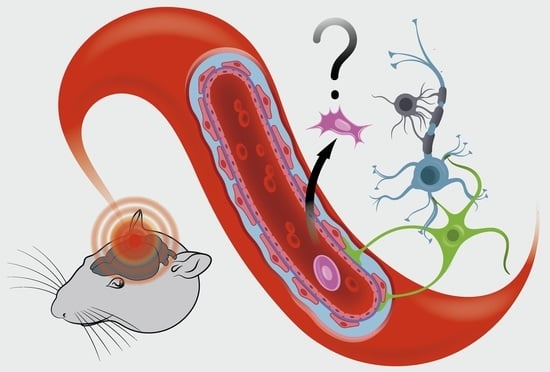
Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood–Brain Barrier?
Abstract
:1. Introduction
2. Blood–Brain Barrier in Stroke and Its Response to MSC Transplantation
3. In Vitro Studies of the MSC Transmigration through the BBB
4. Transmigration of the Intra-Arterially Transplanted MSCs cross the BBB in Experimental Stroke
5. Clinical Trials of the Intra-Arterial MSC Infusion in Patients with Ischemic Stroke
6. Conclusions
- The NVU and the BBB as its part play an important role in the pathogenesis of stroke and during the post-stroke recovery.
- Understanding the details of interactions of transplanted MSCs with all components of the BBB and the NVU is essential for deciphering the mechanisms of MSC therapeutic effects.
- The results of the reviewed in vitro experiments show the ability of MSCs to pass through the monolayer of brain endothelium and substantiate the hypothesis that the BBB can be permeable for MSCs in vivo.
- In vitro MSCs likely pass through the monolayer of endothelium by the paracellular route using transient gaps formed in response to signals induced by ischemic conditions.
- Data of the in vivo experiments on transmigration of the IA transplanted MSCs across the BBB in experimental stroke are not fully consistent. The fates of transplanted cells vary: (1) Most cells just pass-through brain capillaries and presumably to the general circulation. (2) Some cells adhere to the endotheliocytes for up to several days. (3) Other cells adhere to the endothelium, then get through the endothelial layer presumably through intercellular gaps, and home in the perivascular space for no more than several days. Probably they are later destroyed by the microglial cells. (4) Cells adhere to the endothelium, pass through the BBB, and can be found in brain parenchyma for several weeks. We did not find any confirmations of their neural differentiation.
- Transmigration across the BBB is not necessary for induction of therapeutic effects, though, of course, it may change the parameters of the therapeutic response.
- Most likely, the transitory presence of the transplanted MSCs in brain blood vessels may trigger a range of therapeutic and restorative responses through paracrine secretion of an array of biologically active molecules in free form or enclosed in extracellular vesicles.
- Immunosuppression-free transplantation of human MSCs into rat arterial systems may be an in vivo model suitable for studying paracrine effects of MSCs in stroke.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.; Thayabaranathan, T.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; Owolabi, M.; Pandian, J.; et al. Global Stroke Statistics 2019. Int. J. Stroke 2020, 15, 819–838. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke A. Stroke 2019, 50, E344–E418. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Ma, H.; Ringleb, P.A.; Parsons, M.W.; Churilov, L.; Bendszus, M.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Fatar, M.; et al. Extending thrombolysis to 4·5–9 h and wake-up stroke using perfusion imaging: A systematic review and meta-analysis of individual patient data. Lancet 2019, 394, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 h after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pandey, P.K.; Bhasin, A.; Padma, M.V.; Mohanty, S. Application of Stem Cells in Stroke: A Multifactorial Approach. Front. Neurosci. 2020, 14, 473. [Google Scholar] [CrossRef]
- Suda, S.; Nito, C.; Yokobori, S.; Sakamoto, Y.; Nakajima, M.; Sowa, K.; Obinata, H.; Sasaki, K.; Savitz, S.I.; Kimura, K. Recent Advances in Cell-Based Therapies for Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 6718. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.Z.; Lin, Y.H.; Su, L.J.; Wu, M.S.; Jeng, H.Y.; Chang, H.C.; Huang, Y.H.; Ling, T.Y. Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. J. Biomed. Sci. 2021, 28, 1–38. [Google Scholar] [CrossRef]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell. Neurosci. 2021, 15, 1–12. [Google Scholar]
- Rascón-Ramírez, F.J.; Esteban-García, N.; Barcia, J.A.; Trondin, A.; Nombela, C.; Sánchez-Sánchez-Rojas, L. Are We Ready for Cell Therapy to Treat Stroke? Front. Cell Dev. Biol. 2021, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci. 2019, 20, 2406. [Google Scholar] [CrossRef] [Green Version]
- Ichim, T.E.; Alexandrescu, D.T.; Solano, F.; Lara, F.; Campion, R.D.N.; Paris, E.; Woods, E.J.; Murphy, M.P.; Dasanu, C.A.; Patel, A.N.; et al. Mesenchymal stem cells as anti-inflammatories: Implications for treatment of Duchenne muscular dystrophy. Cell. Immunol. 2010, 260, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.-L.L.; Zhang, Y.; Li, X.; Fu, Q.-L.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and clinical applications of mesenchymal stem cells state-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahi, K.C.; Klein, G.; Avci-Adali, M.; Sievert, K.D.; Macneil, S.; Aicher, W.K. Human mesenchymal stromal cells from different sources diverge in their expression of cell surface proteins and display distinct differentiation patterns. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Harman, R.M.; Patel, R.S.; Fan, J.C.; Park, J.E.; Rosenberg, B.R.; Van de Walle, G.R. Single-cell RNA sequencing of equine mesenchymal stromal cells from primary donor-matched tissue sources reveals functional heterogeneity in immune modulation and cell motility. Stem Cell Res. Ther. 2020, 11, 524. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Thakor, A.S. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience 2019, 15, 421–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaffari-Nazari, H. The known molecules involved in MSC homing and migration. J. Stem Cell Res. Med. 2018, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Kholodenko, I.V.; Konieva, A.A.; Kholodenko, R.V.; Yarygin, K.N. Molecular mechanisms of migration and homing of intravenously transplanted mesenchymal stem cells. J. Regen. Med. Tissue Eng. 2013, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Krabbe, C.; Zimmer, J.; Meyer, M. Neural transdifferentiation of mesenchymal stem cells—A critical review. APMIS 2005, 113, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Kuo, T.K.C.; Whang-Peng, J.; Chung, Y.F.; Lin, C.T.; Chou, S.H.; Chen, J.R.; Chen, Y.P.; Lee, O.K.S. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004, 40, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Kurbatov, L.K.; Kholodenko, R.V.; Manukyan, G.V.; Yarygin, K.N. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope? Cells 2019, 8, 1127. [Google Scholar] [CrossRef] [Green Version]
- Phadnis, S.M.; Joglekar, M.V.; Dalvi, M.P.; Muthyala, S.; Nair, P.D.; Ghaskadbi, S.M.; Bhonde, R.R.; Hardikar, A.A. Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo. Cytotherapy 2011, 13, 279–293. [Google Scholar] [CrossRef]
- Choudhary, P.; Gupta, A.; Singh, S. Therapeutic Advancement in Neuronal Transdifferentiation of Mesenchymal Stromal Cells for Neurological Disorders. J. Mol. Neurosci. 2021, 71, 889–901. [Google Scholar] [CrossRef]
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 2019, 10, 1191. [Google Scholar] [CrossRef] [Green Version]
- Surugiu, R.; Olaru, A.; Hermann, D.M.; Glavan, D.; Catalin, B.; Popa-Wagner, A. Recent Advances in Mono- and Combined Stem Cell Therapies of Stroke in Animal Models and Humans. Int. J. Mol. Sci. 2019, 20, 6029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babenko, V.A.; Silachev, D.N.; Popkov, V.A.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules 2018, 23, 687. [Google Scholar] [CrossRef] [Green Version]
- Cheung, T.S.; Galleu, A.; Von Bonin, M.; Bornhäuser, M.; Dazzi, F. Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: Implications for the monitoring of mesenchymal stromal cell activity. Haematologica 2019, 104, 438–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; Von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boltze, J.; Arnold, A.; Walczak, P.; Jolkkonen, J.; Cui, L.; Wagner, D.-C. The dark side of the force–constraints and complications of cell therapies for stroke. Front. Neurol. 2015, 6, 155. [Google Scholar] [CrossRef] [Green Version]
- Guzman, R.; Janowski, M.; Walczak, P. Intra-arterial delivery of cell therapies for stroke. Stroke 2018, 49, 1075–1082. [Google Scholar] [CrossRef]
- Janowski, M.; Lyczek, A.; Engels, C.; Xu, J.; Lukomska, B.; Bulte, J.W.M.; Walczak, P. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J. Cereb. Blood Flow Metab. 2013, 33, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos-dos-Santos, A.; Rosado-de-Castro, P.H.; Lopes de Souza, S.A.; Da Costa Silva, J.; Ramos, A.B.; Rodriguez de Freitas, G.; Barbosa da Fonseca, L.M.; Gutfilen, B.; Mendez-Otero, R. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Garza, D.M.; Cantú-Rodríguez, O.G.; Jaime-Pérez, J.C.; Gutiérrez-Aguirre, C.H.; Góngora-Rivera, J.F.; Gómez-Almaguer, D. Current state and perspectives of stem cell therapy for stroke. Med. Univ. 2016, 18, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Eckert, M.A.; Riazifar, H.; Kang, D.-K.; Agalliu, D.; Zhao, W.; Liu, L.; Eckert, M.A.; Riazifar, H.; Kang, D.-K.; et al. From blood to the brain: Can systemically transplanted mesenchymal stem cells cross the blood–brain barrier? Stem Cells Int. 2013, 2013, 435093. [Google Scholar] [CrossRef]
- Hajal, C.; Le Roi, B.; Kamm, R.D.; Maoz, B.M. Biology and Models of the Blood–brain Barrier. Annu. Rev. Biomed. Eng. 2021, 23, 359–384. [Google Scholar] [CrossRef]
- Dove, A. Breaching the barrier. Nat. Biotechnol. 2008, 26, 1213–1215. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2020, 13, 1–19. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood–Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, K.E.; Witt, K.A. Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Heidari, P.; Blayney, S.; Hitomi, E.; Luby, M.; Leigh, R. Blood–brain barrier integrity of stroke patients presenting in an extended time window. BMC Neurol. 2020, 20, 54. [Google Scholar] [CrossRef]
- Horsch, A.D.; Dankbaar, J.W.; van Seeters, T.; Niesten, J.M.; Luitse, M.J.A.; Vos, P.C.; van der Schaaf, I.C.; Biessels, G.J.; van der Graaf, Y.; Kappelle, L.J.; et al. Relation between stroke severity, patient characteristics and CT-perfusion derived blood–brain barrier permeability measurements in acute ischemic stroke. Clin. Neuroradiol. 2016, 26, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Rosenberg, G.A. Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 2011, 42, 3323–3328. [Google Scholar] [CrossRef] [Green Version]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [Green Version]
- Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F.; Andjelkovic, A.V. Involvement of epigenetic mechanisms and non-coding RNAs in blood–brain barrier and neurovascular unit injury and recovery after stroke. Front. Neurosci. 2019, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Laaker, C.; Hsu, M.; Cismaru, P.; Sandor, M.; Fabry, Z. Molecular Mechanisms of Neuroimmune Crosstalk in the Pathogenesis of Stroke. Int. J. Mol. Sci. 2021, 22, 9486. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Wu, C.-C.; Chiang, Y.-H.; Hu, C.-J.; Chen, K.-Y. Mesenchymal Stem/Stromal Cell Therapy in Blood–Brain Barrier Preservation Following Ischemia: Molecular Mechanisms and Prospects. Int. J. Mol. Sci. 2021, 22, 10045. [Google Scholar] [CrossRef]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Pillai, D.R.; Dittmar, M.S.; Baldaranov, D.; Heidemann, R.M.; Henning, E.C.; Schuierer, G.; Bogdahn, U.; Schlachetzki, F. Cerebral ischemia-reperfusion injury in rats—A 3 T MRI study on biphasic blood–brain barrier opening and the dynamics of edema formation. J. Cereb. Blood Flow Metab. 2009, 29, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Durukan, A.; Marinkovic, I.; Strbian, D.; Pitkonen, M.; Pedrono, E.; Soinne, L.; Abo-Ramadan, U.; Tatlisumak, T. Post-ischemic blood–brain barrier leakage in rats: One-week follow-up by MRI. Brain Res. 2009, 1280, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, C.; Cheung, W.M.; Lin, M.H.; Chen, J.J.; Hsu, C.Y.; Chen, J.H.; Lin, T.N. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: Evaluation with contrast-enhanced magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2008, 28, 1491–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spronk, E.; Sykes, G.; Falcione, S.; Munsterman, D.; Joy, T.; Kamtchum-Tatuene, J.; Jickling, G.C. Hemorrhagic Transformation in Ischemic Stroke and the Role of Inflammation. Front. Neurol. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Khatri, R.; McKinney, A.M.; Swenson, B.; Janardhan, V. Blood–brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79, S52–S57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sladojevic, N.; Stamatovic, S.M.; Johnson, A.M.; Choi, J.; Hu, A.; Dithmer, S.; Blasig, I.E.; Keep, R.F.; Andjelkovic, A.V. Claudin-1-dependent destabilization of the blood–brain barrier in chronic stroke. J. Neurosci. 2019, 39, 743–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, H.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Zhang, L.; Morris, D.; Gregg, S.R.; Wu, Z.; Jiang, A.; Lu, M.; et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J. Cereb. Blood Flow Metab. 2008, 28, 764–771. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar]
- Ögren, J.; Irewall, A.L.; Bergström, L.; Mooe, T. Intracranial Hemorrhage after Ischemic Stroke: Incidence, Time Trends, and Predictors in a Swedish Nationwide Cohort of 196 765 Patients. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A critical role in ischemic stroke. CNS Neurosci. Ther. 2021, 27, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Chang, M.S.; Koh, S.H.; Choi, Y.K. Repair mechanisms of the neurovascular unit after ischemic stroke with a focus on vegf. Int. J. Mol. Sci. 2021, 22, 8543. [Google Scholar] [CrossRef]
- Gao, L.; Song, Z.; Mi, J.; Hou, P.; Xie, C.; Shi, J.; Li, Y.; Manaenko, A. The Effects and Underlying Mechanisms of Cell Therapy on Blood–brain Barrier Integrity After Ischemic Stroke. Curr. Neuropharmacol. 2020, 18, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Namioka, T.; Namioka, A.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Nakazaki, M.; Onodera, R.; Suzuki, J.; Sasaki, Y.; Nagahama, H.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a rat model of chronic cerebral infarction. J. Neurosurg. 2019, 131, 1289–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, L.; Huang, Y.; Mao, Y.; Wu, K.; Zhang, L.; Nan, G. Tail vein infusion of adipose-derived mesenchymal stem cell alleviated inflammatory response and improved blood brain barrier condition by suppressing endoplasmic reticulum stress in a middle cerebral artery occlusion rat model. Med. Sci. Monit. 2018, 24, 3946–3957. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, J.; Cai, J.; Qiu, Y.; Zheng, H.; Lai, X.; Sui, X.; Wang, Y.; Lu, Q.; Zhang, Y.; et al. Targeted homing of CCR2-overexpressing mesenchymal stromal cells to ischemic brain enhances post-stroke recovery partially through PRDX4-mediated blood–brain barrier preservation. Theranostics 2018, 8, 5929–5944. [Google Scholar] [CrossRef]
- Chung, T.N.; Kim, J.H.; Choi, B.Y.; Chung, S.P.; Kwon, S.W.; Suh, S.W. Adipose-Derived Mesenchymal Stem Cells Reduce Neuronal Death After Transient Global Cerebral Ischemia Through Prevention of Blood–brain Barrier Disruption and Endothelial Damage. Stem Cells Transl. Med. 2015, 4, 178–185. [Google Scholar] [CrossRef]
- Nakazaki, M.; Sasaki, M.; Kataoka-Sasaki, Y.; Oka, S.; Suzuki, J.; Sasaki, Y.; Nagahama, H.; Hashi, K.; Kocsis, J.D.; Honmou, O. Intravenous infusion of mesenchymal stem cells improves impaired cognitive function in a cerebral small vessel disease model. Neuroscience 2019, 408, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Faezi, M.; Nasseri Maleki, S.; Aboutaleb, N.; Nikougoftar, M. The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. J. Chem. Neuroanat. 2018, 94, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Savitz, S.I.; Satani, N. Mesenchymal Stem Cell Derived Extracellular Vesicles for Repairing the Neurovascular Unit after Ischemic Stroke. Cells 2021, 10, 767. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.; Tran, M.; Linninger, A.A. Dynamic regulation of aquaporin-4 water channels in neurological disorders. Croat. Med. J. 2015, 56, 401–421. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Li, Q.; Feng, Z.; Mu, D. The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia 2007, 55, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Fujimura, M.; Ma, T.; Noshita, N.; Filiz, F.; Bollen, A.W.; Chan, P.; Verkman, A.S. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med. 2000, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, Y.; Zhang, Z.; Lu, Y.; Wang, Y.; Huang, J.; Li, Y.; Chen, X.; Gu, X.; Wang, Y.; et al. Mesenchymal stem cells maintain blood–brain barrier integrity by inhibiting aquaporin-4 upregulation after cerebral ischemia. Stem Cells 2014, 32, 3150–3162. [Google Scholar] [CrossRef]
- Mezey, É.; Szalayova, I.; Hogden, C.T.; Brady, A.; Dósa, Á.; Sótonyi, P.; Palkovits, M. An immunohistochemical study of lymphatic elements in the human brain. Proc. Natl. Acad. Sci. USA 2021, 118, e2002574118. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.Y. Mesenchymal stem cells attenuate blood–brain barrier leakage after cerebral ischemia in mice. J. Neuroinflamm. 2018, 15, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.S.; Kwon, I.; Lee, B.H.; Kim, H.; Kim, J.; An, S.; Lee, O.H.; Lee, P.H.; Kim, H.O.; Namgoong, H.; et al. Effects of mesenchymal stem cell treatment on the expression of matrix metalloproteinases and angiogenesis during ischemic stroke recovery. PLoS ONE 2015, 10, e0144218. [Google Scholar] [CrossRef] [Green Version]
- Almalki, S.G.; Agrawal, D.K. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Zehendner, C.M.; White, R.; Hedrich, J.; Luhmann, H.J. A Neurovascular Blood–Brain Barrier In Vitro Model. Methods Mol. Biol. 2014, 1135, 403–413. [Google Scholar]
- Czupalla, C.J.; Liebner, S.; Devraj, K. Vitro Models of the Blood–Brain Barrier. Methods Mol. Biol. 2014, 1135, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Canfield, S.G.; Stebbins, M.J.; Morales, B.S.; Asai, S.W.; Vatine, G.D.; Svendsen, C.N.; Palecek, S.P.; Shusta, E.V. An isogenic blood–brain barrier model comprising brain endothelial cells, astrocytes, and neurons derived from human induced pluripotent stem cells. J. Neurochem. 2017, 140, 874–888. [Google Scholar] [CrossRef]
- Erickson, M.A.; Wilson, M.L.; Banks, W.A. In vitro modeling of blood–brain barrier and interface functions in neuroimmune communication. Fluids Barriers CNS 2020, 17, 1–16. [Google Scholar] [CrossRef]
- Nitzsche, F.; Müller, C.; Lukomska, B.; Jolkkonen, J.; Deten, A.; Boltze, J. Concise Review: MSC Adhesion Cascade—Insights into Homing and Transendothelial Migration. Stem Cells 2017, 35, 1446–1460. [Google Scholar] [CrossRef] [Green Version]
- Joó, F. Endothelial cells of the brain and other organ systems: Some similarities and differences. Prog. Neurobiol. 1996, 48, 255–257. [Google Scholar] [CrossRef]
- Kurmann, L.; Okoniewski, M.; Ogunshola, O.O.; Leeners, B.; Imthurn, B.; Dubey, R.K. Transcryptomic Analysis of Human Brain-Microvascular Endothelial Response to -Pericytes: Cell Orientation Defines Barrier Function. Cells 2021, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.C.D.; Rustenhoven, J.; Scotter, E.L.; Schweder, P.; Faull, R.L.M.; Park, T.I.H.; Dragunow, M. Markers for human brain pericytes and smooth muscle cells. J. Chem. Neuroanat. 2018, 92, 48–60. [Google Scholar] [CrossRef]
- Bohannon, D.G.; Long, D.; Kim, W.K. Understanding the heterogeneity of human pericyte subsets in blood–brain barrier homeostasis and neurological diseases. Cells 2021, 10, 890. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, L.; Meng, H.; Xu, Y. Abstract 137: Vascular Smooth Muscle Cell MYPT1 Deficiency Induces Phenotype Switching and Disrupts Blood–brain Barrier After Stroke. Stroke 2020, 51, 1–22. [Google Scholar] [CrossRef]
- Poittevin, M.; Lozeron, P.; Hilal, R.; Levy, B.I.; Merkulova-Rainon, T.; Kubis, N. Smooth Muscle Cell Phenotypic Switching in Stroke. Transl. Stroke Res. 2014, 5, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.; Keep, R.; Andjelkovic, A. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carman, C.V. Mechanisms for transcellular diapedesis: Probing and pathfinding by “invadosome-like protrusions”. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, H.; Fazakas, C.; Haskó, J.; Molnár, K.; Mészáros, Á.; Nyúl-Tóth, Á.; Szabó, G.; Erdélyi, F.; Ardelean, A.; Hermenean, A.; et al. Paracellular and transcellular migration of metastatic cells through the cerebral endothelium. J. Cell. Mol. Med. 2019, 23, 2619–2631. [Google Scholar] [CrossRef] [Green Version]
- Teo, G.S.L.; Ankrum, J.A.; Martinelli, R.; Boetto, S.E.; Simms, K.; Sciuto, T.E.; Dvorak, A.M.; Karp, J.M.; Carman, C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells 2012, 30, 2472–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, M.N.; Shang, D.S.; Sun, W.; Li, B.; Xu, X.; Fang, W.G.; Zhao, W.D.; Cao, L.; Chen, Y.H. Involvement of PI3K and ROCK signaling pathways in migration of bone marrow-derived mesenchymal stem cells through human brain microvascular endothelial cell monolayers. Brain Res. 2013, 1513, 1–8. [Google Scholar] [CrossRef]
- Matsushita, T.; Kibayashi, T.; Katayama, T.; Yamashita, Y.; Suzuki, S.; Kawamata, J.; Honmou, O.; Minami, M.; Shimohama, S. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci. Lett. 2011, 502, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chi, L.; Guo, L.; Liu, X.; Luo, C.; Zhang, S.; He, G. The interactions between brain microvascular endothelial cells and mesenchymal stem cells under hypoxic conditions. Microvasc. Res. 2008, 75, 59–67. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Van Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.J.; Kim, N.E.; Kwon, I.; Choi, D.; Kim, J.; Heo, J.H. Fc-saxatilin inhibits VEGF-induced permeability by regulating claudin-5 expression in human brain microvascular endothelial cells. Microvasc. Res. 2020, 128, 103953. [Google Scholar] [CrossRef]
- Eliceiri, B.P.; Paul, R.; Schwartzberg, P.L.; Hood, J.D.; Leng, J.; Cheresh, D.A. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 1999, 4, 915–924. [Google Scholar] [CrossRef]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood–brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Xu, G.; Xu, M.; Li, Q.; Qin, X. Inhibition of Src phosphorylation reduces damage to the blood–brain barrier following transient focal cerebral ischemia in rats. Int. J. Mol. Med. 2014, 34, 1473–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.L.; Nam, J.O.; Jean, C.; Lawson, C.; Walsh, C.T.; Goka, E.; Lim, S.T.; Tomar, A.; Tancioni, I.; Uryu, S.; et al. VEGF-Induced Vascular Permeability Is Mediated by FAK. Dev. Cell 2012, 22, 146–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front. Cell. Neurosci. 2016, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svedin, P.; Hagberg, H.; Sävman, K.; Zhu, C.; Mallard, C. Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. J. Neurosci. 2007, 27, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Dejonckheere, E.; Vandenbroucke, R.E.; Libert, C. Matrix metalloproteinases as drug targets in ischemia/reperfusion injury. Drug Discov. Today 2011, 16, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Jiang, X.; Paul, D.; Song, L.; Wang, X.; Pachter, J.S. Human ES-derived MSCs correct TNF-α-mediated alterations in a blood–brain barrier model. Fluids Barriers CNS 2019, 16, 18. [Google Scholar] [CrossRef]
- Deng, C.; Liu, G. The PI3K/Akt Signalling Pathway Plays Essential Roles in Mesenchymal Stem Cells. Br. Biomed. Bull. 2017, 5, 301. [Google Scholar]
- Chen, J.; Crawford, R.; Chen, C.; Xiao, Y. The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng. Part B Rev. 2013, 19, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Zhang, M.; Jing Wang, S.; Zhang, R.; Nan Zhang, L.; Cun Zhang, T. The effect of Rho/ROCK signal pathway on the migration of mesenchymal stem cells induced by IL-1β. J. Chem. Pharm. Res. 2015, 7, 760–764. [Google Scholar]
- Lin, C.Y.; Zu, C.H.; Yang, C.C.; Tsai, P.J.; Shyu, J.F.; Chen, C.P.; Weng, Z.C.; Chen, T.H.; Wang, H.S. IL-1 β-induced mesenchymal stem cell migration involves MLCK activation via PKC signaling. Cell Transplant. 2015, 24, 2011–2028. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.M.; Yuan, J.; Li, Q.; Wang, J.N.; Kong, X.; Zheng, F.; Zhang, L.; Chen, L.; Guo, L.Y.; Huang, Y.H.; et al. Acetylcholine induces mesenchymal stem cell migration via Ca 2+/PKC/ERK1/2 signal pathway. J. Cell. Biochem. 2012, 113, 2704–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Yu, H.M.; Shang, D.S.; Fang, W.G.; He, Z.Y.; Chen, Y.H. The involvement of CXCL11 in bone marrow-derived mesenchymal stem cell migration through human brain microvascular endothelial cells. Neurochem. Res. 2014, 39, 700–706. [Google Scholar] [CrossRef]
- Satani, N.; Cai, C.; Giridhar, K.; McGhiey, D.; George, S.; Parsha, K.; Nghiem, D.M.; Valenzuela, K.S.; Riecke, J.; Vahidy, F.S.; et al. World-wide efficacy of bone marrow derived mesenchymal stromal cells in preclinical ischemic stroke models: Systematic review and meta-analysis. Front. Neurol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Xie, X.-F.; Xiong, Y.-Q.; Liu, S.-M.; Hu, G.-Z.; Cao, W.-F.; Wu, X.-M. Comparisons of the therapeutic effects of three different routes of bone marrow mesenchymal stem cell transplantation in cerebral ischemic rats. Brain Res. 2018, 1680, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [Green Version]
- Leibacher, J.; Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells Mesenchymal Stem/Stromal Cells-An update. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Argibay, B.; Trekker, J.; Himmelreich, U.; Beiras, A.; Topete, A.; Taboada, P.; Pérez-Mato, M.; Vieites-Prado, A.; Iglesias-Rey, R.; Rivas, J.; et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci. Rep. 2017, 7, 40758. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; Zhang, J.; Gilad, A.A.; Kedziorek, D.A.; Ruiz-Cabello, J.; Young, R.G.; Pittenger, M.F.; Van Zijl, P.C.M.; Huang, J.; Bulte, J.W.M. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke 2008, 39, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Mitkari, B.; Kerkelä, E.; Nystedt, J.; Korhonen, M.; Mikkonen, V.; Huhtala, T.; Jolkkonen, J. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp. Neurol. 2013, 239, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Oja, S.; Komulainen, P.; Penttilä, A.; Nystedt, J.; Korhonen, M. Automated image analysis detects aging in clinical-grade mesenchymal stromal cell cultures. Stem Cell Res. Ther. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.H.; Wu, Y. The Size of Mesenchymal Stem Cells is a Significant Cause of Vascular Obstructions and Stroke. Stem Cell Rev. Rep. 2014, 10, 295–303. [Google Scholar] [CrossRef]
- Chi, O.Z.; Mellender, S.J.; Kiss, G.K.; Liu, X.; Weiss, H.R. Blood -brain barrier disruption was less under isoflurane than pentobarbital anesthesia via a PI3K/Akt pathway in early cerebral ischemia. Brain Res. Bull. 2017, 131, 1–6. [Google Scholar] [CrossRef]
- Choi, C.; Kim, H.M.; Shon, J.; Park, J.; Kim, H.T.; Kang, S.H.; Oh, S.H.; Kim, N.K.; Kim, O.J. The combination of mannitol and temozolomide increases the effectiveness of stem cell treatment in a chronic stroke model. Cytotherapy 2018, 20, 820–829. [Google Scholar] [CrossRef]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H.; et al. Anesthesia and surgery impair blood–brain barrier and cognitive function in mice. Front. Immunol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ni, C.; Li, Z.; Li, L.; Liu, Y.; Wang, C.; Zhong, Y.; Cui, D.; Guo, X. Isoflurane anesthesia results in reversible ultrastructure and occludin tight junction protein expression changes in hippocampal blood–brain barrier in aged rats. Neurosci. Lett. 2015, 587, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I. Osmotic opening of the blood–brain barrier: Principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Schaffenrath, J.; Huang, S.F.; Wyss, T.; Delorenzi, M.; Keller, A. Characterization of the blood–brain barrier in genetically diverse laboratory mouse strains. Fluids Barriers CNS 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; Wojtkiewicz, J.; Nowakowski, A.; Habich, A.; Holak, P.; Xu, J.; Adamiak, Z.; Chehade, M.; Pearl, M.S.; Gailloud, P.; et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J. Cereb. Blood Flow Metab. 2017, 37, 2346–2358. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.L.; Nitzsche, F.; Pryazhnikov, E.; Tibeykina, M.; Tolppanen, L.; Rytkönen, J.; Huhtala, T.; Mu, J.W.; Khiroug, L.; Boltze, J.; et al. Integrin α4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke 2017, 48, 2895–2900. [Google Scholar] [CrossRef]
- Yavagal, D.R.; Lin, B.; Raval, A.P.; Garza, P.S.; Dong, C.; Zhao, W.; Rangel, E.B.; McNiece, I.; Rundek, T.; Sacco, R.L.; et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS ONE 2014, 9, e93735. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Horie, N.; Satoh, K.; Yamaguchi, S.; Morofuji, Y.; Hiu, T.; Izumo, T.; Hayashi, K.; Nishida, N.; Nagata, I. Intra-Arterial Transplantation of Low-Dose Stem Cells Provides Functional Recovery Without Adverse Effects After Stroke. Cell. Mol. Neurobiol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Ishizaka, S.; Horie, N.; Satoh, K.; Fukuda, Y.; Nishida, N.; Nagata, I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013, 44, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Subash, M.; Yoon, J.S.; Jo, D.; Han, J.; Hong, J.M.; Kim, S.S.; Suh-Kim, H. Neurogenin-1 overexpression increases the therapeutic effects of mesenchymal stem cells through enhanced engraftment in an ischemic rat brain. Int. J. Stem Cells 2020, 13, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keimpema, E.; Fokkens, M.R.; Nagy, Z.; Agoston, V.; Luiten, P.G.M.; Nyakas, C.; Boddeke, H.W.G.M.; Copray, J.C.V.M. Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol. Appl. Neurobiol. 2009, 35, 89–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejewska, A.; Dabrowska, S.; Nowak, B.; Walczak, P.; Lukomska, B.; Janowski, M. Mesenchymal stem cells injected into carotid artery to target focal brain injury home to perivascular space. Theranostics 2020, 10, 6615–6628. [Google Scholar] [CrossRef]
- Khabbal, J.; Kerkelä, E.; Mitkari, B.; Raki, M.; Nystedt, J.; Mikkonen, V.; Bergström, K.; Laitinen, S.; Korhonen, M.; Jolkkonen, J. Differential clearance of rat and human bone marrow-derived mesenchymal stem cells from the brain after intra-arterial infusion in rats. Cell Transplant. 2015, 24, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namestnikova, D.D.; Gubskiy, I.L.; Revkova, V.A.; Sukhinich, K.K.; Melnikov, P.A.; Gabashvili, A.N.; Cherkashova, E.A.; Vishnevskiy, D.A.; Kurilo, V.V.; Burunova, V.V.; et al. Intra-Arterial Stem Cell Transplantation in Experimental Stroke in Rats: Real-Time MR Visualization of Transplanted Cells Starting With Their First Pass Through the Brain With Regard to the Therapeutic Action. Front. Neurosci. 2021, 15, 641970. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Shen, F.; Lu, J.; Liang, F.; Zhang, Y.; Xie, Z.; Dong, Y. WS635 Attenuates the Anesthesia/Surgery-Induced Cognitive Impairment in Mice. Front. Aging Neurosci. 2021, 13, 688587. [Google Scholar] [CrossRef] [PubMed]
- Masterson, C.H.; Curley, G.F.; Laffey, J.G. Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: Knowns and unknowns. Intensive Care Med. Exp. 2019, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Dotoli, G.M.; De Santis, G.C.; Orellana, M.D.; De Lima Prata, K.; Caruso, S.R.; Fernandes, T.R.; Rensi Colturato, V.A.; Kondo, A.T.; Hamerschlak, N.; Simões, B.P.; et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017, 52, 859–862. [Google Scholar] [CrossRef]
- Hadi Kartiko, B.; Milas Siswanto, F.; Purwata, T.E. Mesenchymal stem cell (MSC) as a potential cell therapy for immune related disease. Bali Med. J. 2017, 6, 38. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Mesenchymal stem cells in multiple sclerosis: Recent evidence from pre-clinical to clinical studies. Int. J. Mol. Sci. 2020, 21, 8662. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged no more: Measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Zangi, L.; Margalit, R.; Reich-Zeliger, S.; Bachar-Lustig, E.; Beilhack, A.; Negrin, R.; Reisner, Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Kerkelä, E.; Bakreen, A.; Nitzsche, F.; Andrzejewska, A.; Nowakowski, A.; Janowski, M.; Walczak, P.; Boltze, J.; Lukomska, B.; et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res. Ther. 2015, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, A.; Yasuhara, T.; Kameda, M.; Morimoto, J.; Takeuchi, H.; Wang, F.; Sasaki, T.; Sasada, S.; Shinko, A.; Wakamori, T.; et al. Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms. PLoS ONE 2015, 10, e0127302. [Google Scholar] [CrossRef] [PubMed]
- Sukhinich, K.K.; Namestnikova, D.D.; Gubskii, I.L.; Gabashvili, A.N.; Mel’nikov, P.A.; Vitushev, E.Y.; Vishnevskii, D.A.; Revkova, V.A.; Solov’eva, A.A.; Voitkovskaya, K.S.; et al. Distribution and Migration of Human Placental Mesenchymal Stromal Cells in the Brain of Healthy Rats after Stereotaxic or Intra-Arterial Transplantation. Bull. Exp. Biol. Med. 2020, 168, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Berlet, R.; Anthony, S.; Brooks, B.; Wang, Z.J.; Sadanandan, N.; Shear, A.; Cozene, B.; Gonzales-Portillo, B.; Parsons, B.; Salazar, F.E.; et al. Combination of stem cells and rehabilitation therapies for ischemic stroke. Biomolecules 2021, 11, 1316. [Google Scholar] [CrossRef]
- Moniche, F.; Escudero, I.; Zapata-Arriaza, E.; Usero-Ruiz, M.; Prieto-León, M.; de la Torre, J.; Gamero, M.A.; Tamayo, J.A.; Ochoa-Sepúlveda, J.J.; Maestre, J.; et al. Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): Protocol of a phase II, randomized, dose-finding, controlled multicenter trial. Int. J. Stroke 2015, 10, 1149–1152. [Google Scholar] [CrossRef]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients With Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205. [Google Scholar] [CrossRef]
- Battistella, V.; De Freitas, G.R.; Da Fonseca, L.M.B.; Mercante, D.; Gutfilen, B.; Goldenberg, R.C.S.; Vieira Dias, J.; Kasai-Brunswick, T.H.; Wajnberg, E.; Rosado-De-Castro, P.H.; et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen. Med. 2011, 6, 45–52. [Google Scholar] [CrossRef]
- Barbosa da Fonseca, L.M.; Gutfilen, B.; Rosado de Castro, P.H.; Battistella, V.; Goldenberg, R.C.S.; Kasai-Brunswick, T.; Chagas, C.L.R.; Wajnberg, E.; Maiolino, A.; Salles Xavier, S.; et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp. Neurol. 2010, 221, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Rosado-De-Castro, P.H.; Schmidt, F.R.; Battistella, V.; Lopes De Souza, S.A.; Gutfilen, B.; Goldenberg, R.C.; Kasai-Brunswick, T.H.; Vairo, L.; Silva, R.M.; Wajnberg, E.; et al. Biodistribution of bone marrow mononuclear cells after intra-arterial or intravenous transplantation in subacute stroke patients. Regen. Med. 2013, 8, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Ghali, A.A.; Yousef, M.K.; Ragab, O.A.A.; ElZamarany, E.A. Intra-arterial infusion of autologous bone marrow mononuclear stem cells in subacute ischemic stroke patients. Front. Neurol. 2016, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.A.G.; Martins, M.P.; Araújo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; El Ammar, J.S.; Machado, D.C.; da Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012, 21, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Le, V.C.; Nguyen, N.H.; Le, S.H. Intra-arterial infusion of autologous bone marrow mononuclear cells combined with intravenous injection of cerebrolysin in the treatment of middle cerebral artery ischemic stroke: Case report. SAGE Open Med. Case Rep. 2021, 9, 2050313X211002313. [Google Scholar] [CrossRef] [PubMed]

| Study | Stroke Model | Transplanted Cell Type and Species | Cell Delivery Route and Dose | Thromboembolism Control | Immunosuppression | Time of Delivery | BBB Crossing | Location of the Transplanted Cells | Therapeutics Effects on Stroke |
|---|---|---|---|---|---|---|---|---|---|
| Walczak et al. [123] | Transient 2 h MCAO | Rat bone marrow MSCs into rats | 1 × 106 in 1 mL PBS, rate 1 mL/min, through the ipsilateral ICA | No | No | 30 min after MCAO | Yes | 1 day after transplantation MSCs were located within the brain capillaries and 10 days after in the brain parenchyma | High intracerebral engraftment correlated with significant morbidity (cerebral embolism?) |
| Cui et al. [136] | Transient 2 h MCAO | Integrin α4 positive rat bone marrow MSCs into rats | 0.5 × 106 in 0.5 mL PBS during 3 min through the ipsilateral ECA | Yes | No | 24 h after MCAO | Yes | 2–72 h after transplantation cells were found within the capillaries, after 72 h rare cells were located in the brain perivascular niche or parenchyma | Not described |
| Yavagal et al. [137] | Transient 90 min MCAO | Rat bone marrow MSCs into rats | 1 × 106, 5 × 105, 2 × 105, 1 × 105, 5 × 104 in 0.5 mL PBS manual injection during 3 min through the ipsilateral ICA | Yes | No | 60 min and 24 h after MCAO | Yes | 3–5 days after transplantation cells were located partly inside the vessels and in the adjacent brain parenchyma | Cell dose 1 × 105 and below did not cause embolism; 1 × 105 MSC injected 24 h after MCAO improved neurodeficit score and reduced the mean infarct volume at one month |
| Fukuda et al. [138] | Transient 75 min MCAO | Human bone marrow MSCs into rats | 1 × 104, 1 × 106 in 300 μL PBS, rate 100 μL/min, through the ipsilateral ICA with maintenance of the blood flow | Yes | Cyclosporine A | 24 h after MCAO | Yes | MSCs were found in the vessels’ lumen and the brain parenchyma in the peri-infarct area at 24 h post-transplantation | High- or low- dose MSCs induced behavioral recovery and microglial activation suppression at 8 days after MCAO; mortality was significantly higher in the high-dose group |
| Toyoshima et al. [139] | Transient 75 min MCAO | Human MSCs into rats | 1 × 106 in 300 μL, rate 100 μL/min, thought the ipsilateral ICA with maintenance of the blood flow | Yes | Cyclosporine-A | 1, 4, 7 days after MCAO | Yes | 3 h after transplantation MSCs were distributed throughout the peri-infarction zone and the infarct core, 7 days after only very few MSCs had reached the brain parenchyma | MSCs transplanted 1 and 7 days after MCAO enhanced functional recovery at 7-, 14-, and 21-days post stroke; transplantation 1 day after MCAO caused reduction in brain atrophy |
| Andrzejewska et al. [142] | Stroke-like focal brain injury model | ITGA4 human bone marrow MSCs into rats | 5 × 105 in 1 mL of PBS, rate 0.2 mL/min through the ipsilateral ICA | Yes | No | 48 h after stroke modeling | No | Cells remained inside the vascular lumen over the first 2 days after IA infusion; 3 days after MSCs homed to perivascular space in the injury region; 72 h after transplantation many cells were phagocytosed | Not described |
| Kim et al. [140] | Transient 2 h MCAO | Human bone marrow MSCs with neurogenin 1 overexpression into rats | 1 × 106 in 1.2 mL saline during 5 min through the ipsilateral ICA with maintenance of the blood | No | No | 3 days after MCAO | Yes | 4 h after injection MSCs were mostly detected in the vascular lumen and 1 day after extravasated into the brain parenchyma | Reduction of neuronal cell death and inflammation, enhanced functional recovery in 28 days period |
| Toyoshima et al. [154] | Transient 90 min MCAO | Rat bone marrow MSCs into rats | 1 × 106 in 1 mL PBS, rate 1 mL/2 min, manual injection through the ipsilateral ICA | No | No | 1 h, 6 h, 24 h, 48 h after MCAO | Yes | 7 days after MCAO MSCs were mainly detected in the brain parenchyma in ischemic penumbra | 24 h group displayed the best therapeutic effects: functional recovery, reduction of infarct volumes, the highest number of integrated MSCs over 7 days period |
| Mitkari et. [124] | Transient 90 min MCAO | Human bone marrow MSCs and pronase-detached MSCs into rats | 1.1 × 106, 0.5 × 106 cells in 500 μL slowly through the ipsilateral ICA with maintenance of the blood | Yes | No | Acute phase after MCAO | No | Cells were entrapped within the brain capillaries immediately after transplantation and after 24 h the majority of MSCs disappeared | Not described |
| Keimpema et al. [141] | Transient 60 min MCAO | Rat bone marrow MSCs into rats | 1 × 106 in 100 μL slowly through the ipsilateral ECA | No | No | 1 h after MCAO | Yes | During first 12 h MSCs were detected in the cerebral blood vessels inside and around the ischemic lesion zone. Their number started to decrease after 24 h and within 2 weeks only sporadic cells were detected in the brain parenchyma and blood vessels | A significant reduction of 50% of the ischemic lesion 2 weeks after MCAO; microglia activation |
| Khabbal et al. [143] | Transient 60 min MCAO | Rat and human bone marrow MSCs into rats | 2 × 106 in 500 µL saline during 2 min through the ipsilateral ECA with maintenance of the blood | Yes | No | 24 h after MCAO | No | Both types of MSCs were located within the brain capillaries in the ipsilateral hemisphere 20 min after infusion | No data |
| Namestnikova et al. [144] | Transient 90 min MCAO | Human placenta MSCs into rats | 5 × 105 in 2 mL saline during 20 min through the ipsilateral ECA with maintenance of the blood | Yes | No | 24 h after MCAO | No | MSCs were located inside the cerebral blood vessels closely sticking to their walls for no longer than 3 days after administration | Improving of the neurological deficit and survival rate of animals 14 days after transplantation |
| No. | ClinicalTrials.gov Identifier | Title | Recruitment Status | Intervention/Treatment | Phase |
|---|---|---|---|---|---|
| 1 | NCT04434768 | Evaluate the Safety and Explore Efficacy of Umbilical Cord Mesenchymal Stem Cells in Acute Ischemic Stroke | Recruiting | One dose of IV administration of UC-MSCs | Evaluate the Safety and Explore Efficacy of Umbilical Cord Mesenchymal Stem Cells in Acute Ischemic Stroke |
| 2 | NCT02178657 [158] | Intra-Arterial Bone-Marrow Mononuclear Cells Infusion for Acute Ischemic Stroke | Recruiting | Intra-arterial autologous bone marrow mononuclear cells injection | II |
| 3 | NCT01273337 [159] | Study of ALD-401 via Intracarotid Infusion in Ischemic Stroke Subjects | Unknown | 3 mL ALDHbr cells (a cellular population that expresses high levels of aldehyde dehydrogenase) isolated from autologous bone marrow given as a one-time infusion via intracarotid infusion. | II |
| 4 | NCT03080571 [160] | Intraarterial Stem Cells in Subacute Ischemic Stroke | Completed | Autologous BMMNC injected in the ipsilateral MCA | I |
| 5 | NCT00473057 [160,161,162] | Study of Autologous Stem Cell Transplantation for Patients with Ischemic Stroke | Completed | Intra-arterial or intravenous delivery of autologous bone marrow cells | I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarygin, K.N.; Namestnikova, D.D.; Sukhinich, K.K.; Gubskiy, I.L.; Majouga, A.G.; Kholodenko, I.V. Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood–Brain Barrier? Cells 2021, 10, 2997. https://doi.org/10.3390/cells10112997
Yarygin KN, Namestnikova DD, Sukhinich KK, Gubskiy IL, Majouga AG, Kholodenko IV. Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood–Brain Barrier? Cells. 2021; 10(11):2997. https://doi.org/10.3390/cells10112997
Chicago/Turabian StyleYarygin, Konstantin N., Daria D. Namestnikova, Kirill K. Sukhinich, Ilya L. Gubskiy, Alexander G. Majouga, and Irina V. Kholodenko. 2021. "Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood–Brain Barrier?" Cells 10, no. 11: 2997. https://doi.org/10.3390/cells10112997
APA StyleYarygin, K. N., Namestnikova, D. D., Sukhinich, K. K., Gubskiy, I. L., Majouga, A. G., & Kholodenko, I. V. (2021). Cell Therapy of Stroke: Do the Intra-Arterially Transplanted Mesenchymal Stem Cells Cross the Blood–Brain Barrier? Cells, 10(11), 2997. https://doi.org/10.3390/cells10112997