Amniotic Epithelial Stem Cells Counteract Acidic Degradation By-Products of Electrospun PLGA Scaffold by Improving Their Immunomodulatory Profile In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(L-lactide-co-glycolide) Scaffolds
2.3. In Vitro Degradation
2.4. Characterization of the Degradation Media: pH and Conductivity Measurements
2.5. Characterization of the Fabricated Poly(L-lactide-co-glycolide) Scaffolds during Degradation
2.5.1. Wettability and Mass Loss Changes
2.5.2. Gel Permeation Chromatography (GPC)
2.5.3. Scanning Electron Microscopy (SEM)
2.6. Mechanical Characterization
2.7. Ethical Statement
2.8. Isolation of Ovine AECs
2.9. Media Preparation and pH Measurements
2.10. Cell Culture
2.11. In Vitro Cytocompatibility
2.11.1. MTT Assay
2.11.2. Morphological Evaluation of the Cells
2.11.3. Reverse Transcription and Real-Time Polymerase Chain Reaction (RT-PCR)
2.12. Statistical Analysis
3. Results
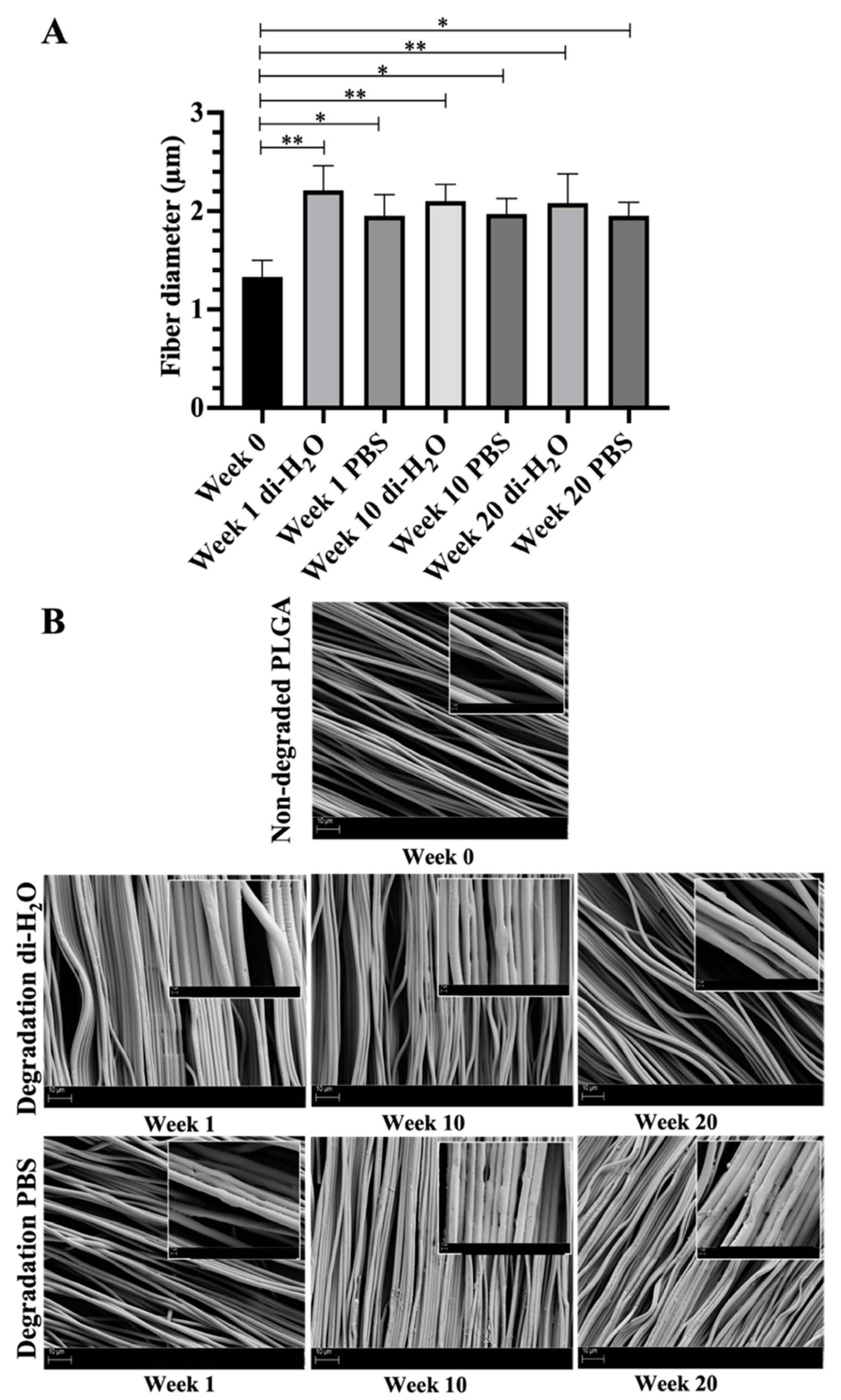
3.1. Changes of Ultrastructural Morphology after In Vitro Degradation on Electrospun Aligned PLGA Scaffold
3.2. Weight Loss and Liquid Uptake of Electrospun PLGA Scaffolds
3.3. pH and Conductivity Changes of the Degradation Media
3.4. Change of Molecular Weight and Its Distribution of Electrospun PLGA Scaffolds Subjected to Degradation
3.5. Change of Mechanical Properties of Electrospun PLGA Scaffolds during Degradation
3.6. Effect of PLGA Degradation By-Products on oAECs
3.6.1. MTT Assay on oAECs Treated with Non-Buffered and Buffered By-Products
3.6.2. oAECs’ Morphology Evaluation after Treatment with Non-Buffered and Buffered By-Products
3.6.3. Immunomodulatory Response of oAECs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Wang, S.; Qi, N. Controllable Structure, Properties, and Degradation of the Electrospun PLGA/PLA-Blended Nanofibrous Scaffolds. J. Appl. Polym. Sci. 2012, 125, 449–456. [Google Scholar] [CrossRef]
- Sensini, A.; Cristofolini, L. Biofabrication of Electrospun Scaffolds for the Regeneration of Tendons and Ligaments. Materials 2018, 11, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Xu, J.; Wang, A.; Zheng, M. Scaffolds for Tendon and Ligament Repair: Review of the Efficacy of Commercial Products. Expert Rev. Med. Devices 2009, 6, 61–73. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, J. Poly(Lactide-Co-Glycolide) Porous Scaffolds for Tissue Engineering and Regenerative Medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sensini, A.; Gualandi, C.; Zucchelli, A.; Boyle, L.A.; Kao, A.P.; Reilly, G.C.; Tozzi, G.; Cristofolini, L.; Focarete, M.L. Tendon Fascicle-Inspired Nanofibrous Scaffold of Polylactic Acid/Collagen with Enhanced 3D-Structure and Biomechanical Properties. Sci. Rep. 2018, 8, 17167. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Gualandi, C.; Cristofolini, L.; Tozzi, G.; Dicarlo, M.; Teti, G.; Mattioli-Belmonte, M.; Focarete, M.L. Biofabrication of Bundles of Poly(Lactic Acid)-Collagen Blends Mimicking the Fascicles of the Human Achille Tendon. Biofabrication 2017, 9, 015025. [Google Scholar] [CrossRef]
- Chen, M.; Patra, P.K.; Warner, S.B.; Bhowmick, S. Role of Fiber Diameter in Adhesion and Proliferation of NIH 3T3 Fibroblast on Electrospun Polycaprolactone Scaffolds. Tissue Eng. 2007, 13, 579–587. [Google Scholar] [CrossRef]
- Jana, S.; Bhagia, A.; Lerman, A. Optimization of Polycaprolactone Fibrous Scaffold for Heart Valve Tissue Engineering. Biomed. Mater. 2019, 14, 065014. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Stojanovic, D.B.; Simic, V.; Grkovic, M.; Bjelovic, M.; Uskokovic, P.S.; Kojic, M. Preparation and Modeling of Three-layered PCL/PLGA/PCL Fibrous Scaffolds for Prolonged Drug Release. Sci. Rep. 2020, 10, 11126. [Google Scholar] [CrossRef]
- Russo, V.; El Khatib, M.; Di Marcantonio, L.; Ancora, M.; Wyrwa, R.; Mauro, A.; Walter, T.; Weisser, J.; Citeroni, M.R.; Lazzaro, F.; et al. Tendon Biomimetic Electrospun PLGA Fleeces Induce an Early Epithelial-Mesenchymal Transition and Tenogenic Differentiation on Amniotic Epithelial Stem Cells. Cells 2020, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Vinhas, A.; Almeida, A.F.; Gonçalves, A.I.; Rodrigues, M.T.; Gomes, M.E. Magnetic Stimulation Drives Macrophage Polarization in Cell to–Cell Communication with Il-1β Primed Tendon Cells. Int. J. Mol. Sci. 2020, 21, 5441. [Google Scholar] [CrossRef]
- Vinhas, A.; Rodrigues, M.T.; Gonçalves, A.I.; Reis, R.L.; Gomes, M.E. Magnetic Responsive Materials Modulate the Inflammatory Profile of IL-1β Conditioned Tendon Cells. Acta Biomater. 2020, 117, 235–245. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, M.; Mauro, A.; Wyrwa, R.; Di Mattia, M.; Turriani, M.; Di Giacinto, O.; Kretzschmar, B.; Seemann, T.; Valbonetti, L.; Berardinelli, P.; et al. Fabrication and Plasma Surface Activation of Aligned Electrospun PLGA Fiber Fleeces with Improved Adhesion and Infiltration of Amniotic Epithelial Stem Cells Maintaining Their Teno-Inductive Potential. Molecules 2020, 25, 3176. [Google Scholar] [CrossRef]
- El Khatib, M.; Mauro, A.; Di Mattia, M.; Wyrwa, R.; Schweder, M.; Ancora, M.; Lazzaro, F.; Berardinelli, P.; Valbonetti, L.; Di Giacinto, O.; et al. Electrospun PLGA Fiber Diameter and Alignment of Tendon Biomimetic Fleece Potentiate Tenogenic Differentiation and Immunomodulatory Function of Amniotic Epithelial Stem Cells. Cells 2020, 9, 1207. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable Surface Modification of Poly(Lactic-Co-Glycolic Acid) (PLGA) by Hydrolysis or Aminolysis I: Physical, Chemical, and Theoretical Aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Ayyoob, M.; Kim, Y.J. Effect of Chemical Composition Variant and Oxygen Plasma Treatments on Thewettability of PLGA Thin Films, Synthesized by Direct Copolycondensation. Polymers 2018, 10, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirazi, R.N.; Aldabbagh, F.; Erxleben, A.; Rochev, Y.; McHugh, P. Nanomechanical Properties of Poly(Lactic-Co-Glycolic) Acid Film during Degradation. Acta Biomater. 2014, 10, 4695–4703. [Google Scholar] [CrossRef]
- Shirazi, R.N.; Ronan, W.; Rochev, Y.; McHugh, P. Modelling the Degradation and Elastic Properties of Poly(Lactic-Co-Glycolic Acid) Films and Regular Open-Cell Tissue Engineering Scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 54, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Holy, C.E.; Dang, S.M.; Davies, J.E.; Shoichet, M.S. In Vitro Degradation of a Novel Poly ( Lactide-Co-Glycolide ) 75/25 Foam. Biomaterials 1999, 20, 1177–1185. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Ontoria-Oviedo, I.; Castellano, D.; Marco, B.; Rabal, A.; Bou, J.J.; Serra, A.; Monreal, L.; Blanes, M.; et al. In Vitro Validation of Biomedical Polyester-Based Scaffolds: Poly(Lactide-Co-Glycolide) as Model-Case. Polym. Test. 2018, 66, 256–267. [Google Scholar] [CrossRef]
- Landes, C.A.; Ballon, A.; Roth, C. In-Patient versus in Vitro Degradation of P(L/DL)LA and PLGA. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2006, 76, 403–411. [Google Scholar] [CrossRef]
- Pamula, E.; Kokoszka, J.; Cholewa-Kowalska, K.; Laczka, M.; Kantor, L.; Niedzwiedzki, L.; Reilly, G.C.; Filipowska, J.; Madej, W.; Kolodziejczyk, M.; et al. Degradation, Bioactivity, and Osteogenic Potential of Composites Made of PLGA and Two Different Sol-Gel Bioactive Glasses. Ann. Biomed. Eng. 2011, 39, 2114–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Feng, X.; Jia, X.; Fan, Y. Influences of Tensile Load on in Vitro Degradation of an Electrospun Poly(L-Lactide-Co-Glycolide) Scaffold. Acta Biomater. 2010, 6, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly (Lactic- Co -Glycolic) Acid (PLGA) -Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Hasan, A. Buffer Systems. In Handbook of Blood Gas/Acid–Base Interpretation; Springer: London, UK, 2009; pp. 143–164. ISBN 978-1-84800-333-0. [Google Scholar]
- Edwards, S.L. Pathophysiology of Acid Base Balance: The Theory Practice Relationship. Intensive Crit. Care Nurs. 2008, 24, 28–40. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for Regenerative Medicine: A Review of the Main Topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Lao, L.; Zhu, Y.; Zhang, Y.; Gao, Z.; Zhou, F.; Chen, L.; Ouyang, H.; Gao, C. Mineralization of Collagen-Coated Electrospun Poly(Lactide-Co-Glycolide) Nanofibrous Mesh to Enhance Growth and Differentiation of Osteoblasts and Bone Marrow Mesenchymal Stem Cells. Adv. Eng. Mater. 2012, 14, B123–B137. [Google Scholar] [CrossRef]
- Ma, Y.; Song, J.; Almassri, H.N.S.; Zhang, D.; Zhang, T.; Cheng, Y.; Wu, X. Minocycline-Loaded PLGA Electrospun Membrane Prevents Alveolar Bone Loss in Experimental Peridontitis. Drug Deliv. 2020, 27, 151–160. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; He, W.; Huang, Q.; Zhang, R.; Feng, Q. Hydroxyapatite/Collagen Coating on PLGA Electrospun Fibers for Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 2018, 106, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Erisken, C.; Zhang, X.; Moffat, K.L.; Levine, W.N.; Lu, H.H. Scaffold Fiber Diameter Regulates Human Tendon Fibroblast Growth and Differentiation. Tissue Eng. Part A 2013, 19, 519–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashur, C.A.; Shaffer, R.D.; Dahlgren, L.A.; Guelcher, S.A.; Goldstein, A.S. Effect of Fiber Diameter and Alignment of Electrospun Polyurethane Meshes on Mesenchymal Progenitor Cells. Tissue Eng. Part A 2009, 15, 2435–2445. [Google Scholar] [CrossRef]
- Sahoo, S.; Lok Toh, S.; Hong Goh, J.C. PLGA Nanofiber-Coated Silk Microfibrous Scaffold for Connective Tissue Engineering. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2010, 95, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Toh, S.L.; Goh, J.C.H. A BFGF-Releasing Silk/PLGA-Based Biohybrid Scaffold for Ligament/Tendon Tissue Engineering Using Mesenchymal Progenitor Cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef]
- Sahoo, S.; Goh Cho-Hong, J.; Siew-Lok, T. Development of Hybrid Polymer Scaffolds for Potential Applications in Ligament and Tendon Tissue Engineering. Biomed. Mater. 2007, 2, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Full, S.M.; Delman, C.; Gluck, J.M.; Abdmaulen, R.; Shemin, R.J.; Heydarkhan-Hagvall, S. Effect of Fiber Orientation of Collagen-Based Electrospun Meshes on Human Fibroblasts for Ligament Tissue Engineering Applications. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2015, 103, 39–46. [Google Scholar] [CrossRef]
- Subramony, S.D.; Dargis, B.R.; Castillo, M.; Azeloglu, E.U.; Tracey, M.S.; Su, A.; Lu, H.H. The Guidance of Stem Cell Differentiation by Substrate Alignment and Mechanical Stimulation. Biomaterials 2013, 34, 1942–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.M.; Erisken, C.; Iskratsch, T.; Sheetz, M.; Levine, W.N.; Lu, H.H. Polymer Fiber-Based Models of Connective Tissue Repair and Healing. Biomaterials 2017, 112, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, N.; Ramakrishna, S.; Tian, L.; Mo, X. The Effect of Plasma Treated PLGA/MWCNTs-COOH Composite Nanofibers on Nerve Cell Behavior. Polymers 2017, 9, 713. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, T.J.; Avila, C.O.C.; Sundararaghavan, H.G. Combining Growth Factor Releasing Microspheres within Aligned Nanofibers Enhances Neurite Outgrowth. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Foraida, Z.I.; Kamaldinov, T.; Nelson, D.A.; Larsen, M.; Castracane, J. Elastin-PLGA Hybrid Electrospun Nanofiber Scaffolds for Salivary Epithelial Cell Self-Organization and Polarization. Acta Biomater. 2017, 62, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface Modification of Electrospun PLGA Scaffold with Collagen for Bioengineered Skin Substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Lee, J.H.; Lee, M.H.; Kwon, B.J.; Park, J.C. Evaluation of Electrospun (1,3)-(1,6)-β-D-Glucans/Biodegradable Polymer as Artificial Skin for Full-Thickness Wound Healing. Tissue Eng.—Part A 2012, 18, 2315–2322. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Xiao, J.; Wang, B.; Li, L.; Kong, X.; Liao, J. The Immune Reaction and Degradation Fate of Scaffold in Cartilage/Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 104, 109927. [Google Scholar] [CrossRef]
- Park, S.-H.; Seok Gil, E.; Shi, H.; Joo Kim, H.; Lee, K.; Kaplan, D.L. Relationships between Degradabiility of Silk Scaffolds and Osteogenesis. Biomaterials 2010, 31, 6162–6172. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Cao, Y. Application of Scaffold Materials in Tissue Reconstruction in Immunocompetent Mammals: Our Experience and Future Requirements. Biomaterials 2007, 28, 5078–5086. [Google Scholar] [CrossRef]
- Docheva, D.; Majewski, M.; Evans, C.H.; Surgery, E.; Maximilians, L.; Medicine, R.; Clinic, M. Biologics of Tendon Repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [Green Version]
- Citeroni, M.R.; Mauro, A.; Ciardulli, M.C.; Di Mattia, M.; El Khatib, M.; Russo, V.; Turriani, M.; Santer, M.; Della Porta, G.; Maffulli, N.; et al. Amnion-Derived Teno-Inductive Secretomes: A Novel Approach to Foster Tendon Differentiation and Regeneration in an Ovine Model. Front. Bioeng. Biotechnol. 2021, 9, 649288. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Russo, V.; Curini, V.; Mauro, A.; Martelli, A.; Muttini, A.; Bernabò, N.; Valbonetti, L.; Marchisio, M.; Di Giacinto, O.; et al. Achilles Tendon Regeneration Can Be Improved by Amniotic Epithelial Cell Allotransplantation. Cell Transplant. 2012, 21, 2377–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canciello, A.; Teti, G.; Mazzotti, E.; Falconi, M.; Russo, V.; Giordano, A.; Barboni, B. Progesterone Prolongs Viability and Anti-Inflammatory Functions of Explanted Preterm Ovine Amniotic Membrane. Front. Bioeng. Biotechnol. 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, E.; Pascual-Gil, S.; Prosper, F.; Blanco-Prieto, M.J. Bioresorbable Polymers for Next-Generation Cardiac Scaffolds. In Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Perale, G., Hilborn, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 445–467. ISBN 9780081002667. [Google Scholar]
- Campos, D.M.; Gritsch, K.; Salles, V.; Attik, G.N.; Grosgogeat, B. Surface Entrapment of Fibronectin on Electrospun PLGA Scaffolds for Periodontal Tissue Engineering. BioResearch Open Access 2014, 3, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Puerto, I.; Ribeiro, S.; Lanceros-Mendez, S.; Barandiarán, J.M. The Influence of Copolymer Composition on PLGA/NHA Scaffolds’ Cytotoxicity and in Vitro Degradation. Nanomaterials 2017, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Jiang, H.; Chen, W. The Biodegradability of Electrospun Dextran/PLGA Scaffold in a Fibroblast/Macrophage Co-Culture. Biomaterials 2008, 29, 1583–1592. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.; Yang, F.; Seyednejad, H.; Chen, Z.; Hennink, W.E.; Anderson, J.M.; van den Beucken, J.J.J.P.; Jansen, J.A. Biocompatibility and Degradation Characteristics of PLGA-Based Electrospun Nanofibrous Scaffolds with Nanoapatite Incorporation. Biomaterials 2012, 33, 6604–6614. [Google Scholar] [CrossRef]
- Chor, A.; Gonçalves, R.P.; Costa, A.M.; Farina, M.; Ponche, A.; Sirelli, L.; Schrodj, G.; Gree, S. In Vitro Degradation of Electrospun Poly(Lactic-Co-Glycolic Acid) (PLGA) for Oral Mucosa Regeneration. Polymers 2020, 12, 1853. [Google Scholar] [CrossRef]
- Sadeghi-avalshahr, A.R.; Khorsand-Ghayeni, M.; Nokhasteh, S.; Molavi, A.M.; Naderi-Meshkin, H. Synthesis and Characterization of PLGA/Collagen Composite Scaffolds as Skin Substitute Produced by Electrospinning through Two Different Approaches. J. Mater. Sci. Mater. Med. 2017, 28, 14. [Google Scholar] [CrossRef]
- Puhl, D.L.; Funnell, J.L.; D’Amato, A.R.; Bao, J.; Zagorevski, D.V.; Pressman, Y.; Morone, D.; Haggerty, A.E.; Oudega, M.; Gilbert, R.J. Aligned Fingolimod-Releasing Electrospun Fibers Increase Dorsal Root Ganglia Neurite Extension and Decrease Schwann Cell Expression of Promyelinating Factors. Front. Bioeng. Biotechnol. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Micro and Nanoparticles; Springer: Singapore, 2019; Volume 49, ISBN 0123456789. [Google Scholar]
- Riggin, C.N.; Qu, F.; Hwa Kim, D.; Huegel, J.; Steinberg, D.R.; Kuntz, A.F.; Soslowsky, L.J.; Mauch, R.L.; Bernstein, J. Electrospun PLGA Nanofiber Scaffolds Release Ibuprofen Faster and Degrade Slower after In Vivo Implantation. Ann. Biomed. Eng. 2017, 45, 2348–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, C.; Wang, F.; Pang, M.; Sun, L.; Chen, R.; Sun, Y. Suspended, Shrinkage-Free, Electrospun PLGA Nanofibrous Scaffold for Skin Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 10872–10877. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, J. In Vitro Degradation of Three-Dimensional Porous Poly(D,L-Lactide-Co- Glycolide) Scaffolds for Tissue Engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Yang, F.; Cheng, X.; Frank Walboomers, X.; Jansen, J.A. The Effect of Electrospun Fibre Alignment on the Behaviour of Rat Periodontal Ligament Cells. Eur. Cell. Mater. 2010, 19, 180–192. [Google Scholar] [CrossRef]
- Sangsanoh, P.; Waleetomcheepsawat, S.; Suwantong, O.; Wutticharoenmongkol, P.; Weeranantanapan, O.; Chuenjitbuntaworn, B.; Cheepsunthom, P.; Pavasant, P.; Supaphol, P. In Vitro Biocompatability of Schwann Cells on Surfaces of Biocompatible Polymeric Electrospun Fibrous and Solution-Cast Film Scaffolds. Biomacromolecules 2007, 8, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Vey, E.; Roger, C.; Meehan, L.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation Mechanism of Poly(Lactic-Co-Glycolic) Acid Block Copolymer Cast Films in Phosphate Buffer Solution. Polym. Degrad. Stab. 2008, 93, 1869–1876. [Google Scholar] [CrossRef]
- Vey, E.; Rodger, C.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation Kinetics of Poly(Lactic-Co-Glycolic) Acid Block Copolymer Cast Films in Phosphate Buffer Solution as Revealed by Infrared and Raman Spectroscopies. Polym. Degrad. Stab. 2011, 96, 1882–1889. [Google Scholar] [CrossRef]
- Dong, Y.; Liao, S.; Ngiam, M.; Chan, C.K.; Ramakrishna, S. Degradation Behaviors of Electrospun Resorbable Polyester Nanofibers. Tissue Eng.—Part B Rev. 2009, 15, 333–351. [Google Scholar] [CrossRef]
- Farahani, T.D.; Entezami, A.A.; Mobedi, H.; Abtahi, M. Degradation of Poly (D, L-Lactide-Co-Glycolide) 50: 50 Implant in Aqueous Medium. Iran. Polym. J. 2005, 14, 753–763. [Google Scholar]
- Dong, Y.; Sc, B.; Yong, T.; Ph, D.; Liao, S.; Chan, C.K. Distinctive Degradation Behaviors of Electrospun polyglycolide, poly(DL-lactide-co-glycolide), and poly(L-lactide-co-epsilon-caprolactone) nanofibers cultured with/without porcine smooth muscle cells. Tissue Eng. Part A 2010, 16, 283–298. [Google Scholar]
- Magiera, A.; Markowski, J.; Pilch, J.; Blazewicz, S. Degradation Behavior of Electrospun PLA and PLA/CNT Nanofibres in Aqueous Environment. J. Nanomater. 2018, 2018, 8796583. [Google Scholar] [CrossRef] [Green Version]
- Silini, A.R.; Magatti, M.; Cargnoni, A.; Parolini, O. Is Immune Modulation the Mechanism Underlying the Beneficial Effects of Amniotic Cells and Their Derivatives in Regenerative Medicine? Cell Transplant. 2017, 26, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, B.Z. Inflammation Fires up Cancer Metastasis. Semin. Cancer Biol. 2017, 47, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Front. Immunol. 2017, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Barboni, B.; Russo, V.; Berardinelli, P.; Mauro, A.; Valbonetti, L.; Sanyal, H.; Canciello, A.; Greco, L.; Muttini, A.; Gatta, V.; et al. Placental Stem Cells from Domestic Animals: Translational Potential and Clinical Relevance. Cell Transplant. 2018, 27, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Flores-Espinosa, P.; Pineda-Torres, M.; Vega-Sánchez, R.; Estrada-Gutiérrez, G.; Espejel-Nuñez, A.; Flores-Pliego, A.; Maida-Claros, R.; Paredes-Vivas, Y.; Morales-Méndez, I.; Sosa-González, I.; et al. Progesterone Elicits an Inhibitory Effect upon LPS-Induced Innate Immune Response in Pre-Labor Human Amniotic Epithelium. Am. J. Reprod. Immunol. 2014, 71, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The Immunomodulatory Properties of Amniotic Cells: The Two Sides of the Coin. Cell Transplant. 2018, 27, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Manuelpillai, U.; Tchongue, J.; Lourensz, D.; Vaghjiani, V.; Samuel, C.S.; Liu, A.; Williams, E.D.; Sievert, W. Transplantation of Human Amnion Epithelial Cells Reduces Hepatic Fibrosis in Immunocompetent CCl4-Treated Mice. Cell Transplant. 2010, 19, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Manuelpillai, U.; Lourensz, D.; Vaghjiani, V.; Tchongue, J.; Lacey, D.; Tee, J.-Y.; Murthi, P.; Chan, J.; Hodge, A.; Sievert, W. Human Amniotic Epithelial Cell Transplantation Induces Markers of Alternative Macrophage Activation and Reduces Established Hepatic Fibrosis. PLoS ONE 2012, 7, e38631. [Google Scholar] [CrossRef] [Green Version]
- Wolbank, S.; Hildner, F.; Redl, H.; van Griensven, M.; Gabriel, C.; Hennerbichler, S. Impact of Human Amniotic Membrane Preparation on Release of Angiogenic Factors. J. Tissue Eng. Regen. Med. 2009, 3, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Kronsteiner, B.; Wolbank, S.; Peterbauer, A.; Hackl, C.; Redl, H.; van Griensven, M.; Gabriel, C. Human Mesenchymal Stem Cells from Adipose Tissue and Amnion Influence T-Cells Depending on Stimulation Method and Presence of Other Immune Cells. Stem Cells Dev. 2011, 20, 2115–2126. [Google Scholar] [CrossRef]
- Barboni, B.; Russo, V.; Gatta, V.; Bernabò, N.; Berardinelli, P.; Mauro, A.; Martelli, A.; Valbonetti, L.; Muttini, A.; Di Giacinto, O.; et al. Therapeutic Potential of HAECs for Early Achilles Tendon Defect Repair through Regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1594–e1608. [Google Scholar] [CrossRef] [PubMed]
- Greco, L.; Russo, V.; Rapino, C.; Di Germanio, C.; Fezza, F.; Bernabò, N.; Berardinelli, P.; Peserico, A.; Fazio, D.; Maccarrone, M.; et al. Characterization of Endocannabinoid System and Interleukin Profiles in Ovine AEC: Cannabinoid Receptors Type-1 and Type-2 as Key Effectors of Pro-Inflammatory Response. Cells 2020, 9, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, D.; Pianta, S.; Magatti, M.; Sedlmayr, P.; Parolini, O. Characterization of the Conditioned Medium from Amniotic Membrane Cells: Prostaglandins as Key Effectors of Its Immunomodulatory Activity. PLoS ONE 2012, 7, e46956. [Google Scholar] [CrossRef]
- Canciello, A.; Russo, V.; Berardinelli, P.; Bernabò, N.; Muttini, A.; Mattioli, M.; Barboni, B. Progesterone Prevents Epithelial-Mesenchymal Transition of Ovine Amniotic Epithelial Cells and Enhances Their Immunomodulatory Properties. Sci. Rep. 2017, 7, 3761. [Google Scholar] [CrossRef] [Green Version]
- Paradowska, E.; Blach-Olszewska, Z.; Gejdel, E. Constitutive and Induced Cytokine Production by Human Placenta and Amniotic Membrane at Term. Placenta 1997, 18, 441–446. [Google Scholar] [CrossRef]
- Meyer, F.; Wardale, J.; Best, S.; Cameron, R.; Rushton, N.; Brooks, R. Effects of Lactic Acid and Glycolic Acid on Human Osteoblasts: A Way to Understand PLGA Involvement in PLGA/Calcium Phosphate Composite Failure. J. Orthop. Res. 2012, 30, 864–871. [Google Scholar] [CrossRef]
- Saleh, A.M.; Rombola, G.; Batlle, D.C. Intracellular H+ Buffering Power and Its Dependency on Intracellular PH. Kidney Int. 1991, 39, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Burton, R.F. Intracellular Buffering. Respir. Physiol. 1978, 33, 51–58. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, Z.; Qian, K.; Wang, J.; Zhu, H. Harnessing the Properties of Biomaterial to Enhance the Immunomodulation of Mesenchymal Stem Cells. Tissue Eng. Part B Rev. 2019, 25, 492–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauro, A.; Russo, V.; Di Marcantonio, L.; Berardinelli, P.; Martelli, A.; Muttini, A.; Mattioli, M.; Barboni, B. M1 and M2 Macrophage Recruitment during Tendon Regeneration Induced by Amniotic Epithelial Cell Allotransplantation in Ovine. Res. Vet. Sci. 2016, 105, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Nerlich, M.; Docheva, D. Tendon Injuries: Basic Science and New Repair Proposals. EFORT Open Rev. Gen. Orthop. 2017, 2, 332–342. [Google Scholar] [CrossRef] [PubMed]












| Concentration (mM) | pH on Non-Buffered Media | ||
|---|---|---|---|
| LA | GA | LA:GA | |
| 5 | 6.70 | 6.74 | 6.32 |
| 10 | 6.34 | 6.33 | 5.47 |
| 20 | 5.60 | 5.40 | 4.03 |
| 40 | 4.07 | 4.01 | 3.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khatib, M.; Russo, V.; Prencipe, G.; Mauro, A.; Wyrwa, R.; Grimm, G.; Di Mattia, M.; Berardinelli, P.; Schnabelrauch, M.; Barboni, B. Amniotic Epithelial Stem Cells Counteract Acidic Degradation By-Products of Electrospun PLGA Scaffold by Improving Their Immunomodulatory Profile In Vitro. Cells 2021, 10, 3221. https://doi.org/10.3390/cells10113221
El Khatib M, Russo V, Prencipe G, Mauro A, Wyrwa R, Grimm G, Di Mattia M, Berardinelli P, Schnabelrauch M, Barboni B. Amniotic Epithelial Stem Cells Counteract Acidic Degradation By-Products of Electrospun PLGA Scaffold by Improving Their Immunomodulatory Profile In Vitro. Cells. 2021; 10(11):3221. https://doi.org/10.3390/cells10113221
Chicago/Turabian StyleEl Khatib, Mohammad, Valentina Russo, Giuseppe Prencipe, Annunziata Mauro, Ralf Wyrwa, Gabriele Grimm, Miriam Di Mattia, Paolo Berardinelli, Matthias Schnabelrauch, and Barbara Barboni. 2021. "Amniotic Epithelial Stem Cells Counteract Acidic Degradation By-Products of Electrospun PLGA Scaffold by Improving Their Immunomodulatory Profile In Vitro" Cells 10, no. 11: 3221. https://doi.org/10.3390/cells10113221










