Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy
Abstract
:1. Introduction
2. Craniofacial Bone Tissue Engineering: Current Approaches and Challenges
3. Biomaterials for Craniofacial Bone Regeneration
3.1. Autologous Bone Graft
3.2. Allogeneic Bone Graft
3.3. Xenogeneic Bone Graft
3.4. Alloplastic Bone Graft Substitute
4. Mesenchymal Stromal Cells: Successes and Challenges
4.1. Bone Marrow Mesenchymal Stromal Cells (BMSCs)
4.2. Dental Pulp Mesenchymal Stromal Cells (DPSCs)
5. Protein-Based Therapy: Current Approaches and Potential Therapy
5.1. Bone Morphogenetic Protein-Based Therapy
5.2. Platelet-Rich Plasma (PRP)
6. Inducing MKs via Thrombopoietin: A Potential Therapy for Craniofacial Bone Defects
7. Conclusions and Future Insights
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, S.B.; Kemp, S.S.; Oh, K.S. Radiographic manifestations of congenital anomalies of the skull. Radiol. Clin. N. Am. 1991, 29, 195–218. [Google Scholar]
- Hoff, S.R.; Edwards, M.S.; Bailey, C.M.; Koltai, P.J. The transpalatal approach to repair of congenital Basal skull base cephaloceles. J. Neurol. Surg. B Skull Base. 2014, 75, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Chamieh, F.; Collignon, A.-M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.-L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumanian, Z.P.; Tollemar, V.; Ye, J.; Lu, M.; Zhu, Y.; Liao, J.; Ameer, G.A.; He, T.-C.; Reid, R.R. Repair of critical sized cranial defects with BMP9-transduced calvarial cells delivered in a thermoresponsive scaffold. PLoS ONE 2017, 12, e0172327. [Google Scholar] [CrossRef]
- Decesare, G.E.; Deleyiannis, F.W.; Losee, J.E. Reconstruction of osteomyelitis defects of the craniofacial skeleton. Semin. Plast. Surg. 2009, 23, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K. Unfavourable results in craniofacial surgery. Indian J. Plast. Surg. 2013, 46, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Kuether, J.; Fong, A.; Reid, R. Cranioplasty for large-sized calvarial defects in the pediatric population: A review. Craniomaxillofac. Trauma Reconstr. 2015, 8, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Edvinsson, J.C.A.; Viganò, A.; Alekseeva, A.; Alieva, E.; Arruda, R.; De Luca, C.; D’Ettore, N.; Frattale, I.; Kurnukhina, M.; Macerola, N.; et al. The fifth cranial nerve in headaches. J. Headache Pain 2020, 21, 65. [Google Scholar] [CrossRef] [PubMed]
- Zanoletti, E.; Mazzoni, A.; Martini, A.; Abbritti, R.V.; Albertini, R.; Alexandre, E.; Baro, V.; Bartolini, S.; Bernardeschi, D.; Bivona, R.; et al. Surgery of the lateral skull base: A 50-year endeavour. Acta Otorhinolaryngol. Ital. 2019, 39, S1–S146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özge, A.; Abu-Arafeh, I.; Gelfand, A.A.; Goadsby, P.J.; Cuvellier, J.C.; Valeriani, M.; Sergeev, A.; Barlow, K.; Uludüz, D.; Yalın, O.; et al. Experts’ opinion about the pediatric secondary headaches diagnostic criteria of the ICHD-3 beta. J. Headache Pain 2017, 18, 113. [Google Scholar] [CrossRef] [Green Version]
- Dennis, E.L.; Humphreys, K.L.; King, L.S.; Thompson, P.M.; Gotlib, I.H. Irritability and brain volume in adolescents: Cross-sectional and longitudinal associations. Soc. Cogn. Affect. Neurosci. 2019, 14, 687–698. [Google Scholar] [CrossRef]
- Townsend, J.M.; Dennis, S.C.; Whitlow, J.; Feng, Y.; Wang, J.; Andrews, B.; Nudo, R.J.; Detamore, M.S.; Berkland, C.J. Colloidal Gels with Extracellular Matrix Particles and Growth Factors for Bone Regeneration in Critical Size Rat Calvarial Defects. AAPS J. 2017, 19, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Maraldi, T.; Pisciotta, A.; La Sala, G.B.; Ferrari, A.; Bruzzesi, G.; Motta, A.; Migliaresi, C.; De Pol, A. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng. Part A 2012, 18, 1006–1013. [Google Scholar] [CrossRef]
- Luo, E.; Liu, H.; Zhao, Q.; Shi, B.; Chen, Q. Dental-craniofacial manifestation and treatment of rare diseases. Int. J. Oral. Sci. 2019, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Kao, M.C. Cranioplasty for patients with severe depressed skull bone defect after cerebrospinal fluid shunting. J. Clin. Neurosci. 2002, 9, 553–555. [Google Scholar] [CrossRef]
- Ueda, K.; Oba, S.; Omiya, Y.; Okada, M. Cranial-bone defects with depression deformity treated with ceramic implants and free-flap transfers. Br. J. Plast. Surg. 2001, 54, 403–408. [Google Scholar] [CrossRef]
- Schiffer, J.; Gur, R.; Nisim, U.; Pollak, L. Symptomatic patients after craniectomy. Surg. Neurol. 1997, 47, 231–237. [Google Scholar] [CrossRef]
- Matarán-Peñarrocha, G.A.; Castro-Sánchez, A.M.; García, G.C.; Moreno-Lorenzo, C.; Carreño, T.P.; Zafra, M.D. Influence of craniosacral therapy on anxiety, depression and quality of life in patients with fibromyalgia. Evid. Based Complement Altern. Med 2011, 2011, 178769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, R.S.; Brigato, R.; Madureira, J.F.; Cruz, A.A.; de Mello Filho, F.V.; Alonso, N.; Machado, H.R. Reconstruction of a large complex skull defect in a child: A case report and literature review. Childs Nerv. Syst. 2007, 23, 1097–1102. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.J.; Lee, J.W.; Jeong, H.S.; Suh, I.S. Staged reconstruction of large skull defects with soft tissue infection after craniectomy using free flap and cranioplasty with a custom-made titanium mesh constructed by 3D-CT-guided 3D printing technology: Two case reports. Medicine 2019, 98, e13864. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [Green Version]
- Emara, A.; Shah, R. Recent update on craniofacial tissue engineering. J. Tissue Eng. 2021, 12, 20417314211003735. [Google Scholar] [CrossRef]
- Aghali, A. Poly(ethylene glycol) and Co-polymer Based-Hydrogels for Craniofacial Bone Tissue Engineering. In Orthopedic Biomaterials; Springer: Cham, Switzerland, 2017; pp. 225–246. [Google Scholar]
- Aghali, A.; Arman, H.E. Photoencapsulated-mesenchymal stromal cells in biodegradable thiol-acrylate hydrogels enhance regeneration of craniofacial bone tissue defects. Regen. Med. 2020, 15, 2115–2127. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, G.; Zhao, Z.; Yin, C.; Zhao, Q.; Xu, H.; Wang, J.; Zhang, J.; Zhang, X.; Zhang, Y.; et al. Individualized plasticity autograft mimic with efficient bioactivity inducing osteogenesis. Int. J. Oral Sci. 2021, 13, 14. [Google Scholar] [CrossRef]
- Sheyn, D.; Cohn Yakubovich, D.; Kallai, I.; Su, S.; Da, X.; Pelled, G.; Tawackoli, W.; Cook-Weins, G.; Schwarz, E.M.; Gazit, D.; et al. PTH promotes allograft integration in a calvarial bone defect. Mol. Pharm. 2013, 10, 4462–4471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.P.; Chiodo, C.P. Autologous Bone Graft in Foot and Ankle Surgery. Foot Ankle Clin. 2016, 21, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Mao, F.; Yuan, B.; Ren, G.; Liu, H.; Peng, C. Minimally Invasive Percutaneous Plate Osteosynthesis (MIPPO) Combined with Onionskin-Like Autologous Bone Grafting: A New Technique for Treatment of Tibial Nonunion. Med. Sci. Monit. 2019, 25, 5997–6006. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Sun, R.; Wang, L.; Zhou, J.; Wan, L.; Zhou, T.; Hu, Y. A New Method for Xenogeneic Bone Graft Deproteinization: Comparative Study of Radius Defects in a Rabbit Model. PLoS ONE 2016, 10, e0146005. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Jahan, K.; Manickam, G.; Tabrizian, M.; Murshed, M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci. Rep. 2020, 10, 11603. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Pokhrel, A.; Agarwal, A.K.; Vijay, V. Current status of bone cementing and bone grafting for giant cell tumour of bone: A systemic review. Ann. R. Coll. Surg. Engl. 2019, 101, 79–85. [Google Scholar] [CrossRef]
- Van de Vijfeijken, S.; Münker, T.; Spijker, R.; Karssemakers, L.H.E.; Vandertop, W.P.; Becking, A.G.; Ubbink, D.T. Autologous Bone Is Inferior to Alloplastic Cranioplasties: Safety of Autograft and Allograft Materials for Cranioplasties, a Systematic Review. World Neurosurg. 2018, 117, 443–452. [Google Scholar] [CrossRef]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Jahan, K.; Tabrizian, M. Composite biopolymers for bone regeneration enhancement in bony defects. Biomater. Sci. 2016, 4, 25–39. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef]
- Marchac, D.; Greensmith, A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 744–752. [Google Scholar] [CrossRef]
- Foster, K.A.; Shin, S.S.; Prabhu, B.; Fredrickson, A.; Sekula, R.F., Jr. Calcium Phosphate Cement Cranioplasty Decreases the Rate of Cerebrospinal Fluid Leak and Wound Infection Compared with Titanium Mesh Cranioplasty: Retrospective Study of 672 Patients. World Neurosurg. 2016, 95, 414–418. [Google Scholar] [CrossRef]
- Khashaba, R.M.; Moussa, M.M.; Mettenburg, D.J.; Rueggeberg, F.A.; Chutkan, N.B.; Borke, J.L. Polymeric-Calcium Phosphate Cement Composites-Material Properties: In Vitro and In Vivo Investigations. Int. J. Biomater. 2010, 2010, 691452. [Google Scholar] [CrossRef] [Green Version]
- Leach, J.K.; Whitehead, J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 1115–1127. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.M.; Cai, A.; Dippold, D.; Boccaccini, A.R.; Schubert, D.W.; Hardt, M.; Lange, C.; Arkudas, A.; et al. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Chai, Y. Stem Cells in Teeth and Craniofacial Bones. J. Dent. Res. 2015, 94, 1495–1501. [Google Scholar] [CrossRef] [Green Version]
- Mebarki, M.; Coquelin, L.; Layrolle, P.; Battaglia, S.; Tossou, M.; Hernigou, P.; Rouard, H.; Chevallier, N. Enhanced human bone marrow mesenchymal stromal cell adhesion on scaffolds promotes cell survival and bone formation. Acta Biomater. 2017, 59, 94–107. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part. B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Blache, U.; Stevens, M.M.; Gentleman, E. Harnessing the secreted extracellular matrix to engineer tissues. Nat. Biomed. Eng. 2020, 4, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Aghali, A.; Arman, H.E. Photoencapsulated-BMP2 in visible light-cured thiol-acrylate hydrogels for craniofacial bone tissue engineering. Regen. Med. 2020, 15, 2099–2113. [Google Scholar] [CrossRef]
- Machado, E.; Fernandes, M.H.; de Sousa Gomes, P. Dental stem cells for craniofacial tissue engineering. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2012, 113, 728–733. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. 2019, 10, 68. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, K.C.; Tsai, S.J.; Chang, H.H.; Lin, C.P. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J. Med. Assoc. 2014, 113, 956–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Gilbert, J.R.; Zhang, X.; Zhao, B.; Ker, D.F.E.; Cooper, G.M. Calvarial Versus Long Bone: Implications for Tailoring Skeletal Tissue Engineering. Tissue Eng. Part. B Rev. 2020, 26, 46–63. [Google Scholar] [CrossRef]
- Chai, Y.; Maxson, R.E., Jr. Recent advances in craniofacial morphogenesis. Dev. Dyn. 2006, 235, 2353–2375. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult craniofacial stem cells: Sources and relation to the neural crest. Stem Cell Rev. Rep. 2012, 8, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel Chu, T.M.; Goebel, W.S. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [CrossRef]
- Marie, P.J.; Fromigue, O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen. Med. 2006, 1, 539–548. [Google Scholar] [CrossRef]
- Sununliganon, L.; Peng, L.; Singhatanadgit, W.; Cheung, L.K. Osteogenic efficacy of bone marrow concentrate in rabbit maxillary sinus grafting. J. Craniomaxillofac. Surg. 2014, 42, 1753–1765. [Google Scholar] [CrossRef]
- Zakharov Iu, M.; Makarova, E.B. Regulation of osteogenic differentiation of mesenchimal stem sells of bone marrow. Ross. Fiziol. Zh. Im. I. M. Sechenova 2013, 99, 417–432. [Google Scholar]
- Vilquin, J.T.; Rosset, P. Mesenchymal stem cells in bone and cartilage repair: Current status. Regen. Med. 2006, 1, 589–604. [Google Scholar] [CrossRef]
- Zhou, D.A.; Zheng, H.X.; Wang, C.W.; Shi, D.; Li, J.J. Influence of glucocorticoids on the osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. BMC Musculoskelet. Disord. 2014, 15, 239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Shao, H.; Xu, K.Q.; Kuang, L.T.; Chen, R.F.; Xiu, H.H. Midazolam suppresses osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Eur. Rev. Med. Pharm. Sci. 2014, 18, 1411–1418. [Google Scholar]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, K.; Kaito, T.; Furuya, M.; Seno, S.; Okuzaki, D.; Kikuta, J.; Tsukazaki, H.; Matsuda, H.; Yoshikawa, H.; Ishii, M. In vivo dynamic analysis of BMP-2-induced ectopic bone formation. Sci. Rep. 2020, 10, 4751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddi, A.H.; Reddi, A. Bone morphogenetic proteins (BMPs): From morphogens to metabologens. Cytokine Growth Factor Rev. 2009, 20, 341–342. [Google Scholar] [CrossRef]
- Reddi, A.H. Bone morphogenetic proteins: An unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev. 1997, 8, 11–20. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Mishina, Y. New insights on the roles of BMP signaling in bone-A review of recent mouse genetic studies. Biofactors 2011, 37, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Luu, H.H.; Song, W.X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Li, C.; Yu, Y. Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol. Med. Rep. 2015, 12, 4230–4237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Luo, Q.; Shu, Y.; Zeng, Z.; Huang, B.; Feng, Y.; Zhang, B.; Wang, X.; Lei, Y.; Ye, Z.; et al. Transcriptomic landscape regulated by the 14 types of bone morphogenetic proteins (BMPs) in lineage commitment and differentiation of mesenchymal stem cells (MSCs). Genes Dis. 2019, 6, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Wongwitwichot, P.; Kaewsrichan, J. Osteogenic differentiation of mesenchymal stem cells is impaired by bone morphogenetic protein 7. Adv. Med. Sci. 2017, 62, 266–272. [Google Scholar] [CrossRef]
- Kumar, S.; Wan, C.; Ramaswamy, G.; Clemens, T.L.; Ponnazhagan, S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol. Ther. 2010, 18, 1026–1034. [Google Scholar] [CrossRef]
- Sammons, J.; Ahmed, N.; El-Sheemy, M.; Hassan, H.T. The role of BMP-6, IL-6, and BMP-4 in mesenchymal stem cell-dependent bone development: Effects on osteoblastic differentiation induced by parathyroid hormone and vitamin D(3). Stem Cells Dev. 2004, 13, 273–280. [Google Scholar] [CrossRef]
- Mizrahi, O.; Sheyn, D.; Tawackoli, W.; Kallai, I.; Oh, A.; Su, S.; Da, X.; Zarrini, P.; Cook-Wiens, G.; Gazit, D.; et al. BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene 2013, 20, 370–377. [Google Scholar] [CrossRef]
- Vukicevic, S.; Grgurevic, L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009, 20, 441–448. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Gagne, K.; Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.T.; Zhao, Z.; Wang, Z.; Lewis, I.S.; Krebsbach, P.H.; Franceschi, R.T. Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J. Dent. Res. 2008, 87, 845–849. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.S.; Huang, B.J.; Kaltz, S.R.; Sur, S.; Newcomb, C.J.; Stock, S.R.; Shah, R.N.; Stupp, S.I. Bone regeneration with low dose BMP-2 amplified by biomimetic supramolecular nanofibers within collagen scaffolds. Biomaterials 2013, 34, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Duan, H.; Xu, L.; Witman, N.; Yan, B.; Yu, Z.; Wang, H.; Tan, Y.; Lin, L.; Li, D.; et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Kim, K. Bone Tissue Engineering Strategies in Co-Delivery of Bone Morphogenetic Protein-2 and Biochemical Signaling Factors. Adv. Exp. Med. Biol. 2018, 1078, 233–244. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Rihn, J.A.; Patel, R.; Makda, J.; Hong, J.; Anderson, D.G.; Vaccaro, A.R.; Hilibrand, A.S.; Albert, T.J. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009, 9, 623–629. [Google Scholar] [CrossRef]
- Choudhry, O.J.; Christiano, L.D.; Singh, R.; Golden, B.M.; Liu, J.K. Bone morphogenetic protein-induced inflammatory cyst formation after lumbar fusion causing nerve root compression. J. Neurosurg. Spine 2012, 16, 296–301. [Google Scholar] [CrossRef]
- Chen, N.F.; Smith, Z.A.; Stiner, E.; Armin, S.; Sheikh, H.; Khoo, L.T. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J. Neurosurg. Spine 2010, 12, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, H. High-dose bone morphogenetic protein-induced ectopic abdomen bone growth. Spine J. 2010, 10, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.A.; Kumar, A.; Jatana, S.; Ghiselli, G.; Wong, K. Neurologic impairment from ectopic bone in the lumbar canal: A potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008, 8, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Than, K.D.; Rahman, S.U.; McKeever, P.E.; Wang, A.C.; La Marca, F.; Park, P. Symptomatic calcified perineural cyst after use of bone morphogenetic protein in transforaminal lumbar interbody fusion: A case report. Spine J. 2013, 13, e31–e35. [Google Scholar] [CrossRef]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, M.; Sawai, H.; Tsuji, Y.; Okamura, H.; Koyama, K. Bone morphogenetic protein-2 counterregulates interleukin-18 mRNA and protein in MC3T3-E1 mouse osteoblastic cells. Connect Tissue Res. 2006, 47, 124–132. [Google Scholar] [CrossRef]
- Lee, K.B.; Taghavi, C.E.; Song, K.J.; Sintuu, C.; Yoo, J.H.; Keorochana, G.; Tzeng, S.T.; Fei, Z.; Liao, J.C.; Wang, J.C. Inflammatory characteristics of rhBMP-2 in vitro and in an in vivo rodent model. Spine 2011, 36, E149–E154. [Google Scholar] [CrossRef]
- Shen, J.; James, A.W.; Zara, J.N.; Asatrian, G.; Khadarian, K.; Zhang, J.B.; Ho, S.; Kim, H.J.; Ting, K.; Soo, C. BMP2-induced inflammation can be suppressed by the osteoinductive growth factor NELL-1. Tissue Eng. Part A 2013, 19, 2390–2401. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.B.; Taghavi, C.E.; Murray, S.S.; Song, K.J.; Keorochana, G.; Wang, J.C. BMP induced inflammation: A comparison of rhBMP-7 and rhBMP-2. J. Orthop. Res. 2012, 30, 1985–1994. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J. Bone Jt. Surg. Am. 2013, 95, 1537–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Beachler, D.C.; Yanik, E.L.; Martin, B.I.; Pfeiffer, R.M.; Mirza, S.K.; Deyo, R.A.; Engels, E.A. Bone Morphogenetic Protein Use and Cancer Risk Among Patients Undergoing Lumbar Arthrodesis: A Case-Cohort Study Using the SEER-Medicare Database. J. Bone Jt. Surg. Am. Vol. 2016, 98, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Zhao, J.; Brochmann, E.J.; Wang, J.C.; Murray, S.S. Bone morphogenetic protein-2 and tumor growth: Diverse effects and possibilities for therapy. Cytokine Growth Factor Rev. 2017, 34, 73–91. [Google Scholar] [CrossRef]
- Huang, P.; Chen, A.; He, W.; Li, Z.; Zhang, G.; Liu, Z.; Liu, G.; Liu, X.; He, S.; Xiao, G.; et al. BMP-2 induces EMT and breast cancer stemness through Rb and CD44. Cell Death Discov. 2017, 3, 17039. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.H.; Park, H.J.; Lee, S.K. The Dual Role of Bone Morphogenetic Proteins in Cancer. Mol. Oncolytics 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kaushansky, K. Thrombopoietin: The primary regulator of megakaryocyte and platelet production. Thromb. Haemost. 1995, 74, 521–525. [Google Scholar] [CrossRef]
- Zucker-Franklin, D.; Kaushansky, K. Effect of thrombopoietin on the development of megakaryocytes and platelets: An ultrastructural analysis. Blood 1996, 88, 1632–1638. [Google Scholar] [CrossRef] [Green Version]
- Malara, A.; Abbonante, V.; Di Buduo, C.A.; Tozzi, L.; Currao, M.; Balduini, A. The secret life of a megakaryocyte: Emerging roles in bone marrow homeostasis control. Cell Mol. Life Sci. 2015, 72, 1517–1536. [Google Scholar] [CrossRef] [Green Version]
- Nakamura-Ishizu, A.; Matsumura, T.; Stumpf, P.S.; Umemoto, T.; Takizawa, H.; Takihara, Y.; O’Neil, A.; Majeed, A.; MacArthur, B.D.; Suda, T. Thrombopoietin Metabolically Primes Hematopoietic Stem Cells to Megakaryocyte-Lineage Differentiation. Cell Rep. 2018, 25, 1772–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, M.B.; Xu, L.; Childress, P.J.; Maupin, K.A.; Mohamad, S.F.; Chitteti, B.R.; Himes, E.; Olivos, D.J., 3rd; Cheng, Y.H.; Conway, S.J.; et al. Megakaryocyte and Osteoblast Interactions Modulate Bone Mass and Hematopoiesis. Stem Cells Dev. 2018, 27, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Kaushansky, K. Mplandthehematopoieticstemcell. Nat. Publ. Grou. 2002, 2, 738–739. [Google Scholar]
- Besancenot, R.; Roos-Weil, D.; Tonetti, C.; Abdelouahab, H.; Lacout, C.; Pasquier, F.; Willekens, C.; Rameau, P.; Lecluse, Y.; Micol, J.B.; et al. JAK2 and MPL protein levels determine TPO-induced megakaryocyte proliferation vs differentiation. Blood 2014, 124, 2104–2115. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, B.; Wang, S.; Zhang, J.; Liu, Y.; Wang, J.; Fan, Z.; Lv, Y.; Zhang, X.; He, L.; et al. Recombinant human thrombopoietin promotes hematopoietic reconstruction after severe whole body irradiation. Sci. Rep. 2015, 5, 12993. [Google Scholar] [CrossRef] [Green Version]
- Kacena, M.A.; Shivdasani, R.A.; Wilson, K.; Xi, Y.; Troiano, N.; Nazarian, A.; Gundberg, C.M.; Bouxsein, M.L.; Lorenzo, J.A.; Horowitz, M.C. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Kacena, M.A.; Eleniste, P.P.; Cheng, Y.H.; Huang, S.; Shivanna, M.; Meijome, T.E.; Mayo, L.D.; Bruzzaniti, A. Megakaryocytes regulate expression of Pyk2 isoforms and caspase-mediated cleavage of actin in osteoblasts. J. Biol. Chem. 2012, 287, 17257–17268. [Google Scholar] [CrossRef] [Green Version]
- Ciovacco, W.A.; Cheng, Y.H.; Horowitz, M.C.; Kacena, M.A. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J. Cell Biochem. 2010, 109, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Kwak, M.K.; Moon, S.A.; Choi, Y.J.; Baek, J.E.; Park, S.Y.; Kim, B.J.; Lee, S.H.; Koh, J.M. Regulation of bone metabolism by megakaryocytes in a paracrine manner. Sci. Rep. 2020, 10, 2277. [Google Scholar] [CrossRef] [Green Version]
- Bethel, M.; Barnes, C.L.; Taylor, A.F.; Cheng, Y.H.; Chitteti, B.R.; Horowitz, M.C.; Bruzzaniti, A.; Srour, E.F.; Kacena, M.A. A novel role for thrombopoietin in regulating osteoclast development in humans and mice. J. Cell Physiol. 2015, 230, 2142–2151. [Google Scholar] [CrossRef] [Green Version]
- Wakikawa, T.; Shioi, A.; Hino, M.; Inaba, M.; Nishizawa, Y.; Tatsumi, N.; Morii, H.; Otani, S. Thrombopoietin inhibits in vitro osteoclastogenesis from murine bone marrow cells. Endocrinology 1997, 138, 4160–4166. [Google Scholar] [CrossRef] [PubMed]
- Meijome, T.E.; Baughman, J.T.; Hooker, R.A.; Cheng, Y.H.; Ciovacco, W.A.; Balamohan, S.M.; Srinivasan, T.L.; Chitteti, B.R.; Eleniste, P.P.; Horowitz, M.C.; et al. C-Mpl Is Expressed on Osteoblasts and Osteoclasts and Is Important in Regulating Skeletal Homeostasis. J. Cell Biochem. 2016, 117, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivos, D.J., 3rd; Alvarez, M.; Cheng, Y.H.; Hooker, R.A.; Ciovacco, W.A.; Bethel, M.; McGough, H.; Yim, C.; Chitteti, B.R.; Eleniste, P.P.; et al. Lnk Deficiency Leads to TPO-Mediated Osteoclastogenesis and Increased Bone Mass Phenotype. J. Cell Biochem. 2017, 118, 2231–2240. [Google Scholar] [CrossRef]
- Brudvik, P.; Rygh, P. Multi-nucleated cells remove the main hyalinized tissue and start resorption of adjacent root surfaces. Eur. J. Orthod. 1994, 16, 265–273. [Google Scholar] [CrossRef]
- Xu, F.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prow, D.; Vadhan-Raj, S. Thrombopoietin: Biology and potential clinical applications. Oncology 1998, 12, 1597–1604. [Google Scholar]
- Shayesteh, Y.S.; Khojasteh, A.; Soleimani, M.; Alikhasi, M.; Khoshzaban, A.; Ahmadbeigi, N. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral. Surg. Oral. Med.Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Alhadlaq, A.; Mao, J.J. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J. Bone Jt. Surg. Am. Vol. 2005, 87, 936–944. [Google Scholar] [CrossRef]
- Emmakah, A.M.; Arman, H.E.; Alvarez, M.B.; Childress, P.J.; Bidwell, J.P.; Goebel, W.S.; Gabriel Chu, T.M.; Kacena, M.A. Megakaryocytes Enhance Mesenchymal Stromal Cells Proliferation and Inhibit Differentiation. J. Cell Biochem. 2017. [Google Scholar] [CrossRef] [Green Version]
- Kuter, D.J.; Mufti, G.J.; Bain, B.J.; Hasserjian, R.P.; Davis, W.; Rutstein, M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood 2009, 114, 3748–3756. [Google Scholar] [CrossRef] [Green Version]
- Hibi, H.; Yamada, Y.; Ueda, M.; Endo, Y. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int. J. Oral. Maxillofac. Surg. 2006, 35, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Marion, N.W.; Hollister, S.; Mao, J.J. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng. Part A 2009, 15, 3923–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Biomed. Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef]
- Ng, J.; Spiller, K.; Bernhard, J.; Vunjak-Novakovic, G. Biomimetic Approaches for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 480–493. [Google Scholar] [CrossRef]
- Emmakah, A.M.; Arman, H.E.; Bragg, J.C.; Greene, T.; Alvarez, M.B.; Childress, P.J.; Goebel, W.S.; Kacena, M.A.; Lin, C.C.; Chu, T.M. A fast-degrading thiol-acrylate based hydrogel for cranial regeneration. Biomed. Mater. 2017, 12, 025011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.N.; Cammisa, F.P.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The Biology of Bone Grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Chircov, C.; Miclea, I.I.; Grumezescu, V.; Grumezescu, A.M. Essential Oils for Bone Repair and Regeneration-Mechanisms and Applications. Materials 2021, 14, 1867. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Alemdar, N.; Annabi, N.; Khademhosseini, A. Oxygen Releasing Biomaterials for Tissue Engineering. Polym. Int. 2013, 62, 843–848. [Google Scholar] [CrossRef] [Green Version]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater. 2019, 11, 65. [Google Scholar] [CrossRef] [Green Version]
- Chenard, K.E.; Teven, C.M.; He, T.C.; Reid, R.R. Bone morphogenetic proteins in craniofacial surgery: Current techniques, clinical experiences, and the future of personalized stem cell therapy. J. Biomed. Biotechnol. 2012, 2012, 601549. [Google Scholar] [CrossRef] [Green Version]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied. Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Touzet, S.; Ferri, J.; Wojcik, T.; Raoul, G. Complications of Calvarial Bone Harvesting for Maxillofacial Reconstructions. J. Craniofacial. Surg. 2011, 22, 178–181. [Google Scholar] [CrossRef]
- Ross, N.; Tacconi, L.T.; Miles, J.B. Heterotopic bone formation causing recurrent donor site pain following iliac crest bone harvesting. J. Neurosurg. 1999, 14, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Metcalfe, A.; Colquitt, J.; Loveman, E.; Smith, N.A.; Royle, P.; Waugh, N. Autograft or allograft for reconstruction of anterior cruciate ligament: A health economics perspective. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1782–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Fabry, G. Allograft Versus Autograft Bone in Idiopathic Scoliosis Surgery: A Multivariate Statistical Analysis. J. Pediatric. Orthop. 1991, 11, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Verret, D.J.; Ducic, Y.; Oxford, L.; Smith, J. Hydroxyapatite cement in craniofacial reconstruction. Otolaryngol. Head Neck Surg. 2005, 133, 897–899. [Google Scholar] [CrossRef]
- Shafiei, Z.; Bigham, A.S.; Dehghani, S.N.; Torabi Nezhad, S. Fresh cortical autograft versus fresh cortical allograft effects on experimental bone healing in rabbits: Radiological, Histopathological and Biomechanical evaluation. Cell Tissue Bank. 2009, 10, 19–26. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Summers, B.N.; Eisenstein, S.M. Donor Site Pain from the Ilium. A complication of Lumbar Spine Fusion. J. Bone Jt. Surg. 1989, 710B, 677–680. [Google Scholar]
- Betz, R.R.; Lavelle, W.F.; Samdani, A.F. Bone Grafting Options in Children. Spine 2010, 35, 1648–1654. [Google Scholar] [CrossRef]
- Cypher, T.J.; Grossman, J.P. Biological Principles of Bone Graft Healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Skaggs, D.L.; Samuelson, M.A.; PAC, J.M.H.; Kay, R.M.; Tolo, V.T. Complications of Posterior Iliac Crest Bone Grafting in Spine Surgery in Children. Spine J. 2000, 25, 2400–2402. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [Green Version]
- Bronner, F.; Farach-Carson, M.C.; Mikos, A.G. Engineering of Functional Skeletal Tissues; Springer: Cham, Switzerland, 2007; Volume 3. [Google Scholar]
- Annu; Manzoor, K.; Ahmad, S.; Soundarajan, A.; Ikram, S.; Ahmed, S. Chapter 30—Chitosan Based Nanomaterials for Biomedical Applications. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 543–562. [Google Scholar]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. 3), S20–S27. [Google Scholar] [CrossRef]
- Wan, D.C.; Aalami, O.O.; Wang, Z.; Nacamuli, R.P.; Lorget, F.; Derynck, R.; Longaker, M.T. Differential gene expression between juvenile and adult dura mater: A window into what genes play a role in the regeneration of membranous bone. Plast. Reconstr. Surg. 2006, 118, 851–861. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.M.; Cooper, G.M.; Afifi, A.M.; Mooney, M.P.; Cray, J.; Rubin, J.P.; Marra, K.G.; Losee, J.E. Regenerative surgery in cranioplasty revisited: The role of adipose-derived stem cells and BMP-2. Plast. Reconstr. Surg. 2011, 128, 1053–1060. [Google Scholar] [CrossRef]
- Eppley, B.L. Alloplastic Implantation. Plast. Reconstr. Surg. 1999, 104, 1761–1783. [Google Scholar] [CrossRef]
- Mah, J.; Hung, J.; Wang, J.; Salih, E. The Efficacy of Various Alloplastic Bone Grafts on the Healing of Rat Calvarial Defects. Eur. J. Orthod. 2004, 26, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Cole, D.W.; Ginn, T.A.; Chen, G.J.; Smith, B.P.; Curl, W.W.; Martin, D.F.; Poehling, G.G. Cost comparison of anterior cruciate ligament reconstruction: Autograft versus allograft. Arthroscopy 2005, 21, 786–790. [Google Scholar] [CrossRef]
- Wasserstein, D.; Sheth, U.; Cabrera, A.; Spindler, K.P. A Systematic Review of Failed Anterior Cruciate Ligament Reconstruction With Autograft Compared With Allograft in Young Patients. Sports Health 2015, 7, 207–216. [Google Scholar] [CrossRef]
- Gosain, A.K. Biomaterials for reconstruction of the cranial vault. Plast. Reconstr. Surg. 2005, 116, 663–666. [Google Scholar] [CrossRef] [PubMed]
- DM, S. BMP-2-based Repair of Large-scale Calvarial Defects in An experimental Model: Regenerative Surgery in Cranioplasty. J. Craniofacial. Surg. 2008, 19, 1315–1322. [Google Scholar]
- Shendre, A.A.; Gattani, D.; Sayed, A.; Rajput, N.S. Alloplastic Bone Grafting Materials. Indian J. Multidiscip. Dent. 2012, 2, 573–576. [Google Scholar]
- Vangsness, C.T., Jr. Allografts: Graft Sterilization and Tissue Banking Safety Issues Graft Sterilization and Tissue Banking Safety Issues. Noyes’ Knee Disord. Surg. Rehabil. Clin. Outcomes 2010, 240–244. [Google Scholar] [CrossRef]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft Bone. The Influence of Processing on Safety and Performance. Orthop. Clin. N. Am. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Klimczak, A.; Siemionow, M. Immune responses in transplantation: Application to composite tissue allograft. Semin. Plast. Surg. 2007, 21, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Nasr, H.; Aichelmann-Reidy, M.E.; Yukna, R. Bone and bone substitutes. Periodontology 1999, 19, 74–86. [Google Scholar] [CrossRef]
- Abt, P.; Shaked, A. The Allograft Immune Response. Graft 2003, 6, 71–79. [Google Scholar] [CrossRef]
- Lomas, R.; Chandrasekar, A.; Board, T.N. Bone allograft in the U.K.: Perceptions and realities. Hip. Int. 2013, 23, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Beer, A.J.; Tauro, T.M.; Redondo, M.L.; Christian, D.R.; Cole, B.J.; Frank, R.M. Use of Allografts in Orthopaedic Surgery: Safety, Procurement, Storage, and Outcomes. Orthop. J. Sports Med. 2019, 7, 2325967119891435. [Google Scholar] [CrossRef] [Green Version]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef]
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based strategies for maxillofacial tumour therapy and bone defect regeneration. Int. J. Oral. Sci. 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Lopez, M.J. Chapter 78-Bone Grafts and Bone Replacements. In Equine Surgery, 5th ed.; Auer, J.A., Stick, J.A., Kümmerle, J.M., Prange, T., Eds.; W.B. Saunders: Philadelphia, NY, USA, 2019; pp. 1314–1326. [Google Scholar]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and mechanobiology of trabecular bone: A review. J. Biomech. Eng. 2015, 137, 0108021–01080215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.; Ren, H.; Li, A.; Liu, B.; cui, C.; Dong, Y.; Tian, Y.; Qiu, D. Novel bioactive glass based injectable bone cement with improved osteoinductivity and its in vivo evaluation. Sci. Rep. 2017, 7, 3622. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nomani, A.; Patel, N.; Nouri, F.S.; Hatefi, A. Bioengineering a non-genotoxic vector for genetic modification of mesenchymal stem cells. Biomaterials 2018, 152, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yoon, D.M.; Spicer, P.P.; Henslee, A.M.; Scott, D.W.; Wong, M.E.; Kasper, F.K.; Mikos, A.G. Characterization of porous polymethylmethacrylate space maintainers for craniofacial reconstruction. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 813–825. [Google Scholar] [CrossRef]
- Dumas, J.E.; Prieto, E.M.; Zienkiewicz, K.J.; Guda, T.; Wenke, J.C.; Bible, J.; Holt, G.E.; Guelcher, S.A. Balancing the rates of new bone formation and polymer degradation enhances healing of weight-bearing allograft/polyurethane composites in rabbit femoral defects. Tissue Eng. Part A 2014, 20, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Vadori, M.; Cozzi, E. The immunological barriers to xenotransplantation. Tissue Antigens 2015, 86, 239–253. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef] [Green Version]
- Bruens, M.L.; Pieterman, H.; de Wijn, J.R.; Vaandrager, J.M. Porous polymethylmethacrylate as bone substitute in the craniofacial area. J. Craniofac. Surg. 2003, 14, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.C.; Krieghoff, J.; Mikos, A.G. Chapter 33—Synthetic Polymers. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 559–590. [Google Scholar]
- Gomez, E.; Martin, M.; Arias, J.; Carceller, F. Clinical applications of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children: Our experience at Hospital La Paz since 2001. J. Oral. Maxillofac. Surg. 2005, 63, 8–14. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O.; Milam, S.B. Reconstruction of bone using calcium phosphate bone cements: A critical review. J. Oral. Maxillofac. Surg. 1999, 57, 1122–1126. [Google Scholar] [CrossRef]
- Li, Z.J.; Lu, C.T.; Feng, Z.Q.; Zhao, Q.T.; Zhou, Z.Y.; Lai, R.F. Antigen-extracted xenogeneic cancellous bone graft with recombinant human bone morphogenetic protein-2 enhances bone regeneration in repair of mandibular defect in rabbits. Kaohsiung J. Med. Sci. 2015, 31, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lin, Z.; Duan, Y.; Shu, X.; Jin, A.; Min, S.; Yi, W. Repair of large segmental bone defects in rabbits using BMP and FGF composite xenogeneic bone. Genet. Mol. Res. 2015, 14, 6395–6400. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Schmitz, J.P.; Mark, D.E.; Seyfer, A.E. Osseous wound healing with xenogeneic bone implants with a biodegradable carrier. Surgery 1990, 107, 50–54. [Google Scholar]
- Chen, W.C.; Ju, C.P.; Wang, J.C.; Hung, C.C.; Chern Lin, J.H. Brittle and ductile adjustable cement derived from calcium phosphate cement/polyacrylic acid composites. Dent. Mater. 2008, 24, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef]
- Vordemvenne, T.; Wähnert, D.; Koettnitz, J.; Merten, M.; Fokin, N.; Becker, A.; Büker, B.; Vogel, A.; Kronenberg, D.; Stange, R.; et al. Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography. Cells 2020, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem cell-based bone and dental regeneration: A view of microenvironmental modulation. Int. J. Oral. Sci. 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.Q.; Kannan, T.P.; Ahmad, A.; Samsudin, A.R. In vitro genotoxicity tests for polyhydroxybutyrate—A synthetic biomaterial. Toxicol. Vitro 2008, 22, 57–67. [Google Scholar] [CrossRef]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Chabalenge, B.; Mwila, C.; Kalungia, A.C.; Nkanga, C.I.; Bapolisi, A.M.; Walker, R.B. Biocompatibility of Biomaterials for Nanoencapsulation: Current Approaches. Nanomaterials 2020, 10, 1649. [Google Scholar] [CrossRef]
- Ivanova, D.G.; Yaneva, Z.L. Antioxidant Properties and Redox-Modulating Activity of Chitosan and Its Derivatives: Biomaterials with Application in Cancer Therapy. Biores. Open Access 2020, 9, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Q.; Fan, C.; Xu, S. Pediatric mesenchymal stromal cells therapy: An update. Minerva Pediatr. 2018, 70, 396–402. [Google Scholar] [CrossRef]
- Shi, M.; Kretlow, J.D.; Spicer, P.P.; Tabata, Y.; Demian, N.; Wong, M.E.; Kasper, F.K.; Mikos, A.G. Antibiotic-releasing porous polymethylmethacrylate/gelatin/antibiotic constructs for craniofacial tissue engineering. J. Control Release 2011, 152, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuhashi, K.; Ono, W.; Matsushita, Y.; Sakagami, N.; Takahashi, A.; Saunders, T.L.; Nagasawa, T.; Kronenberg, H.M.; Ono, N. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature 2018, 563, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 462–482. [Google Scholar]
- Khojasteh, A.; Eslaminejad, M.B.; Nazarian, H. Mesenchymal stem cells enhance bone regeneration in rat calvarial critical size defects more than platelete-rich plasma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 356–362. [Google Scholar] [CrossRef]
- Geffers, M.; Groll, J.; Gbureck, U. Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements. Materials 2015, 8, 2700–2717. [Google Scholar] [CrossRef] [Green Version]
- Wähnert, D.; Koettnitz, J.; Merten, M.; Kronenberg, D.; Stange, R.; Greiner, J.F.W.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Spongostan(™) Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone(®) and Actifuse. Materials 2021, 14, 1961. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Das, B. In vitro Culture of Naïve Human Bone Marrow Mesenchymal Stem Cells: A Stemness Based Approach. Front. Cell Dev. Biol. 2017, 5, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madl, C.M.; Heilshorn, S.C.; Blau, H.M. Bioengineering strategies to accelerate stem cell therapeutics. Nature 2018, 557, 335–342. [Google Scholar] [CrossRef]
- Jackson, L.; Jones, D.R.; Scotting, P.; Sottile, V. Adult mesenchymal stem cells: Differentiation potential and therapeutic applications. J. Postgrad. Med. 2007, 53, 121–127. [Google Scholar] [PubMed]
- Herrmann, R.P.; Sturm, M.J. Adult human mesenchymal stromal cells and the treatment of graft versus host disease. Stem Cells Cloning 2014, 7, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Bentivegna, A.; Roversi, G.; Riva, G.; Paoletta, L.; Redaelli, S.; Miloso, M.; Tredici, G.; Dalprà, L. The Effect of Culture on Human Bone Marrow Mesenchymal Stem Cells: Focus on DNA Methylation Profiles. Stem Cells Int. 2016, 2016, 5656701. [Google Scholar] [CrossRef] [Green Version]
- Ridzuan, N.; Al Abbar, A.; Yip, W.K.; Maqbool, M.; Ramasamy, R. Characterization and Expression of Senescence Marker in Prolonged Passages of Rat Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 8487264. [Google Scholar] [CrossRef] [Green Version]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Longaker, M.T.; Chan, C.K.F. A Revised Perspective of Skeletal Stem Cell Biology. Front. Cell Dev. Biol. 2019, 7, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knopf, F.; Hammond, C.; Chekuru, A.; Kurth, T.; Hans, S.; Weber, C.W.; Mahatma, G.; Fisher, S.; Brand, M.; Schulte-Merker, S.; et al. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev. Cell 2011, 20, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal. Implant. Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; Morente-López, M.; Arufe, M.C. Effect of aging on behaviour of mesenchymal stem cells. World J. Stem Cells 2019, 11, 337–346. [Google Scholar] [CrossRef]
- Gyöngyösi, M.; Blanco, J.; Marian, T.; Trón, L.; Petneházy, O.; Petrasi, Z.; Hemetsberger, R.; Rodriguez, J.; Font, G.; Pavo, I.J.; et al. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ. Cardiovasc. Imaging 2008, 1, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Caplan, A.I. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [Green Version]
- Mauney, J.R.; Volloch, V.; Kaplan, D.L. Role of adult mesenchymal stem cells in bone tissue engineering applications: Current status and future prospects. Tissue Eng. 2005, 11, 787–802. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Zhang, X.; Qian, H.; Zhu, W.; Sun, X.; Hu, J.; Zhou, H.; Chen, Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp. Biol. Med. 2004, 229, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Thanh, V.V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Yeo, G.C.; Weiss, A.S. Soluble matrix protein is a potent modulator of mesenchymal stem cell performance. Proc. Natl. Acad. Sci. USA 2019, 116, 2042–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ercal, P.; Pekozer, G.G.; Kose, G.T. Dental Stem Cells in Bone Tissue Engineering: Current Overview and Challenges. Adv. Exp. Med. Biol. 2018, 1107, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Mendi, A.; Ulutürk, H.; Ataç, M.S.; Yılmaz, D. Stem Cells for the Oromaxillofacial Area: Could they be a promising source for regeneration in dentistry? Adv. Exp. Med. Biol. 2019, 1144, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Han, D.S.; Chang, H.K.; Kim, K.R.; Woo, S.M. Consideration of bone regeneration effect of stem cells: Comparison of bone regeneration between bone marrow stem cells and adipose-derived stem cells. J. Craniofac. Surg. 2014, 25, 196–201. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Dou, H.; Tian, B.; Li, L.; Jin, L.; Zhang, Z.; Hu, L. Bone regeneration capacities of alveolar bone mesenchymal stem cells sheet in rabbit calvarial bone defect. J. Tissue Eng. 2020, 11, 2041731420930379. [Google Scholar] [CrossRef] [PubMed]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; De Rosa, A.; Papaccio, G.; Tirino, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, X.; Wang, W.E.; Zeng, C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Stem Cells Int. 2016, 2016, 9682757. [Google Scholar] [CrossRef] [Green Version]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganathan, K.; Lakshminarayanan, V. Stem cells of the dental pulp. Indian J. Dent. Res. 2012, 23, 558. [Google Scholar] [CrossRef] [PubMed]
- Kress, S.; Neumann, A.; Weyand, B.; Kasper, C. Stem cell differentiation depending on different surfaces. Adv. Biochem. Eng. Biotechnol 2012, 126, 263–283. [Google Scholar] [CrossRef]
- Baghaei, K.; Hashemi, S.M.; Tokhanbigli, S.; Rad, A.A.; Assadzadeh-Aghdaei, H.; Sharifian, A.; Zali, M.R. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol. Hepatol. Bed Bench 2017, 10, 208–213. [Google Scholar]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Zaim, M.; Karaman, S.; Cetin, G.; Isik, S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann. Hematol. 2012, 91, 1175–1186. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, R.-X.; He, X.-T.; Xu, X.-Y.; Wang, J.; Chen, F.-M. Influences of age-related changes in mesenchymal stem cells on macrophages during in-vitro culture. Stem Cell Res. Ther. 2017, 8, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnani, D.; Crippa, S.; Della Volpe, L.; Rossella, V.; Conti, A.; Lettera, E.; Rivis, S.; Ometti, M.; Fraschini, G.; Bernardo, M.E.; et al. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell 2019, 18, e12933. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Lee, M.O.; Moon, B.H.; Shim, S.H.; Fornace, A.J., Jr.; Cha, H.J. Senescent growth arrest in mesenchymal stem cells is bypassed by Wip1-mediated downregulation of intrinsic stress signaling pathways. Stem Cells 2009, 27, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Chen, E.; Pan, Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020, 382, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef] [PubMed]
- McGinley, L.M.; McMahon, J.; Stocca, A.; Duffy, A.; Flynn, A.; O’Toole, D.; O’Brien, T. Mesenchymal stem cell survival in the infarcted heart is enhanced by lentivirus vector-mediated heat shock protein 27 expression. Hum. Gene 2013, 24, 840–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsässer, A.; Suzuki, K.; Lorenz-Meyer, S.; Bode, C.; Schaper, J. The role of apoptosis in myocardial ischemia: A critical appraisal. Basic Res. Cardiol. 2001, 96, 219–226. [Google Scholar] [CrossRef]
- Chang, S.C.; Chuang, H.; Chen, Y.R.; Yang, L.C.; Chen, J.K.; Mardini, S.; Chung, H.Y.; Lu, Y.L.; Ma, W.C.; Lou, J. Cranial repair using BMP-2 gene engineered bone marrow stromal cells. J. Surg. Res. 2004, 119, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Young, F.; Sloan, A.; Song, B. Dental pulp stem cells and their potential roles in central nervous system regeneration and repair. J. Neurosci. Res. 2013, 91, 1383–1393. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Alencar, A.H.; Kitten, G.T.; Vencio, E.F.; Gava, E. Mesenchymal stem cells in the dental tissues: Perspectives for tissue regeneration. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Gao, L.N.; An, Y.; Hu, C.H.; Jin, F.; Zhou, J.; Jin, Y.; Chen, F.M. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 2013, 34, 7033–7047. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Q.; Yuan, Q.; Zhang, S. Spatial Distributions, Characteristics, and Applications of Craniofacial Stem Cells. Stem Cells Int. 2020, 2020, 8868593. [Google Scholar] [CrossRef]
- Takaoka, K.; Koezuka, M.; Nakahara, H. Telopeptide-depleted bovine skin collagen as a carrier for bone morphogenetic protein. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1991, 9, 902–907. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, P.T. Dental mesenchymal stem cells. Development 2016, 143, 2273–2280. [Google Scholar] [CrossRef] [Green Version]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T.; Sonoyama, W.; Chen, J.; Park, S.H. In vitro characterization of human dental pulp cells: Various isolation methods and culturing environments. Cell Tissue Res. 2006, 324, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Sasaki, R.; Aoki, S.; Yamato, M.; Uchiyama, H.; Wada, K.; Okano, T.; Ogiuchi, H. Neurosphere generation from dental pulp of adult rat incisor. Eur. J. Neurosci. 2008, 27, 538–548. [Google Scholar] [CrossRef]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Deng, Z.; Tang, L.; Li, Y.; Shi, J.; Jin, Y. Odontogenic capability: Bone marrow stromal stem cells versus dental pulp stem cells. Biol. Cell 2007, 99, 465–474. [Google Scholar] [CrossRef]
- Huang, G.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Jiang, L.; Li, Z.; Lee, S.; Liu, C.; Wang, J.; Zhang, J. Recombinant human BMP-2 accelerates the migration of bone marrow mesenchymal stem cells via the CDC42/PAK1/LIMK1 pathway in vitro and in vivo. Biomater. Sci. 2018, 7, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Riccio, M.; Pisciotta, A.; Zavatti, M.; Carnevale, G.; Beretti, F.; La Sala, G.B.; Motta, A.; De Pol, A. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res. 2013, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- De Mendonca Costa, A.; Bueno, D.F.; Martins, M.T.; Kerkis, I.; Kerkis, A.; Fanganiello, R.D.; Cerruti, H.; Alonso, N.; Passos-Bueno, M.R. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J. Craniofac. Surg. 2008, 19, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Beretti, F.; Gibellini, L.; Maraldi, T.; Cavallini, G.M.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human serum promotes osteogenic differentiation of human dental pulp stem cells in vitro and in vivo. PLoS ONE 2012, 7, e50542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.C.; E, L.L.; Wang, D.S.; Su, F.; Wu, X.; Shi, Z.P.; Lv, Y.; Wang, J.Z. Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide). Tissue Eng. Part A 2011, 17, 2417–2433. [Google Scholar] [CrossRef] [PubMed]
- Alkaisi, A.; Ismail, A.R.; Mutum, S.S.; Ahmad, Z.A.; Masudi, S.; Abd Razak, N.H. Transplantation of human dental pulp stem cells: Enhance bone consolidation in mandibular distraction osteogenesis. J. Oral. Maxillofac. Surg. 2013, 71, 1758–e1751. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Fukui, M.; Nakagawa, H.; Fujii, T.; Akino, K. Cranial bone defect healing is accelerated by mesenchymal stem cells induced by coadministration of bone morphogenetic protein-2 and basic fibroblast growth factor. Wound Repair. Regen. 2004, 12, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Chen, F.; Yang, Y.; Cheng, X.; Gao, Z.; Yang, H.O.; Wu, W.; Mao, T. Comparative study between coral-mesenchymal stem cells-rhBMP-2 composite and auto-bone-graft in rabbit critical-sized cranial defect model. J. Biomed. Mater. Res. A 2007, 80, 85–93. [Google Scholar] [CrossRef]
- Inoda, H.; Yamamoto, G.; Hattori, T. rh-BMP2-induced ectopic bone for grafting critical size defects: A preliminary histological evaluation in rat calvariae. Int. J. Oral. Maxillofac. Surg. 2007, 36, 39–44. [Google Scholar] [CrossRef]
- Springer, I.N.; Acil, Y.; Kuchenbecker, S.; Bolte, H.; Warnke, P.H.; Abboud, M.; Wiltfang, J.; Terheyden, H. Bone graft versus BMP-7 in a critical size defect–cranioplasty in a growing infant model. Bone 2005, 37, 563–569. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is I PRP and What Is Not PRP? Implant. Dent. 2001, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrior, E.; Ladner, K. Platelet gels and hemostasis in facial plastic surgery. Facial. Plast. Surg. 2011, 27, 308–314. [Google Scholar] [CrossRef]
- Bhanot, S.; Alex, J.C. Current applications of platelet gels in facial plastic surgery. Facial. Plast. Surg. 2002, 18, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, T.W.; Einhorn, T.A. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev. 2009, 20, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett. 2009, 31, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Cao, X. BMP signaling in skeletal development. Biochem. Biophys. Res. Commun. 2005, 328, 651–657. [Google Scholar] [CrossRef]
- Liu, H.W.; Chen, C.H.; Tsai, C.L.; Lin, I.H.; Hsiue, G.H. Heterobifunctional poly(ethylene glycol)-tethered bone morphogenetic protein-2-stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Eng. 2007, 13, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Woo, E.J. Reply: A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 804–805. [Google Scholar] [CrossRef] [PubMed]
- Even, J.; Eskander, M.; Kang, J. Bone morphogenetic protein in spine surgery: Current and future uses. J. Am. Acad. Orthop. Surg. 2012, 20, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral. Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.P.; Freymannx, U.; Vetterlein, S.; Neumann, K.; Endres, M.; Kaps, C. Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus. Med. Hemother. 2013, 40, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Gudas, R.; Kalesinskas, R.J.; Kimtys, V.; Stankevicius, E.; Toliusis, V.; Bernotavicius, G.; Smailys, A. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy 2005, 21, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B. PRP as a new approach to prevent infection: Preparation and in vitro antimicrobial properties of PRP. J. Vis. Exp. 2013, 50351. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Avila, G. Platelet Rich Plasma: Myth or Reality? Eur. J. Dent. 2007, 1, 192–194. [Google Scholar] [CrossRef] [Green Version]
- Landesberg, R.; Moses, M.; Karpatkin, M. Risks of using platelet rich plasma gel. J. Oral. Maxillofac. Surg. 1998, 56, 1116–1117. [Google Scholar] [CrossRef]
- Oley, M.C.; Islam, A.A.; Hatta, M.; Hardjo, M.; Nirmalasari, L.; Rendy, L.; Ana, I.D.; Bachtiar, I. Effects of platelet-rich plasma and carbonated hydroxyapatite combination on cranial defect Bone Regeneration: An animal study. Wound Med. 2018, 21, 12–15. [Google Scholar] [CrossRef]
- Xie, H.; Cao, L.; Ye, L.; Du, J.; Shan, G.; Hu, J.; Jiang, C.; Song, W. Autogenous bone particles combined with platelet-rich plasma can stimulate bone regeneration in rabbits. Exp. Med. 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J. Biology and Chemistry of Thrombopoietic Agents. Semin. Hematol. 2010, 47, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokemueller, H.; Spalthoff, S.; Nolff, M.; Tavassol, F.; Essig, H.; Stuehmer, C.; Bormann, K.-H.; Rücker, M.; Gellrich, N.-C. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: Experimental pilot study in sheep and first clinical application. Int. Assoc. Oral. Maxillofac. Surg. 2010, 39, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Evangelista, M.L.; Amadori, S. Novel thrombopoietic agents: A review of their use in idiopathic thrombocytopenic purpura. Drugs 2008, 68, 901–912. [Google Scholar] [CrossRef]
- Stasi, R.; Bosworth, J.; Rhodes, E.; Shannon, M.S.; Willis, F.; Gordon-Smith, E.C. Thrombopoietic agents. Blood Rev. 2010, 24, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Saito, H. Megakaryocytic cell lines. Baillieres. Clin. Haematol. 1997, 10, 47–63. [Google Scholar] [CrossRef]
- Ripamonti, U.; Ma, S.; Cunningham, N.S.; Yeates, L.; Reddi, A.H. Initiation of Bone Regeneration in Adult Baboons by Osteogenin, a Bone Morphogenetic Protein. Matrix 1992, 12, 369–380. [Google Scholar] [CrossRef]
- Pang, E.K.; Im, S.U.; Kim, C.S.; Choi, S.H.; Chai, J.K.; Kim, C.K.; Han, S.B.; Cho, K.S. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J. Periodontol. 2004, 75, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, I.S.; Kaushansky, K. Thrombopoietin from beginning to end. Br. J. Haematol. 2014, 165, 259–268. [Google Scholar] [CrossRef]
- Ripamonti, U.; Herbst, N.N.; Ramoshebi, L.N. Bone morphogenetic proteins in craniofacial and periodontal tissue engineering: Experimental studies in the non-human primate Papio ursinus. Cytokine Growth Factor Rev. 2005, 16, 357–368. [Google Scholar] [CrossRef]
- Ekanayake, S.; Hall, B.K. The in vivo and in vitro effects of bone morphogenetic protein-2 on the development of the chick mandible. Int. J. Dev. Biol. 1997, 41, 67–81. [Google Scholar]
- Cardier JE, D.J. Thrombopoietin and its receptor, c-mpl, are constitutively expressed by mouse liver endothelial cells: Evidence of thrombopoietin as a growth factor for liver endothelial cells. Blood 1998, 91, 923–929. [Google Scholar] [CrossRef] [Green Version]
- Haddad, A.J.; Peel, S.A.; Clokie, C.M.; Sandor, G.K. Closure of rabbit calvarial critical-sized defects using protective composite allogeneic and alloplastic bone substitutes. J. Craniofac. Surg. 2006, 17, 926–934. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Zhao, H.Y.; Jing, X.B.; Qin, Q.L.; Gu, J.J.; Tian, N.; Huang, D.P. Reconstruction of orbital floor defect with polylacticglycolide acid/recombinant human bone morphogenetic protein 2 compound implanted material in sheep. Zhonghua Yan Ke Za Zhi 2006, 42, 535–539. [Google Scholar]
- Ji, W.; Wang, H.; van den Beucken, J.J.; Yang, F.; Walboomers, X.F.; Leeuwenburgh, S.; Jansen, J.A. Local delivery of small and large biomolecules in craniomaxillofacial bone. Adv. Drug Deliv. Rev. 2012, 64, 1152–1164. [Google Scholar] [CrossRef]
- Fu, Y.; Du, L.; Wang, Q.; Liao, W.; Jin, Y.; Dong, A.; Chen, C.; Li, Z. In vitro sustained release of recombinant human bone morphogenetic protein-2 microspheres embedded in thermosensitive hydrogels. Pharmazie 2012, 67, 299–303. [Google Scholar]
- Cheng, T.L.; Murphy, C.M.; Cantrill, L.C.; Mikulec, K.; Carpenter, C.; Schindeler, A.; Little, D.G. Local delivery of recombinant human bone morphogenetic proteins and bisphosphonate via sucrose acetate isobutyrate can prevent femoral head collapse in Legg-Calve-Perthes disease: A pilot study in pigs. Int. Orthop. 2014, 38, 1527–1533. [Google Scholar] [CrossRef] [Green Version]
- Koo, K.H.; Ahn, J.M.; Lee, J.M.; Kim, B.S.; Kim, C.S.; Im, G.I. Apatite-Coated Collagen Sponge for the Delivery of Bone Morphogenetic Protein-2 in Rabbit Posterolateral Lumbar Fusion. Artif. Organs 2014, 38, 893–899. [Google Scholar] [CrossRef]
- Jelic, M.; Pecina, M.; Haspl, M.; Kos, J.; Taylor, K.; Maticic, D.; McCartney, J.; Yin, S.; Rueger, D.; Vukicevic, S. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth. Factors 2001, 19, 101–113. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Ni, Y.; Yan, X.; Lu, Q.; Xu, H.; Cheng, Q.; Liu, K. Bone morphogenetic protein-2 stimulation of cartilage regeneration in canine tracheal graft. J. Heart Lung. Transpl. 2009, 28, 285–289. [Google Scholar] [CrossRef]
- Aspenberg, P.; Forslund, C. Bone morphogenetic proteins and tendon repair. Scand. J. Med. Sci. Sports 2000, 10, 372–375. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Kim, H.M.; Silva, M.J.; Ntouvali, E.; Manning, C.N.; Potter, R.; Seeherman, H.; Gelberman, R.H. Effect of bone morphogenetic protein 2 on tendon-to-bone healing in a canine flexor tendon model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 1702–1709. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J.; Debiais, F.; Haÿ, E. Regulation of human cranial osteoblast phenotype by FGF-2, FGFR-2 and BMP-2 signaling. Histol. Histopathol. 2002, 17, 877–885. [Google Scholar] [CrossRef]
- Pai, S.R.; Bird, R.C. c-fos expression is required during all phases of the cell cycle during exponential cell proliferation. Anticancer Res. 1994, 14, 985–994. [Google Scholar]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Jt. Surg. Am. Vol. 2008, 90, 48–54. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Nakamura, K.; Tabata, Y.; Ikada, Y.; Aoyama, I.; Anzai, J.; Nakamura, T.; Hiyama, Y.; Tamura, M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J. Clin. Endocrinol. Metab. 2001, 86, 875–880. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Oka, H.; Jingushi, S.; Izumi, T.; Fukunaga, M.; Sato, K.; Matsushita, T.; Nakamura, K. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010, 25, 2735–2743. [Google Scholar] [CrossRef]
- Zeitelhofer, M.; Li, H.; Adzemovic, M.Z.; Nilsson, I.; Muhl, L.; Scott, A.M.; Eriksson, U. Preclinical toxicological assessment of a novel monoclonal antibody targeting human platelet-derived growth factor CC (PDGF-CC) in PDGF-CChum mice. PLoS ONE 2018, 13, e0200649. [Google Scholar] [CrossRef]
- Peng, H.; Wright, V.; Usas, A.; Gearhart, B.; Shen, H.C.; Cummins, J.; Huard, J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Investig. 2002, 110, 751–759. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, M.; Shi, P.; Gao, Y.; Ma, J.; Li, Y.; Huang, L.; Yang, Z.; Yang, L. Polydopamine-modified collagen sponge scaffold as a novel dermal regeneration template with sustained release of platelet-rich plasma to accelerate skin repair: A one-step strategy. Bioact. Mater. 2021, 6, 2613–2628. [Google Scholar] [CrossRef] [PubMed]
- Nikolidakis, D. Effect of Biological Factors on Bone Healing in Implant Dentistry; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2013; p. 104. [Google Scholar]
- Uggeri, J.; Belletti, S.; Guizzardi, S.; Poli, T.; Cantarelli, S.; Scandroglio, R.; Gatti, R. Dose-dependent effects of platelet gel releasate on activities of human osteoblasts. J. Periodontol. 2007, 78, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Lakkaraja, M. Thrombopoietic agents: There is still much to learn. Presse Med. 2014, 43, e69–e78. [Google Scholar] [CrossRef]
- Kuter, D.J. Thrombopoietin: Biology and Clinical Applications. Oncologist 1996, 1, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Kuter, D.J. New thrombopoietic growth factors. Blood 2007, 109, 4607–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, O.; Schlué, J.; Mengel, M.; Büsche, G.; Serinsöz, E.; Kreipe, H. Thrombopoietin receptor (Mpl) expression by megakaryocytes in myeloproliferative disorders. J. Pathol. 2004, 203, 609–615. [Google Scholar] [CrossRef]
- Schmelzer, E.; Deiwick, A.; Bruns, H.; Fiegel, H.C.; Bader, A. Thrombopoietin is a growth factor for rat hepatic progenitors. Eur. J. Gastroenterol. Hepatol. 2008, 20, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.E.; Su, J.; Koprowski, S.; Dhanasekaran, A.; Aufderheide, T.P.; Gross, G.J. Thrombopoietin receptor agonists protect human cardiac myocytes from injury by activation of cell survival pathways. J. Pharm. Exp. Ther. 2015, 352, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Elagib, K.E.; Brock, A.T.; Goldfarb, A.N. Megakaryocyte ontogeny: Clinical and molecular significance. Exp. Hematol. 2018, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Leysi-Derilou, Y.; Robert, A.; Duchesne, C.; Garnier, A.; Boyer, L.; Pineault, N. Polyploid megakaryocytes can complete cytokinesis. Cell Cycle 2010, 9, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Ebbe, S.; Stohlman, F., Jr. Megakaryocytopoiesis in the rat. Blood 1965, 26, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Odell, T.T., Jr.; Jackson, C.W. Polyploidy and maturation of rat megakaryocytes. Blood 1968, 32, 102–110. [Google Scholar] [CrossRef]
- Odell, T.T., Jr.; Jackson, C.W.; Friday, T.J. Megakaryocytopoiesis in rats with special reference to polyploidy. Blood 1970, 35, 775–782. [Google Scholar] [CrossRef]
- Kacena, M.A.; Nelson, T.; Clough, M.E.; Lee, S.K.; Lorenzo, J.A.; Gundberg, C.M.; Horowitz, M.C. Megakaryocyte-mediated inhibition of osteoclast development. Bone 2006, 39, 991–999. [Google Scholar] [CrossRef]
- Yan, X.Q.; Lacey, D.; Fletcher, F.; Hartley, C.; McElroy, P.; Sun, Y.; Xia, M.; Mu, S.; Saris, C.; Hill, D.; et al. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood 1995, 86, 4025–4033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, H.; Hackett, N.R.; Rafii, S.; Crystal, R.G. Thrombopoietin gene transfer-mediated enhancement of angiogenic responses to acute ischemia. Circ. Res. 2005, 97, 337–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Gao, Z.; Chen, X.P.; Zhang, H.Y.; Yang, N.; Wang, F.Y.; Guan, L.X.; Gu, Z.Y.; Zhao, S.S.; Luo, L.; et al. Efficacy and safety of thrombopoietin receptor agonists in patients with primary immune thrombocytopenia: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 39003. [Google Scholar] [CrossRef] [Green Version]
- Branehög, I.; Ridell, B.; Swolin, B.; Weinfeld, A. Megakaryocyte quantifications in relation to thrombokinetics in primary thrombocythaemia and allied diseases. Scand. J. Haematol. 1975, 15, 321–332. [Google Scholar] [CrossRef]
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New Insights Into the Differentiation of Megakaryocytes From Hematopoietic Progenitors. Arter. Thromb. Vasc. Biol. 2019, 39, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
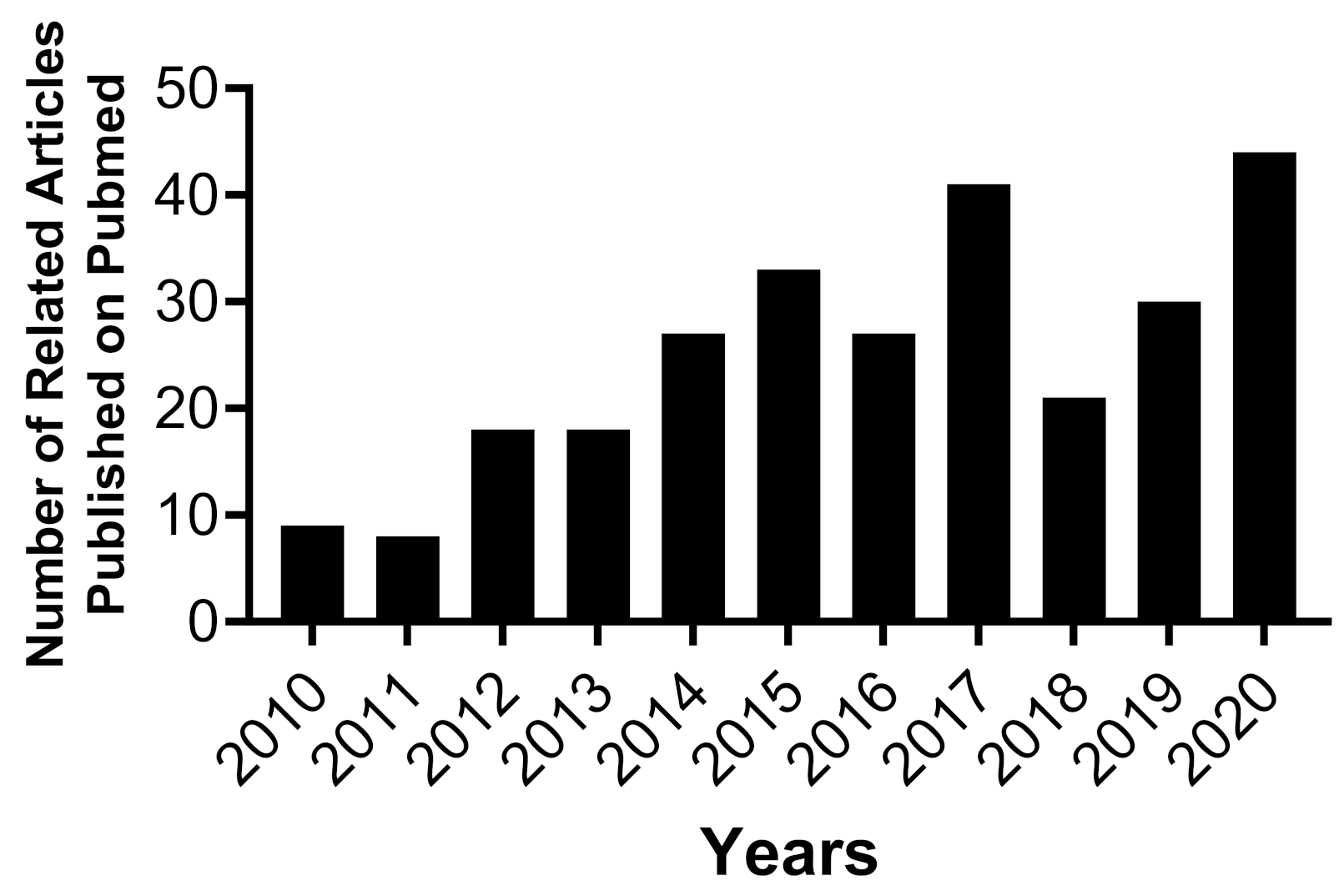




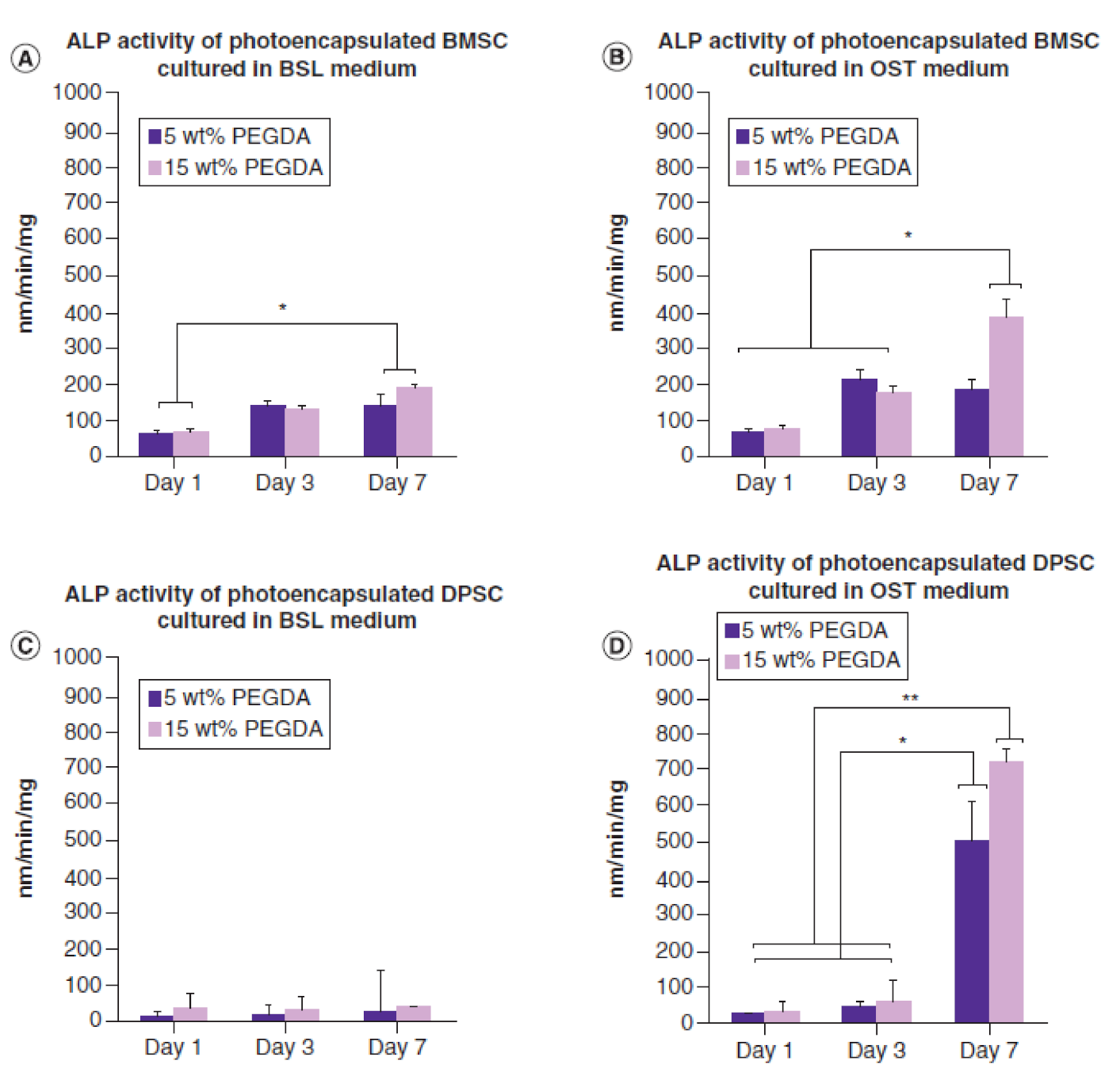




| Bone Grafting | Descriptions | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Autogenous Bone Grafting | Bone graft harvested from patient’s skeleton. | Highly biocompatible. Easy to integrate with surrounding tissues. Inducing osteogenesis, osteoinduction, and osteoconduction. Minimal potential risk of infection. Minimal potential risk of disease transmission. Available immediately for patients. | Requires two surgical procedures. Causes local pain at donor site. Extends patients’ recovery time. Requires long time to full recovery. Can leave a scar at harvested site. Patients may require taking medications for faster recovery. Low cost-effective. | [147,152,158,159,161,166,167] |
| Allogeneic Bone Grafting | Harvested from a donor of same species. | Ability to harvest similar bone tissue to lost bone tissue. Does not require a second surgery site. Eliminates donor site morbidity. Minimize requirements for patients to stay in the hospital for a long time to recover once donor match is found. | High risk of infection and transmitting viruses. Hard to find matching donor. Takes longer time to incorporate with the surrounding native tissue and prolonged healing. Losing mechanical strength during preparations, cleaning, and sterilization processes. | [134,155,166,168,169,170,171] |
| Xenogeneic Bone Grafting | Harvested from different species such as animals and plants. | Reduces potential risk of infection. Reduces potential risk of human disease transmission. No second surgery is required at the site. Unlimited supply. | Potential risk of transmitting diseases from different species such as animals and plants. Potential risk of undesired immune system reaction. Losing mechanical strength due to cleaning and sterilization processes. Poor outcomes have been reported. | [149,166,172,173] |
| Alloplastic Bone Substitutes | Synthetic biomaterials such as polymer, metal, ceramic or composite biomaterials. | Biocompatible, biodegradable, and bioresorbable materials. Minimizes donor sites morbidity and pain. Minimizes potential risk of disease transmissions. Ability to adjust mechanical properties according to desirable bone tissue, shape, and location. Unlimited supply of materials. | Requires longer time for defected bone to heal. Produces less bone volume. Lower osteoinduction and osteogenesis. | [160,161,164,166,170] |
| Proteins | Descriptions | Advantages | Disadvantages | References |
|---|---|---|---|---|
| BMP-2 | A multifunctional growth factor belongs to transforming growth factors (TGF-β) superfamily. | Induces MSCs to differentiate into osteoblasts. Ability to recruit BMSCs into the defect site. Promotes new bone formation. Induces osteoinductive factors during bone healing. Indirectly enhances angiogenesis. | Has a short half-life and challenge to control dose deliveries into the defect site. Higher risk of radiculitis, ectopic bone formation, and osteolysis. Generally poor global outcomes Contraventional regarding its safety. Expensive. | [251,256,267,273,274,281,282,283,284,285] |
| Platelet-rich Plasma | Derived from whole blood. Contains multiple potent growth factors. | Enhances cell migration and proliferation. Promotes angiogenesis. Efficacy in promoting new bone tissue. Reduces risk of virus transmission (autologous). Reduces immune system reactions (autologous). | Not an osteoinductive agent. Requires bovine thrombin and calcium chloride to initiate gelation in vitro. Contains several growth factors into the defect site. May affect surrounding and untargeted tissues. High risk of infection. | [264,277,286,287,288,289,290,291,292,293] |
| TPO | Produced by liver and kidney. TPO receptor (Mpl) has been identified on several cells such as HSCs, MKs, and platelets. | Induces HSCs to differentiate into mature MKs and ultimately into platelets. Increased bone mass via induction of MKs. Enhances osteoblast proliferation and increases bone formation. Enhances angiogenesis by increasing platelet production and stimulating endothelial cell proliferation. Produce high MK count that enhances MSCs proliferation and survival rate. Produce high MK count that maintains MSCs stemness. | Hard to control dose deliveries into defect location. Produces high MK count that inhibits MSC differentiation into osteoclast lineage cells. Induces bone marrow fibrosis by increasing MKs and platelets. Produces high MK count that contributes in inhibiting osteoclastogenesis. | [112,114,117,125,294,295,296,297,298] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghali, A. Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy. Cells 2021, 10, 2993. https://doi.org/10.3390/cells10112993
Aghali A. Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy. Cells. 2021; 10(11):2993. https://doi.org/10.3390/cells10112993
Chicago/Turabian StyleAghali, Arbi. 2021. "Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy" Cells 10, no. 11: 2993. https://doi.org/10.3390/cells10112993





