Challenge Tooth Regeneration in Adult Dogs with Dental Pulp Stem Cells on 3D-Printed Hydroxyapatite/Polylactic Acid Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
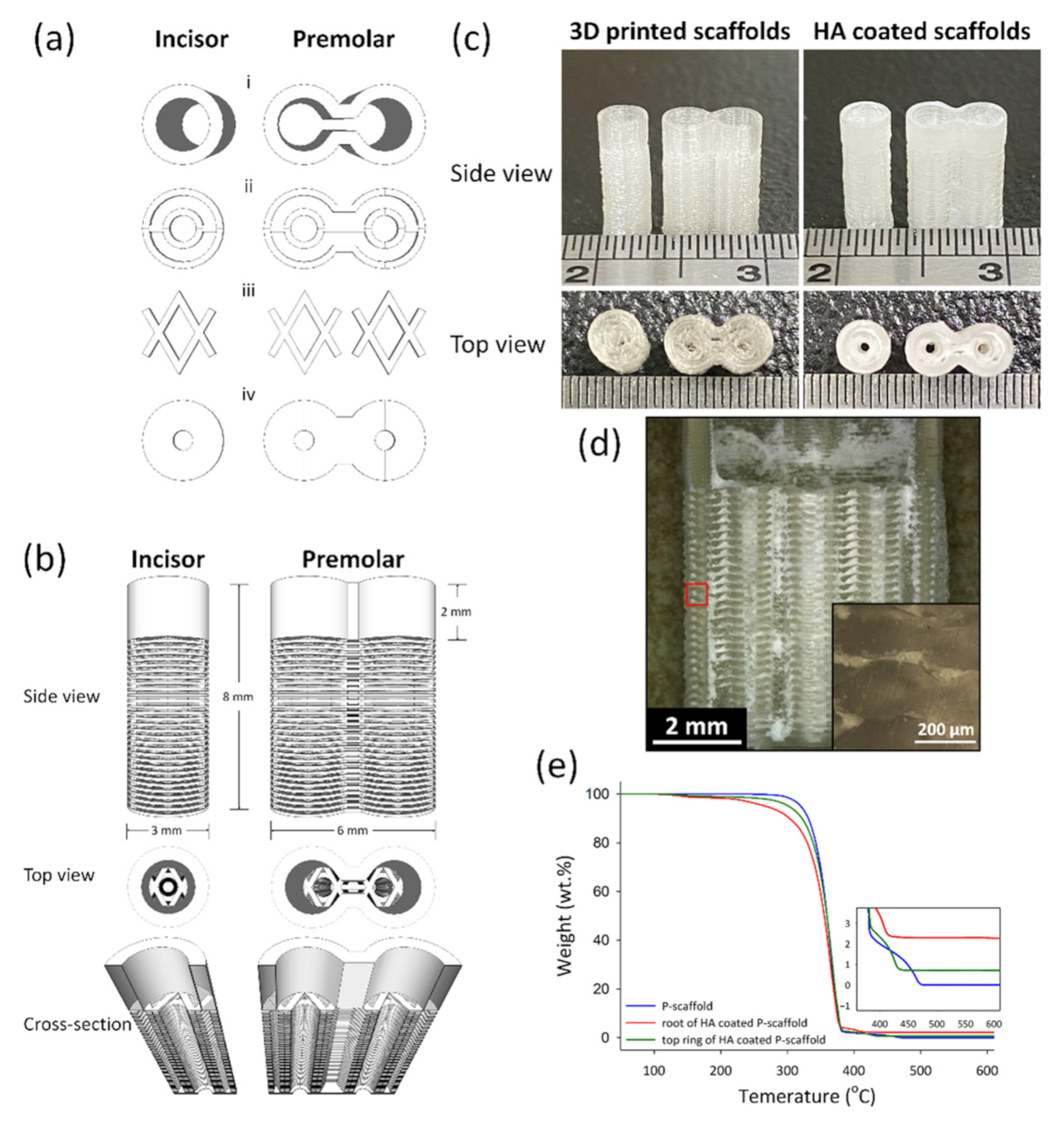
2.1. 3D-Printed PLA Scaffolds
2.2. Dip-Coating HA on PLA Scaffolds
2.3. Thermal Gravimetric Analysis (TGA) of Scaffolds
2.4. Micro Structure Observation
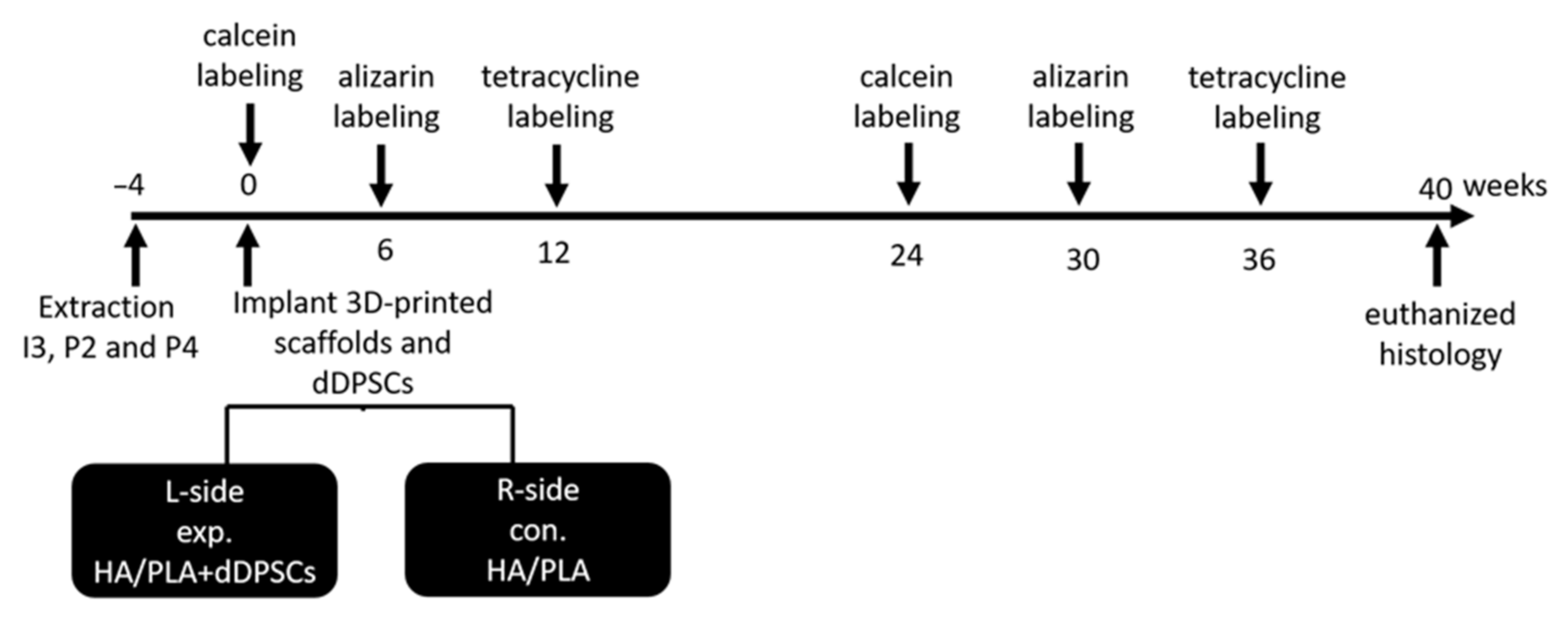
2.5. Animals
2.6. Extraction Tooth and Sequential Fluorochrome Labeling
2.7. Cultivation of Dental Pulp Stem Cells of Dogs
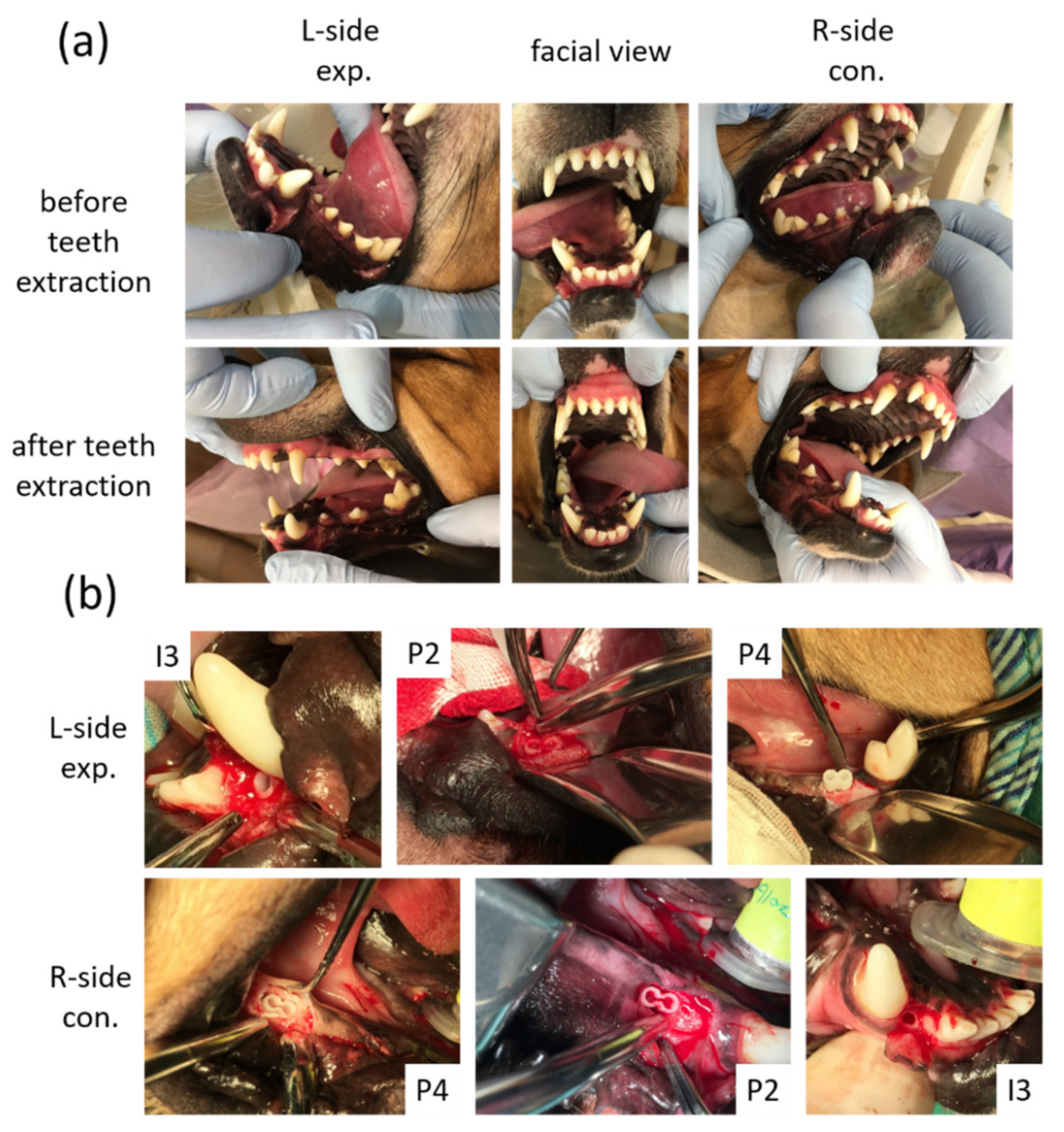
2.8. Surgical Procedures
2.9. Labeling
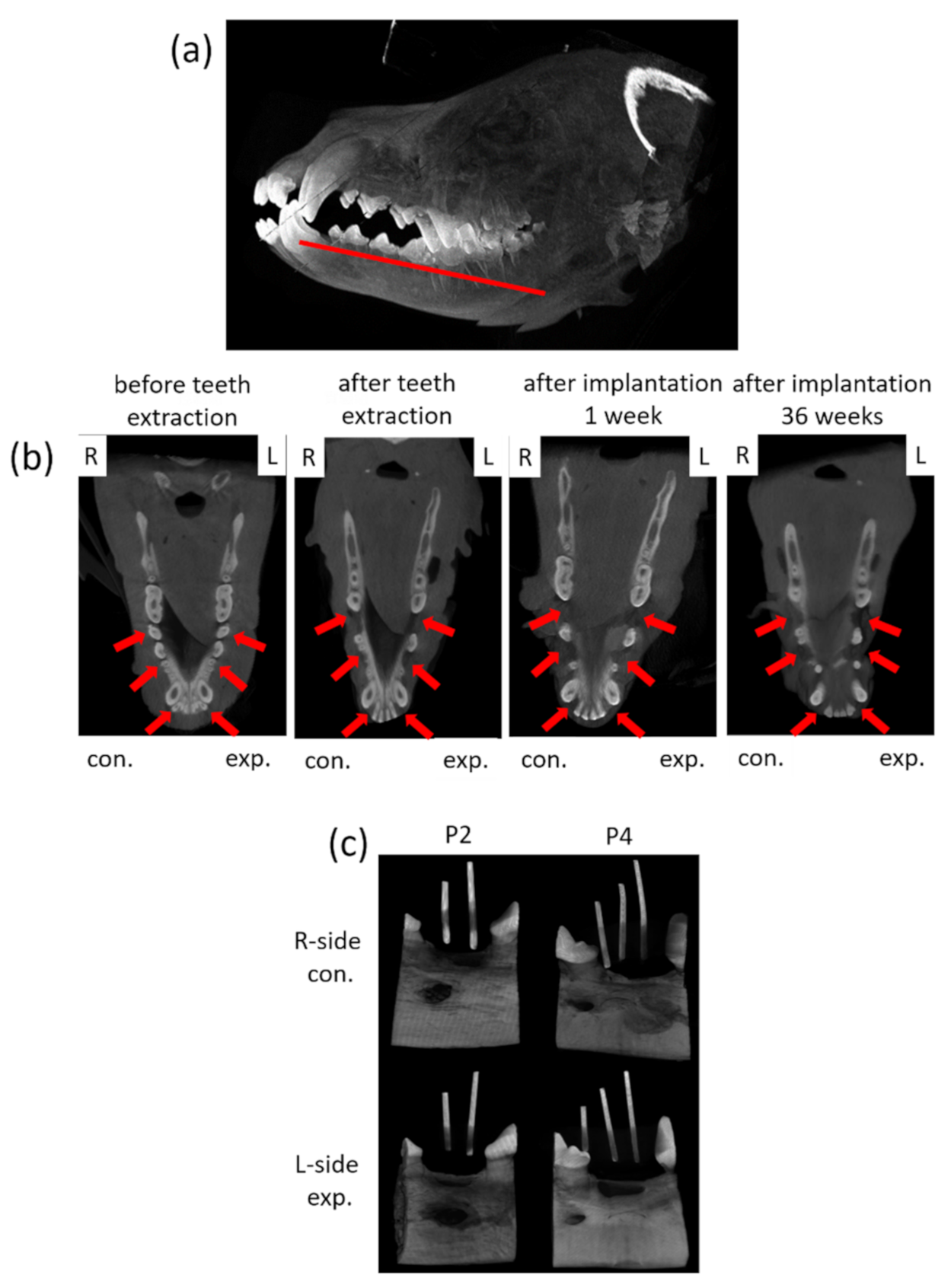
2.9.1. Cone Beam CT and Micro CT Observation
2.9.2. Undecalcified Ground Sections
2.10. Micro-CT and Fluorescence Analysis
2.11. Decalcified Histology
2.12. Statistical Analysis
3. Results
3.1. 3D-Printed HA/PLA Scaffolds
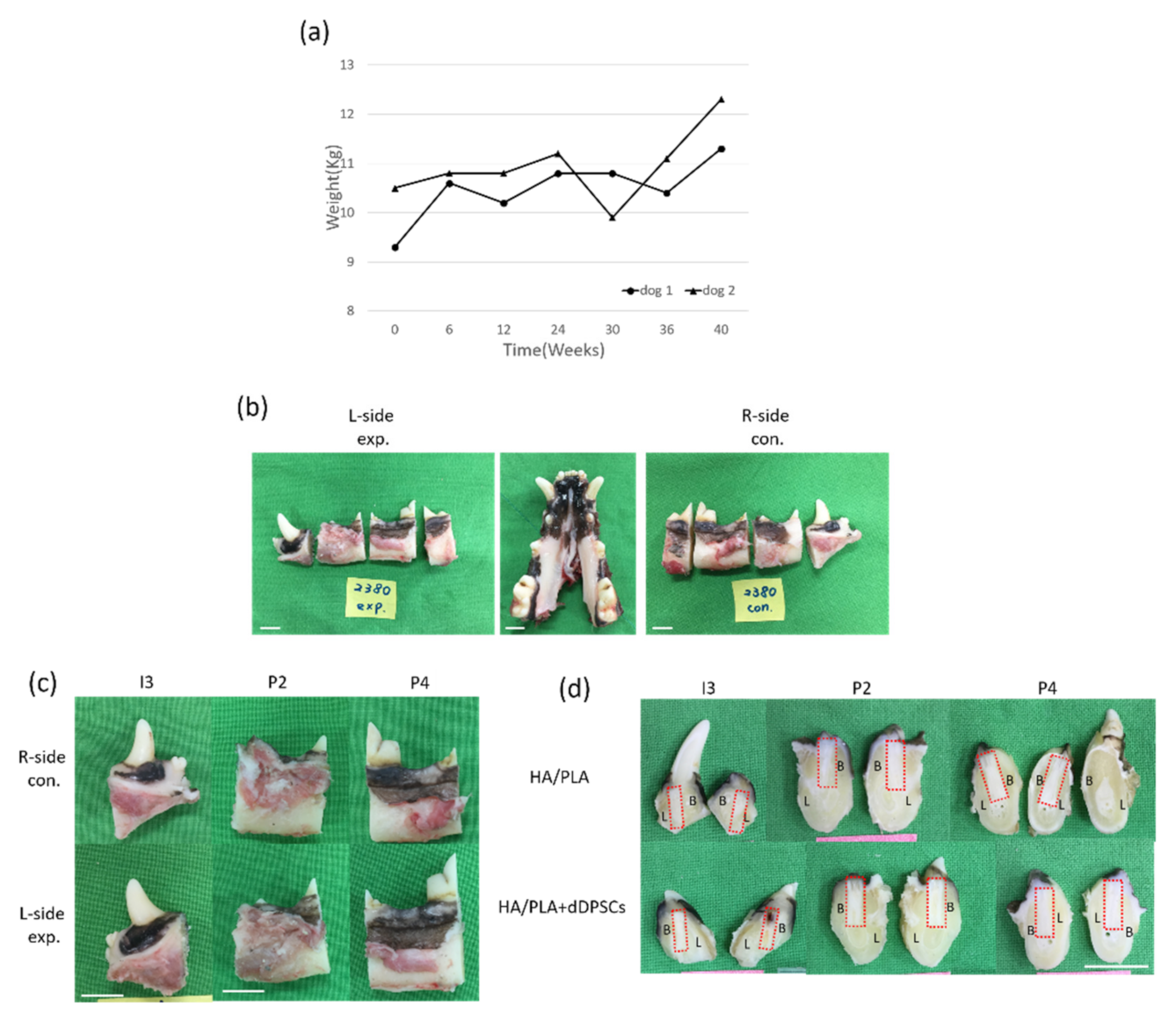
3.2. Clinical Findings
3.3. Micro-CT and Fluorescence Analysis
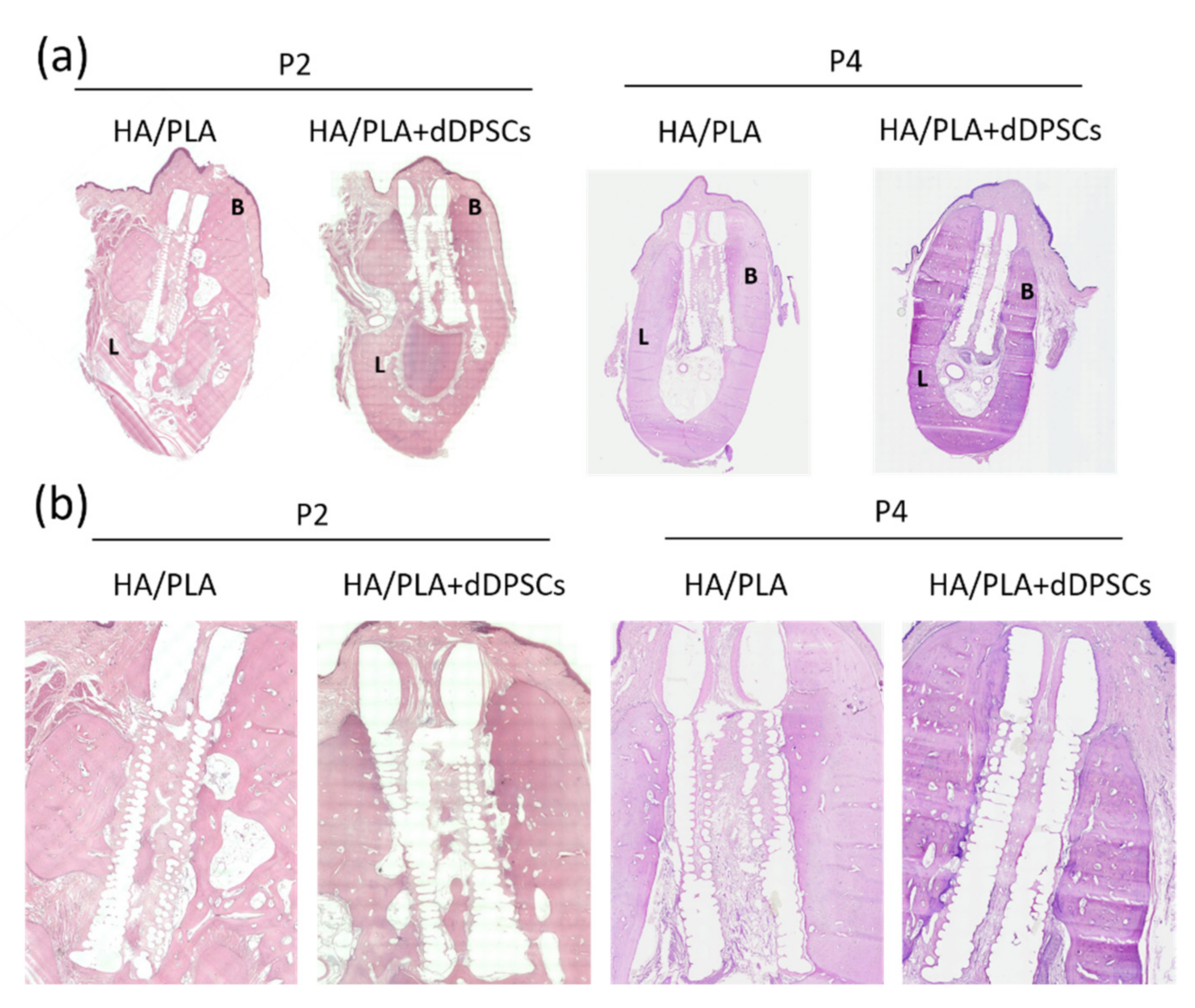
3.4. Histological Observations
4. Discussion
4.1. 3D-Printed Scaffolds
4.2. HA Coating on Scaffolds
4.3. Evaluation
4.4. Clinical Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.; Ren, J.; Li, R.; Guan, C.; Feng, Z.; Bao, B.; Wang, W.; Zhou, C. Tooth Regeneration: Insights from Tooth Development and Spatial-Temporal Control of Bioactive Drug Release. Stem Cell Rev. Rep. 2020, 16, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, T.F.; Huang, A.T.; Chang, H.H.; Lin, F.H.; Chen, S.T.; Chen, R.S.; Chou, C.H.; Lin, H.C.; Chiang, H.; Chen, M.H. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. J. Biomed. Mater. Res. A 2008, 86, 1062–1068. [Google Scholar] [CrossRef]
- Chen, R.S.; Chen, M.H.; Young, T.H. Induction of differentiation and mineralization in rat tooth germ cells on PVA through inhibition of ERK1/2. Biomaterials 2009, 30, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batouli, S.; Miura, M.; Brahim, J.; Tsutsui, T.W.; Fisher, L.W.; Gronthos, S.; Robey, P.G.; Shi, S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J. Dent. Res. 2003, 82, 976–981. [Google Scholar] [CrossRef]
- Tsutsui, T.W.; Inaba, T.; Fisher, L.W.; Robey, P.G.; Tsutsui, T. In vitro chromosome aberration tests using human dental pulp cells to detect the carcinogenic potential of chemical agents. Odontology 2006, 94, 44–50. [Google Scholar] [CrossRef]
- Matsui, M.; Kobayashi, T.; Tsutsui, T.W. CD146 positive human dental pulp stem cells promote regeneration of dentin/pulp-like structures. Hum. Cell 2018, 31, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Prescott, R.S.; Alsanea, R.; Fayad, M.I.; Johnson, B.R.; Wenckus, C.S.; Hao, J.; John, A.S.; George, A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J. Endod. 2008, 34, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Sui, B.; Wu, D.; Xiang, L.; Fu, Y.; Kou, X.; Shi, S. Dental Pulp Stem Cells: From Discovery to Clinical Application. J. Endod. 2020, 46, S46–S55. [Google Scholar] [CrossRef]
- Marin, E.; Briceno, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. Int. J. Nanomed. 2013, 8, 3071–3091. [Google Scholar]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Raveendran, N.T.; Fournier, B.; Ivanovski, S. Fabrication of biocompatible and bioabsorbable polycaprolactone/magnesium hydroxide 3D printed scaffolds: Degradation and in vitro osteoblasts interactions. Compos Part B-Eng 2020, 197, 108158. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, H.; Zhang, S.; Qu, S.; Jiang, Y.; Chen, M. Effects of Magnesium Oxide (MgO) Shapes on In Vitro and In Vivo Degradation Behaviors of PLA/MgO Composites in Long Term. Polymers 2020, 12, 1074. [Google Scholar] [CrossRef]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Lin, H.H.; Tsai, M.H.; Lin, S.P.; Chen, M.H. Zinc chloride for odontogenesis of dental pulp stem cells via metallothionein up-regulation. J. Endod. 2011, 37, 211–216. [Google Scholar] [CrossRef]
- Chang, H.H.; Yeh, C.L.; Wang, Y.L.; Fu, K.K.; Tsai, S.J.; Yang, J.H.; Lin, C.P. Neutralized Dicalcium Phosphate and Hydroxyapatite Biphasic Bioceramics Promote Bone Regeneration in Critical Peri-Implant Bone Defects. Materials 2020, 13, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, Y.L.; Wang, T.M.; Chang, H.H.; Lin, L.D. Effects of low-dose rhBMP-2 on peri-implant ridge augmentation in a canine model. J. Clin. Periodontol. 2021, 48, 734–744. [Google Scholar] [CrossRef]
- Lee, S.J.; Jo, H.H.; Lim, K.S.; Lim, D.; Lee, S.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Lim, J.Y.; Kwon, I.K.; et al. Heparin coating on 3D printed poly (l-lactic acid) biodegradable cardiovascular stent via mild surface modification approach for coronary artery implantation. Chem. Eng. J. 2019, 378, 122116. [Google Scholar] [CrossRef]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef]
- Pautke, C.; Tischer, T.; Vogt, S.; Haczek, C.; Deppe, H.; Neff, A.; Horch, H.H.; Schieker, M.; Kolk, A. New advances in fluorochrome sequential labelling of teeth using seven different fluorochromes and spectral image analysis. J. Anat. 2007, 210, 117–121. [Google Scholar] [CrossRef]
- Cooke, M.E.; Kalscheur, V.L.; Wilson, D.G.; Zdeblick, T.A. Preparation of large calcified bone sections for fluorescence histomorphometry. J. Histotechnol. 1999, 22, 93–96. [Google Scholar] [CrossRef]
- Rahn, B.A.; Perren, S.M. Xylenol Orange, a Fluorochrome Useful in Polychrome Sequential Labeling of Calcifying Tissues. Stain. Technol. 1971, 46, 125–129. [Google Scholar] [CrossRef]
- Malouvier, A.; Martin, F.; Orus, L.; De Pollak, C.; Marie, P.J. Comparative Use of Calcein and Oxytetracycline for the Analysis of Bone Mineralization in Rhesus-Monkeys. Med. Sci. Res. 1993, 21, 423–425. [Google Scholar]
- Kruyt, M.C.; Wilson, C.E.; de Bruijn, J.D.; van Blitterswijk, C.A.; Oner, C.F.; Verbout, A.J.; Dhert, W.J. The effect of cell-based bone tissue engineering in a goat transverse process model. Biomaterials 2006, 27, 5099–5106. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, M.C.; Dhert, W.J.; Oner, F.C.; van Blitterswijk, C.A.; Verbout, A.J.; de Bruijn, J.D. Analysis of ectopic and orthotopic bone formation in cell-based tissue-engineered constructs in goats. Biomaterials 2007, 28, 1798–1805. [Google Scholar] [CrossRef] [Green Version]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.A.; Marques, M.M.; Cavalcanti, S.C.; Pedroni, A.C.; Ferraz, E.P.; Miniello, T.G.; Moreira, M.S.; Jerônimo, T.; Deboni, M.C.; Lascala, C.A. Photobiomodulation as adjunctive therapy for guided bone regeneration. A microCT study in osteoporotic rat model. J. Photochem. Photobiol. B 2020, 213, 112053. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura-Yamada, S.; Konoki, R.; Baba, S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem. Cell Res. Ther. 2020, 11, 175. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.M.; Holmes, B.; Faucett, S.; Zhang, L.G. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng. Part B Rev. 2015, 21, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Legemate, K.; Tarafder, S.; Jun, Y.; Lee, C.H. Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds. J. Dent. Res. 2016, 95, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Hamlet, S.M.; Vaquette, C.; Shah, A.; Hutmacher, D.W.; Ivanovski, S. 3-Dimensional functionalized polycaprolactone-hyaluronic acid hydrogel constructs for bone tissue engineering. J. Clin. Periodontol. 2017, 44, 428–437. [Google Scholar] [CrossRef]
- Huang, K.H.; Lin, Y.H.; Shie, M.Y.; Lin, C.P. Effects of bone morphogenic protein-2 loaded on the 3D-printed MesoCS scaffolds. J. Formos Med. Assoc. 2018, 117, 879–887. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Q.; Wang, M. Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication 2017, 9, 025031. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Yu, J.; Becker, M.L. Enhanced osteogenic activity of poly(ester urea) scaffolds using facile post-3D printing peptide functionalization strategies. Biomaterials 2017, 141, 176–187. [Google Scholar] [CrossRef]
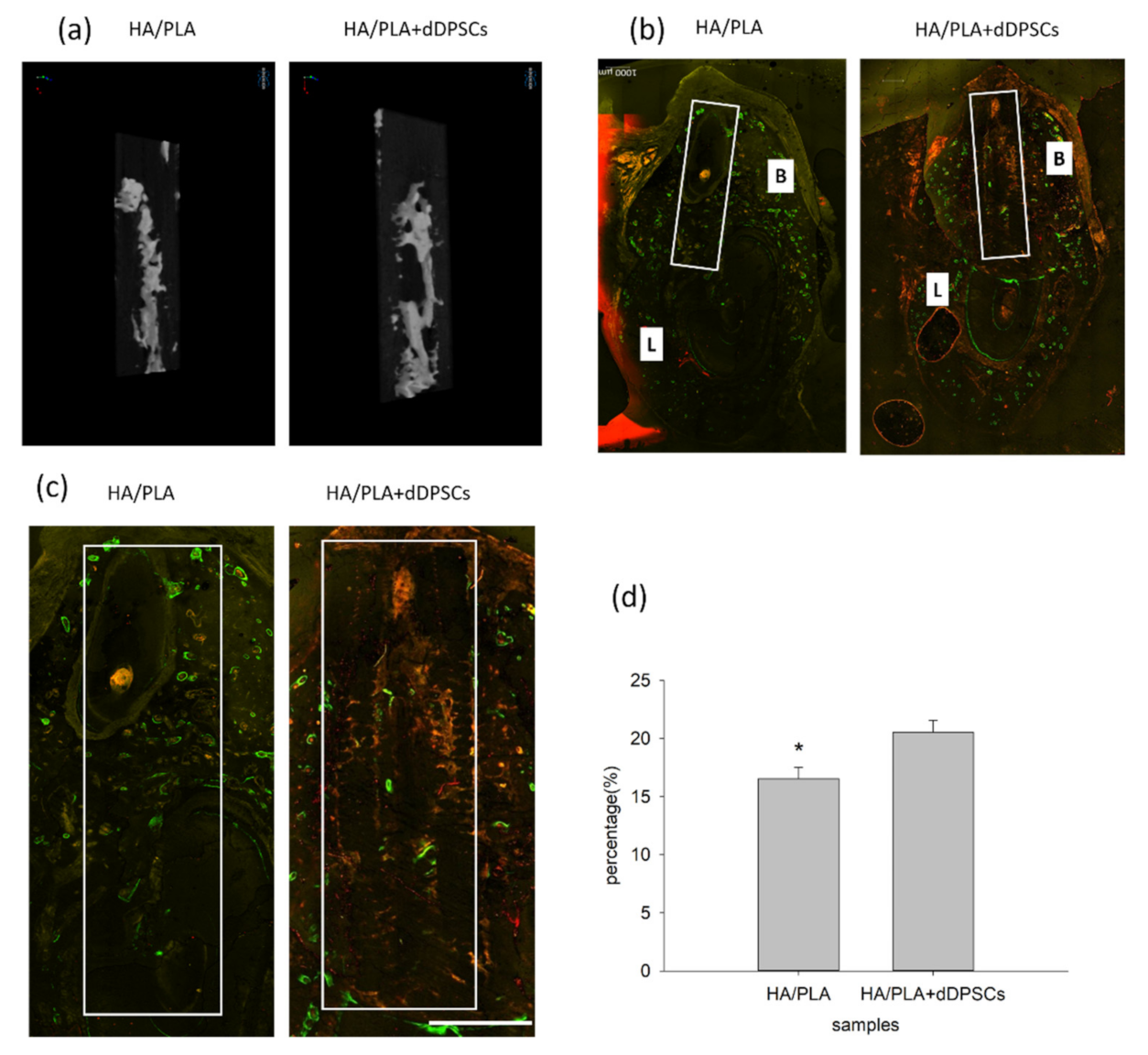
| Mineralization tissue volume/total volume (%) | 10.79 ± 3.29 | 12.93 ± 2.43 |
| Mineralization tissue number (1/mm) | 0.69 ± 0.16 | 0.79 ± 0.12 |
| Structure thickness (mm) | 0.30 ± 0.05 | 0.32 ± 0.03 |
| Structure separation (mm) | 3.03 ± 0.58 | 3.03 ± 017 |
| Structure model index | 3.42 ± 0.80 | 3.27 ± 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.-S.; Hsu, S.-H.; Chang, H.-H.; Chen, M.-H. Challenge Tooth Regeneration in Adult Dogs with Dental Pulp Stem Cells on 3D-Printed Hydroxyapatite/Polylactic Acid Scaffolds. Cells 2021, 10, 3277. https://doi.org/10.3390/cells10123277
Chen R-S, Hsu S-H, Chang H-H, Chen M-H. Challenge Tooth Regeneration in Adult Dogs with Dental Pulp Stem Cells on 3D-Printed Hydroxyapatite/Polylactic Acid Scaffolds. Cells. 2021; 10(12):3277. https://doi.org/10.3390/cells10123277
Chicago/Turabian StyleChen, Rung-Shu, Sheng-Hao Hsu, Hao-Hueng Chang, and Min-Huey Chen. 2021. "Challenge Tooth Regeneration in Adult Dogs with Dental Pulp Stem Cells on 3D-Printed Hydroxyapatite/Polylactic Acid Scaffolds" Cells 10, no. 12: 3277. https://doi.org/10.3390/cells10123277
APA StyleChen, R.-S., Hsu, S.-H., Chang, H.-H., & Chen, M.-H. (2021). Challenge Tooth Regeneration in Adult Dogs with Dental Pulp Stem Cells on 3D-Printed Hydroxyapatite/Polylactic Acid Scaffolds. Cells, 10(12), 3277. https://doi.org/10.3390/cells10123277





