. J. 2011, 22, 344–358. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, L.; Reid, B.; Pu, J.; Hara, T.; Zhao, M. Directing migration of endothelial progenitor cells with applied DC electric fields. Stem Cell Res. 2012, 8, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Guo, A.; Song, B.; Reid, B.; Gu, Y.; Forrester, J.V.; Jahoda, C.A.B.; Zhao, M. Effects of physiological electric fields on migration of human dermal fibroblasts. J. Investig. Dermatol. 2010, 130, 2320–2327. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Lee, M.H.; Kwon, B.J.; Kim, D.; Koo, M.A.; Seon, G.M.; Park, J.C. Homogeneity evaluation of mesenchymal stem cells based on electrotaxis analysis. Sci. Rep. 2017, 7, 9582. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, M.H.; Koo, M.A.; Seon, K.M.; Park, Y.J.; Kim, D.; Park, J.C. Stem cell passage affects directional migration of stem cells in electrotaxis. Stem Cell Res. 2019, 38, 101475. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Long, H.; Ren, X.; Ma, K.; Xiao, Z.; Wang, Y.; Guo, Y. Regulation of adipose-tissue-derived stromal cell orientation and motility in 2D- and 3D-cultures by direct-current electrical field. Dev. Growth Differ. 2017, 59, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, M.H.; Kwon, B.J.; Koo, M.A.; Seon, S.M.; Park, J.C. Golgi polarization plays a role in the directional migration of neonatal dermal fibroblasts induced by the direct current electric fields. Biochem. Biophys. Res. Commun. 2015, 460, 255–260. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, M.H.; Kwon, B.J.; Seo, H.J.; Koo, M.A.; You, K.E.; Kim, D.; Park, J.C. Control of neonatal human dermal fibroblast migration on poly(lactic-co-glycolic acid)-coated surfaces by electrotaxis. J. Tissue Eng. Regen. Med. 2017, 11, 862–868. [Google Scholar] [CrossRef]
- Banks, T.A.; Luckman, P.S.B.; Frith, J.E.; Cooper-White, J.J. Effects of electric fields on human mesenchymal stem cell behaviour and morphology using a novel multichannel device. Integr. Biol. 2015, 7, 693–712. [Google Scholar] [CrossRef]
- Bicer, M.; Sheard, J.; Iandolo, D.; Boateng, S.Y.; Cottrell, G.S.; Widera, D. Electrical stimulation of adipose-derived stem cells in 3D nanofibrillar cellulose increases their osteogenic potential. Biomolecules 2020, 10, 1696. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Gregory, B.W.; Semenov, I.; Pakhomov, A.G. Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS ONE 2013, 8, e70278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakhomov, A.G.; Xiao, S.; Pakhomova, O.N.; Semenov, I.; Kuipers, M.A.; Ibey, B.L. Disassembly of actin structures by nanosecond pulsed electric field is a downstream effect of cell swelling. Bioelectrochemistry 2014, 100, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, Y.Y.; Wang, X.S.; Zhang, Y.; Yao, C.G.; Zhang, X.M.; Xiong, Z.A. Intense picosecond pulsed electric fields induce apoptosis through a mitochondrial-mediated pathway in HeLa cells. Mol. Med. Rep. 2012, 5, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.R.; Palee, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of electrical stimulation on cell proliferation and apoptosis. J. Cell. Physiol. 2018, 233, 1860–1876. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.L.; Wu, H.Y.; Tian, Z.X.; Luo, Z.; Wu, Y.F.; Zhao, J. Electrical stimulation induces mitochondrial autophagy via activating oxidative stress and Sirt3 signaling pathway. Chin. Med. J. 2021, 134, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Palamà, I.E.; D’Amone, S.; Gigli, G. Influence of electrotaxis on cell behavior. Integr. Biol. 2014, 6, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Ozkucur, N.; Monsees, T.K.; Perike, S.; Do, H.Q.; Funk, R.H.W. Local calcium elevation and cell elongation initiate guided motility in electrically stimulated osteoblast-like cells. PLoS ONE 2009, 4, e6131. [Google Scholar] [CrossRef] [PubMed]
- McKasson, M.J.; Huang, L.; Robinson, K.R. Chick embryonic Schwann cells migrate anodally in small electrical fields. Exp. Neurol. 2008, 211, 585–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, G.M.; Mogilner, A.; Theriot, J.A. Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr. Biol. 2013, 23, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, J.; McCaig, C.D.; Cao, L.; Zhao, Z.; Segall, J.E.; Zhao, M. EGF receptor signaling is essential for electric-field-directed migration of breast cancer cells. J. Cell Sci. 2007, 120, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Calafiore, M.; Zeng, Q.; Zhang, X.; Huang, Y.; Li, R.A.; Deng, W.; Zhao, M. Electrically guiding migration of human induced pluriopotent stem cells. Stem Cell Rev. Rep. 2011, 7, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Hammerick, K.E.; James, A.W.; Huang, Z.; Prinz, F.B.; Longaker, M.T. Pulsed direct current electric fields enhance osteogenesis in adipose-derived stromal cells. Tissue Eng. Part A 2010, 16, 917–931. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.F.; Liu, J.; Zhang, X.Z.; Zhang, L.; Jiang, J.Y.; Nolta, J.; Zhao, M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 2012, 30, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Song, B.; Pu, J.; Wada, T.; Reid, B.; Tai, G.; Wang, F.; Guo, A.; Walczysko, P.; Gu, Y.; et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006, 442, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Sato, M.J.; Kuwayama, H.; van Egmond, W.N.; Takayama, A.L.K.; Takagi, H.; van Haastert, P.J.M.; Yanagida, T.; Ueda, M. Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 6667–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkucur, N.; Perike, S.; Sharma, P.; Funk, R.H. Persistent directional cell migration requires ion transport proteins as direction sensors and membrane potential differences in order to maintain directedness. BMC Cell Biol. 2011, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.W.; Hsiao, C.T.; Chen, Y.Q.; Huang, C.M.; Chan, S.I.; Chiou, A.; Kuo, J.C. Affiliations expand Centrosome guides spatial activation of Rac to control cell polarization and directed cell migration. Life Sci. Alliance 2019, 2, e201800135. [Google Scholar] [CrossRef]
- Yadav, S.; Linstedt, A.D. Golgi positioning. Cold Spring Harb. Perspect. Biol. 2011, 3, a005322. [Google Scholar] [CrossRef] [Green Version]
- Natividad, R.J.; Lalli, M.L.; Muthuswamy, S.K.; Asthagiri, A.R. Golgi stabilization, not its front-rear bias, is associated with EMT-enhanced fibrillar migration. Biophys. J. 2018, 115, 2067–2077. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.H.; Park, Y.J.; Hong, S.H.; Koo, M.-A.; Cho, M.; Park, J.-C. Pulsed Electrical Stimulation Enhances Consistency of Directional Migration of Adipose-Derived Stem Cells. Cells 2021, 10, 2846. https://doi.org/10.3390/cells10112846
Lee MH, Park YJ, Hong SH, Koo M-A, Cho M, Park J-C. Pulsed Electrical Stimulation Enhances Consistency of Directional Migration of Adipose-Derived Stem Cells. Cells. 2021; 10(11):2846. https://doi.org/10.3390/cells10112846
Chicago/Turabian StyleLee, Mi Hee, Ye Jin Park, Seung Hee Hong, Min-Ah Koo, Minyoung Cho, and Jong-Chul Park. 2021. "Pulsed Electrical Stimulation Enhances Consistency of Directional Migration of Adipose-Derived Stem Cells" Cells 10, no. 11: 2846. https://doi.org/10.3390/cells10112846





