The Retina: A Window into the Brain
Funding
Acknowledgments
Conflicts of Interest
References
- Baden, T.; Euler, T.; Berens, P. Understanding the retinal basis of vision across species. Nat. Rev. Neurosci. 2019, 21, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Mashige, K.P.; Oduntan, O.A. A review of the human retina with emphasis on nerve fibre layer and macula thicknesses. Afr. Vis. Eye Health 2016, 75, 1–8. [Google Scholar] [CrossRef]
- Demb, J.B.; Singer, J.H. Functional Circuitry of the Retina. Annu. Rev. Vis. Sci. 2015, 1, 263–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cajal, S.R. La retine des vertebres. Cellule 1893, 9, 119–255. [Google Scholar]
- Ishikawa, H.; Stein, D.M.; Wollstein, G.; Beaton, S.; Fujimoto, J.G.; Schuman, J. Macular Segmentation with Optical Coherence Tomography. Investig. Opthalmology Vis. Sci. 2005, 46, 2012–2017. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, C.D. The Constructive Nature of Visual Processing. In Principles of Neural Science, 6th ed.; Kandel, E.R., Schwartz, J.H., Jessell, T.M., Eds.; McGraw-Hill: NewYork, NY, USA, 2021; pp. 556–576. [Google Scholar]
- Wu, C.-C.; Wick, F.A.; Pomplun, M. Guidance of visual attention by semantic information in real-world scenes. Front. Psychol. 2014, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, R.H. Movements of the Eyes, 2nd Rev; Pion Limited: London, UK, 1988. [Google Scholar]
- Fuchs, A.F. Saccadic and smooth pursuit eye movements in the monkey. J. Physiol. 1967, 191, 609–631. [Google Scholar] [CrossRef]
- Diamond, J.S. Inhibitory Interneurons in the Retina: Types, Circuitry, and Function. Annu. Rev. Vis. Sci. 2017, 3, 1–24. [Google Scholar] [CrossRef]
- Franke, K.; Baden, T. General features of inhibition in the inner retina. J. Physiol. 2017, 595, 5507–5515. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, R.H. Interactions between the retinal pigment epithelium and the neural retina. Doc. Ophthalmol. 1985, 60, 327–346. [Google Scholar] [CrossRef]
- Nag, T.C.; Wadhwa, S. Ultrastructure of the human retina in aging and various pathological states. Micron 2012, 43, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Swaroop, A.; Zack, D.J. Transcriptome analysis of the retina. Genome Biol. 2002, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, G.A. Topography of the layer of the rods and cones in the human retima. Acta Ophthalmol 1935, 13, 1–102. [Google Scholar]
- Kottke, T.; Xie, A.; Larsen, D.S.; Hoff, W.D. Photoreceptors Take Charge: Emerging Principles for Light Sensing. Annu. Rev. Biophys. 2018, 47, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Borwein, B. The Retinal Receptor: A Description. Vertebr. Photoreceptor Opt. 1981, 23, 11–81. [Google Scholar] [CrossRef]
- Turner, P.L.; Mainster, M.A. Circadian photoreception: Ageing and the eye’s important role in systemic health. Br. J. Ophthalmol. 2008, 92, 1439–1444. [Google Scholar] [CrossRef]
- Johnson, L.V.; Hageman, G.S.; Blanks, J.C. Interphotoreceptor matrix domains ensheath vertebrate cone photoreceptor cells. Investig. Ophthalmol. Vis. Sci. 1986, 27, 129–135. [Google Scholar]
- Blanks, J.C.; Hageman, G.S.; Johnson, L.V.; Spee, C. Ultrastructural visualization of primate cone photoreceptor matrix sheaths. J. Comp. Neurol. 1988, 270, 288–300. [Google Scholar] [CrossRef]
- Yin, L.; Smith, R.G.; Sterling, P.; Brainard, D.H. Chromatic Properties of Horizontal and Ganglion Cell Responses Follow a Dual Gradient in Cone Opsin Expression. J. Neurosci. 2006, 26, 12351–12361. [Google Scholar] [CrossRef]
- Jacobs, G.H.; Rowe, M.P. Evolution of vertebrate colour vision. Clin. Exp. Optom. 2004, 87, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R. Imaging single cells in the living retina. Vis. Res. 2011, 51, 1379–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Euler, T.; Haverkamp, S.; Schubert, T.; Baden, T. Retinal bipolar cells: Elementary building blocks of vision. Nat. Rev. Neurosci. 2014, 15, 507–519. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Omi, N. Functional allocation of synaptic contacts in microcircuits from rods via rod bipolar to AII amacrine cells in the mouse retina. J. Comp. Neurol. 2013, 521, 3541–3555. [Google Scholar] [CrossRef] [Green Version]
- Wässle, H.; Puller, C.; Müller, F.; Haverkamp, S.; Mueller, F. Cone Contacts, Mosaics, and Territories of Bipolar Cells in the Mouse Retina. J. Neurosci. 2009, 29, 106–117. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Bujan, S.; Haverkamp, S.; Feigenspan, A.; Wässle, H. Types of bipolar cells in the mouse retina. J. Comp. Neurol. 2004, 469, 70–82. [Google Scholar] [CrossRef]
- Breuninger, T.; Puller, C.; Haverkamp, S.; Euler, T. Chromatic Bipolar Cell Pathways in the Mouse Retina. J. Neurosci. 2011, 31, 6504–6517. [Google Scholar] [CrossRef] [PubMed]
- Euler, T.; Masland, R.H. Light-evoked responses of bipolar cells in a mammalian retina. J. Neurophysiol. 2000, 83, 1817–1829. [Google Scholar] [CrossRef]
- Hartveit, E. Functional Organization of Cone Bipolar Cells in the Rat Retina. J. Neurophysiol. 1997, 77, 1716–1730. [Google Scholar] [CrossRef] [Green Version]
- Grünert, U.; Martin, P.R. Cell types and cell circuits in human and non-human primate retina. Prog. Retin. Eye Res. 2020, 78, 100844. [Google Scholar] [CrossRef]
- Watson, A.B. A formula for human retinal ganglion cell receptive field density as a function of visual field location. J. Vis. 2014, 14, 15. [Google Scholar] [CrossRef] [Green Version]
- Herbin, M.; Boire, D.; Ptito, M. Size and distribution of retinal ganglion cells in the St. Kitts green monkey (Cercopithecus aethiops sabeus). J. Comp. Neurol. 1997, 383, 459–472. [Google Scholar] [CrossRef]
- Tan, O.; Li, G.; Lu, A.T.-H.; Varma, R.; Huang, D. Mapping of Macular Substructures with Optical Coherence Tomography for Glaucoma Diagnosis. Ophthalmology 2008, 115, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Kuffler, S.W. Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 1953, 16, 37–68. [Google Scholar] [CrossRef] [Green Version]
- Sanes, J.R.; Masland, R.H. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Baden, T.; Berens, P.; Franke, K.J.; Rosón, M.R.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen-Ba-Charvet, K.T.; Rebsam, A. Neurogenesis and Specification of Retinal Ganglion Cells. Int. J. Mol. Sci. 2020, 21, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattar, S.; Liao, H.-W.; Takao, M.; Berson, D.M.; Yau, K.-W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [Green Version]
- Do, M.T.H.; Yau, K.-W. Intrinsically Photosensitive Retinal Ganglion Cells. Physiol. Rev. 2010, 90, 1547–1581. [Google Scholar] [CrossRef]
- Chew, K.S.; Renna, J.M.; McNeill, D.S.; Fernandez, D.C.; Keenan, W.T.; Thomsen, M.B.; Ecker, J.L.; Loevinsohn, G.S.; Van Dunk, C.; Vicarel, D.C.; et al. A subset of ipRGCs regulates both maturation of the circadian clock and segregation of retinogeniculate projections in mice. eLife 2017, 6, e22861. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.-J.; Gao, F.; Wu, S.M. Light-Evoked Excitatory and Inhibitory Synaptic Inputs to ON and OFF α Ganglion Cells in the Mouse Retina. J. Neurosci. 2003, 23, 6063–6073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Kim, I.-J.; Sanes, J.R.; Meister, M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc. Natl. Acad. Sci. USA 2012, 109, E2391–E2398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.-J.; Zhang, Y.; Yamagata, M.; Meister, M.; Sanes, J.R. Molecular identification of a retinal cell type that responds to upward motion. Nature 2008, 452, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Boije, H.; Shirazi Fard, S.; Edqvist, P.-H.; Hallböök, F. Horizontal Cells, the Odd Ones Out in the Retina, Give Insights into Development and Disease. Front. Neuroanat. 2016, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Zhang, Y.; Chen, M.; Zhou, Z.J. Segregated glycine-glutamate co-transmission from vGluT3 amacrine cells to contrast-suppressed and contrast-enhanced retinal circuits. Neuron 2016, 90, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tien, N.-W.; Kim, T.; Kerschensteiner, D. Target-Specific Glycinergic Transmission from VGluT3-Expressing Amacrine Cells Shapes Suppressive Contrast Responses in the Retina. Cell Rep. 2016, 15, 1369–1375. [Google Scholar] [CrossRef] [Green Version]
- Flores-Herr, N.; Protti, D.A.; Wässle, H. Synaptic Currents Generating the Inhibitory Surround of Ganglion Cells in the Mammalian Retina. J. Neurosci. 2001, 21, 4852–4863. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-J.; Hare, W.A. Temporal Modulation of Scotopic Visual Signals by A17 Amacrine Cells in Mammalian Retina In Vivo. J. Neurophysiol. 2003, 89, 2159–2166. [Google Scholar] [CrossRef]
- Grimes, W.N.; Zhang, J.; Tian, H.; Graydon, C.W.; Hoon, M.; Rieke, F.; Diamond, J.S. Complex inhibitory microcircuitry regulates retinal signaling near visual threshold. J. Neurophysiol. 2015, 114, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Bouskila, J.; Micaelo-Fernandes, C.; Palmour, R.M.; Bouchard, J.-F.; Ptito, M. Transient receptor potential vanilloid type 1 is expressed in the horizontal pathway of the vervet monkey retina. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Gan, L. Development of Retinal Amacrine Cells and Their Dendritic Stratification. Curr. Ophthalmol. Rep. 2014, 2, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Zaghloul, K.A.; Manookin, M.B.; Borghuis, B.G.; Boahen, K.; Demb, J.B. Functional Circuitry for Peripheral Suppression in Mammalian Y-Type Retinal Ganglion Cells. J. Neurophysiol. 2007, 97, 4327–4340. [Google Scholar] [CrossRef] [PubMed]
- Baccus, S.A.; Ölveczky, B.P.; Manu, M.; Meister, M. A Retinal Circuit That Computes Object Motion. J. Neurosci. 2008, 28, 6807–6817. [Google Scholar] [CrossRef] [Green Version]
- Vaney, D.I.; Sivyer, B.; Taylor, W.R. Direction selectivity in the retina: Symmetry and asymmetry in structure and function. Nat. Rev. Neurosci. 2012, 13, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Roska, B.; Werblin, F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 2001, 410, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; McCall, M.A. Receptor targets of amacrine cells. Vis. Neurosci. 2012, 29, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The fundamental plan of the retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Masland, R.H. The tasks of amacrine cells. Vis. Neurosci. 2012, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sheth, B.R.; Young, R. Two Visual Pathways in Primates Based on Sampling of Space: Exploitation and Exploration of Visual Information. Front. Integr. Neurosci. 2016, 10, 37. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021, 7, 1–25. [Google Scholar] [CrossRef]
- Altschwager, P.; Ambrosio, L.; Swanson, E.A.; Moskowitz, A.; Fulton, A.B. Juvenile Macular Degenerations. Semin. Pediatr. Neurol. 2017, 24, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remmer, M.H.; Rastogi, N.; Ranka, M.P.; Ceisler, E.J. Achromatopsia. Curr. Opin. Ophthalmol. 2015, 26, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [Green Version]
- Eckert, K.A.; Carter, M.J.; Lansingh, V.C.; Wilson, D.A.; Furtado, J.M.; Frick, K.D.; Resnikoff, S. A Simple Method for Estimating the Economic Cost of Productivity Loss Due to Blindness and Moderate to Severe Visual Impairment. Ophthalmic Epidemiol. 2015, 22, 349–355. [Google Scholar] [CrossRef]
- Ramrattan, R.S.; Wolfs, R.C.; Panda-Jonas, S.; Jonas, J.B.; Bakker, D.; Pols, H.A.; de Jong, P.T. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning: The Rotterdam Study. Arch. Ophthalmol. 2001, 119, 1788–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, C. Educating learners with vision impairment in inclusive settings. Int. Congr. Ser. 2005, 1282, 922–926. [Google Scholar] [CrossRef]
- Schinazi, V.R.; Thrash, T.; Chebat, D. Spatial navigation by congenitally blind individuals. WIREs Cogn. Sci. 2016, 7, 37–58. [Google Scholar] [CrossRef] [Green Version]
- Ptito, M.; Bleau, M.; Djerourou, I.; Paré, S.; Schneider, F.C.; Chebat, D.-R. Brain-Machine Interfaces to Assist the Blind. Front. Hum. Neurosci. 2021, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E. Development of visual Neuroprostheses: Trends and challenges. Bioelectron. Med. 2018, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chebat, D.-R.; Schneider, F.C.; Ptito, M. Spatial Competence and Brain Plasticity in Congenital Blindness via Sensory Substitution Devices. Front. Neurosci. 2020, 14, 815. [Google Scholar] [CrossRef] [PubMed]
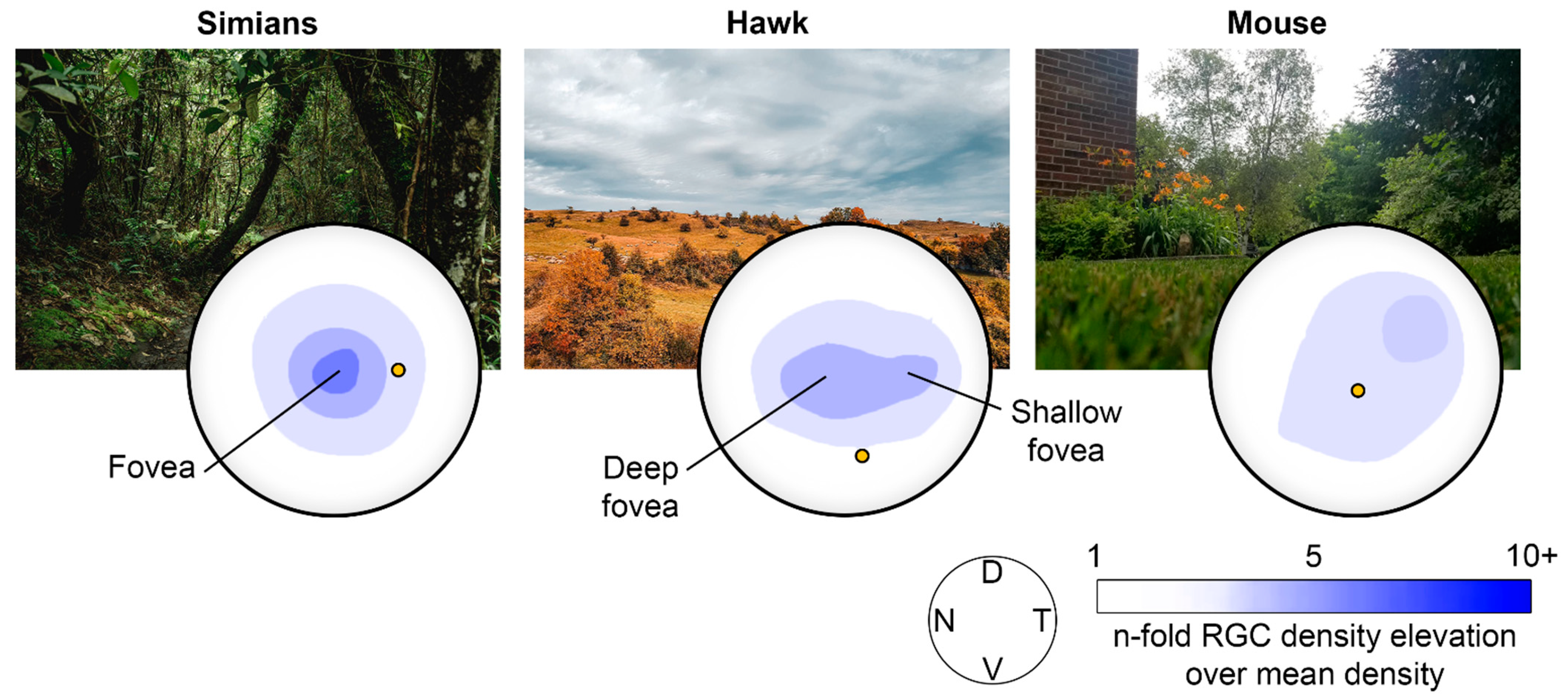
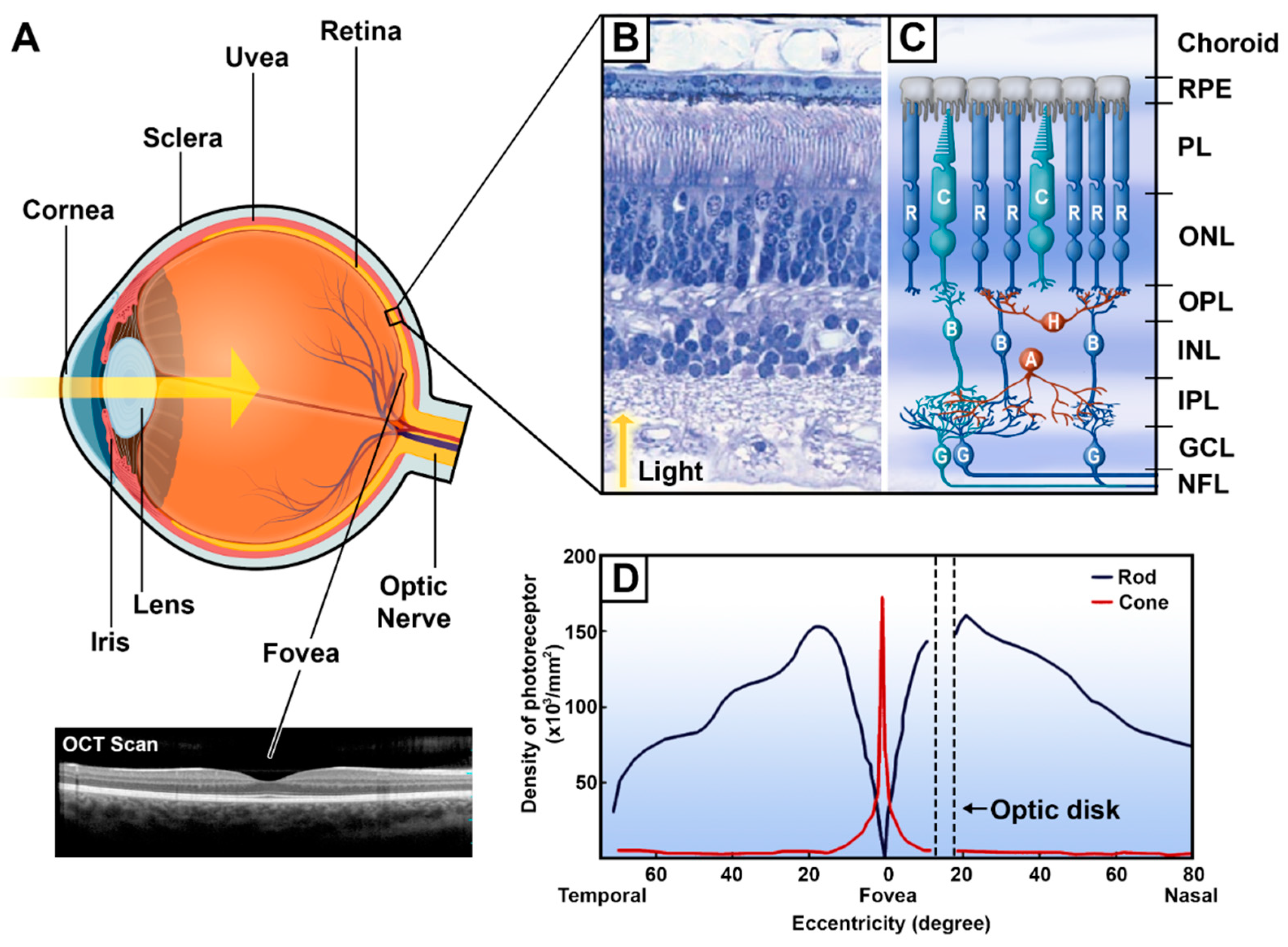
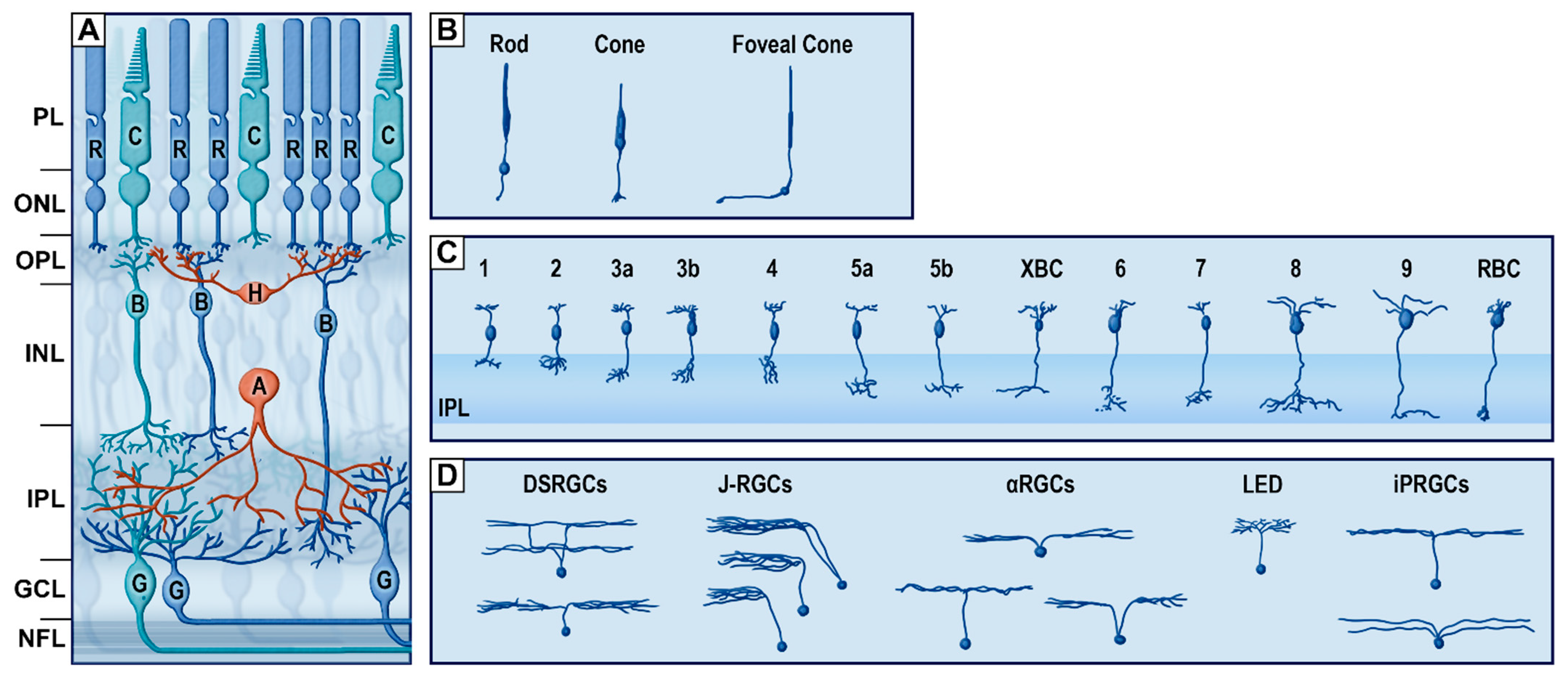
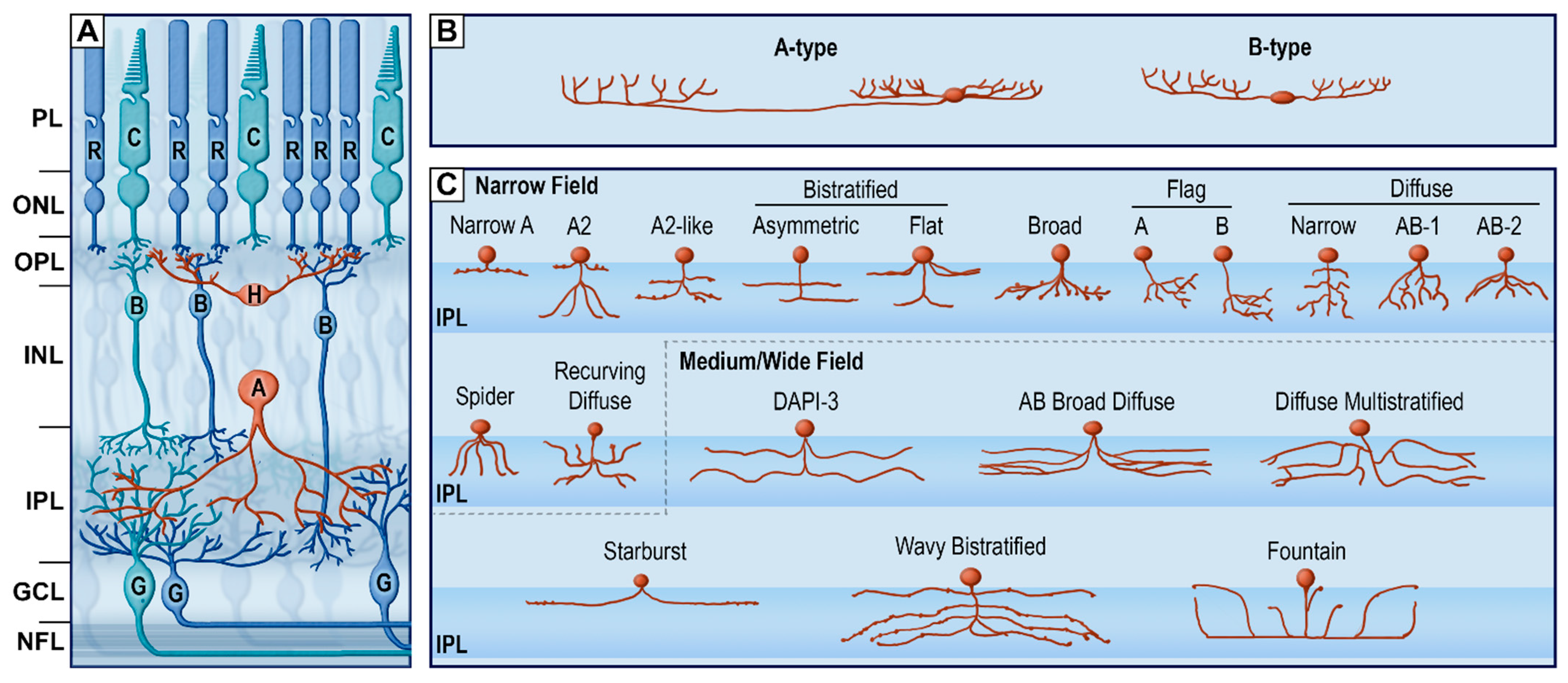
| Pathology | Glare | VF Loss | Scotoma | Night Blindness | Light Adaptation | Nystagmus | Fluctuating Vision | Depth Perception |
|---|---|---|---|---|---|---|---|---|
| Achromatopsia | x | x | x | |||||
| Albinism | x | x | x | |||||
| Coloboma | x | x | ||||||
| Diabetes | x | x | x | x | x | x | ||
| Glaucoma | x | x | x | x | x | x | ||
| Macular degeneration | x | x | x | x | ||||
| Optic atrophy | x | x | x | x | x | x | ||
| Retinal detachment | x | x | x | |||||
| Retinopathy of prematurity | x | x | x | x | x | |||
| Retinitis pigmentosa | x | x | x | x | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ptito, M.; Bleau, M.; Bouskila, J. The Retina: A Window into the Brain. Cells 2021, 10, 3269. https://doi.org/10.3390/cells10123269
Ptito M, Bleau M, Bouskila J. The Retina: A Window into the Brain. Cells. 2021; 10(12):3269. https://doi.org/10.3390/cells10123269
Chicago/Turabian StylePtito, Maurice, Maxime Bleau, and Joseph Bouskila. 2021. "The Retina: A Window into the Brain" Cells 10, no. 12: 3269. https://doi.org/10.3390/cells10123269
APA StylePtito, M., Bleau, M., & Bouskila, J. (2021). The Retina: A Window into the Brain. Cells, 10(12), 3269. https://doi.org/10.3390/cells10123269






