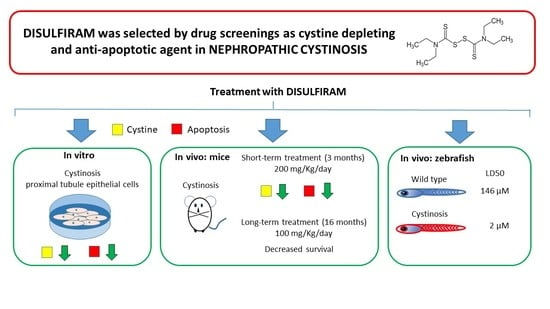
Benefits and Toxicity of Disulfiram in Preclinical Models of Nephropathic Cystinosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Quantitative Determination of Cystine in Cells
2.3. Measurement of Apoptosis in Cells
2.4. Cell Viability Assay
2.5. Redox Status
2.6. Tandem Mass Spectrometry
2.7. Studies on Cystinotic Mice
2.8. Quantitative Determination of Diethyldithiocarbamate (DDC) and Cystine in Tissues
2.9. Measurement of Apoptosis in Tissue
2.10. Zebrafish Assays
2.11. Statistical Analysis
3. Results
3.1. In Vitro DSF Studies
3.2. In Vivo Studies: Murine Treatment with High DSF Dose
3.3. In Vivo Studies: Murine Treatment with Low DSF Dose
3.4. In Vivo Studies: Zebrafish Embryos and Larvae
3.5. N-Acetyl Cysteine Can Rescue Disulfiram Toxicity in Cystinotic Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Elmonem, M.A.; Veys, K.R.; Soliman, N.A.; van Dyck, M.; van den Heuvel, L.P.; Levtchenko, E. Cystinosis: A review. Orphanet J. Rare Dis. 2016, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Emma, F.; Nesterova, G.; Langman, C.; Labbe, A.; Cherqui, S.; Goodyer, P.; Janssen, M.C.; Greco, M.; Topaloglu, R.; Elenberg, E.; et al. Nephropathic cystinosis: An international consensus document. Nephrol. Dial. Transplant. 2014, 29 (Suppl. 4), iv87–iv94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasimer, R.N.; Langman, C.B. Adult complications of nephropathic cystinosis: A systematic review. Pediatr. Nephrol. 2021, 36, 223–236. [Google Scholar] [CrossRef]
- Gaide Chevronnay, H.P.; Janssens, V.; Van Der Smissen, P.; N’Kuli, F.; Nevo, N.; Guiot, Y.; Levtchenko, E.; Marbaix, E.; Pierreux, C.E.; Cherqui, S.; et al. Time course of pathogenic and adaptation mechanisms in cystinotic mouse kidneys. J. Am. Soc. Nephrol. 2014, 25, 1256–1269. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Helip-Wooley, A.; Thoene, J. Lysosomal cystine storage augments apoptosis in cultured human fibroblasts and renal tubular epithelial cells. J. Am. Soc. Nephrol. 2002, 13, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Taranta, A.; Bellomo, F.; Petrini, S.; Polishchuk, E.; De Leo, E.; Rega, L.R.; Pastore, A.; Polishchuk, R.; De Matteis, M.A.; Emma, F. Cystinosin-LKG rescues cystine accumulation and decreases apoptosis rate in cystinotic proximal tubular epithelial cells. Pediatr. Res. 2017, 81, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Jezegou, A.; Llinares, E.; Anne, C.; Kieffer-Jaquinod, S.; O’Regan, S.; Aupetit, J.; Chabli, A.; Sagne, C.; Debacker, C.; Chadefaux-Vekemans, B.; et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc. Natl. Acad. Sci. USA 2012, 109, E3434–E3443. [Google Scholar] [CrossRef] [Green Version]
- Emma, F.; Hoff, W.V.; Hohenfellner, K.; Topaloglu, R.; Greco, M.; Ariceta, G.; Bettini, C.; Bockenhauer, D.; Veys, K.; Pape, L.; et al. An international cohort study spanning five decades assessed outcomes of nephropathic cystinosis. Kidney Int. 2021, 100, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, F.; De Leo, E.; Taranta, A.; Giaquinto, L.; Di Giovamberardino, G.; Montefusco, S.; Rega, L.R.; Pastore, A.; Medina, D.L.; Di Bernardo, D.; et al. Drug repurposing in rare diseases: An integrative study of drug screening and transcriptomic analysis in nephropathic cystinosis. Int. J. Mol. Sci. 2021. Submitted. [Google Scholar]
- Wilmer, M.J.; Saleem, M.A.; Masereeuw, R.; Ni, L.; van der Velden, T.J.; Russel, F.G.; Mathieson, P.W.; Monnens, L.A.; van den Heuvel, L.P.; Levtchenko, E.N. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010, 339, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastore, A.; Lo Russo, A.; Greco, M.; Rizzoni, G.; Federici, G. Semiautomated method for determination of cystine concentration in polymorphonuclear leukocytes. Clin. Chem. 2000, 46, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Jamalpoor, A.; van Gelder, C.A.; Yousef Yengej, F.A.; Zaal, E.A.; Berlingerio, S.P.; Veys, K.R.; Pou Casellas, C.; Voskuil, K.; Essa, K.; Ammerlaan, C.M.; et al. Cysteamine-bicalutamide combination therapy corrects proximal tubule phenotype in cystinosis. EMBO Mol. Med. 2021, 13, e13067. [Google Scholar] [CrossRef] [PubMed]
- Nevo, N.; Chol, M.; Bailleux, A.; Kalatzis, V.; Morisset, L.; Devuyst, O.; Gubler, M.C.; Antignac, C. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol. Dial. Transplant. 2010, 25, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Elmonem, M.A.; Khalil, R.; Khodaparast, L.; Khodaparast, L.; Arcolino, F.O.; Morgan, J.; Pastore, A.; Tylzanowski, P.; Ny, A.; Lowe, M.; et al. Cystinosis (ctns) zebrafish mutant shows pronephric glomerular and tubular dysfunction. Sci. Rep. 2017, 7, 42583. [Google Scholar] [CrossRef] [Green Version]
- Harper, C.; Lawrence, C. The Laboratory Zebrafish, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–274. [Google Scholar]
- Kragh, H. From disulfiram to antabuse: The invention of a drug. Bull. Hist. Chem. 2008, 33, 82–88. [Google Scholar]
- Johansson, B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr. Scand. 1992, 369, 15–26. [Google Scholar] [CrossRef]
- Lipsky, J.J.; Shen, M.L.; Naylor, S. In vivo inhibition of aldehyde dehydrogenase by disulfiram. Chem. Biol. Interact. 2001, 130–132, 93–102. [Google Scholar] [CrossRef]
- Vallari, R.C.; Pietruszko, R. Human aldehyde dehydrogenase: Mechanism of inhibition of disulfiram. Science 1982, 216, 637–639. [Google Scholar] [CrossRef]
- Deng, W.; Yang, Z.; Yue, H.; Ou, Y.; Hu, W.; Sun, P. Disulfiram suppresses NLRP3 inflammasome activation to treat peritoneal and gouty inflammation. Free Radic. Biol. Med. 2020, 152, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, G.; Caiello, I.; Cherqui, S.; Whisenant, T.; Petrini, S.; Emma, F.; De Benedetti, F. Inflammasome activation by cystine crystals: Implications for the pathogenesis of cystinosis. J. Am. Soc. Nephrol. 2014, 25, 1163–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmonem, M.A.; Makar, S.H.; van den Heuvel, L.; Abdelaziz, H.; Abdelrahman, S.M.; Bossuyt, X.; Janssen, M.C.; Cornelissen, E.A.; Lefeber, D.J.; Joosten, L.A.; et al. Clinical utility of chitotriosidase enzyme activity in nephropathic cystinosis. Orphanet J. Rare Dis. 2014, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Park, M.A.; Pejovic, V.; Kerisit, K.G.; Junius, S.; Thoene, J.G. Increased apoptosis in cystinotic fibroblasts and renal proximal tubule epithelial cells results from cysteinylation of protein kinase Cdelta. J. Am. Soc. Nephrol. 2006, 17, 3167–3175. [Google Scholar] [CrossRef]
- De Rasmo, D.; Signorile, A.; De Leo, E.; Polishchuk, E.V.; Ferretta, A.; Raso, R.; Russo, S.; Polishchuk, R.; Emma, F.; Bellomo, F. Mitochondrial Dynamics of Proximal Tubular Epithelial Cells in Nephropathic Cystinosis. Int. J. Mol. Sci. 2019, 21, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, M.; Mitchell, S.J.; Wahl, D.; Diaz, A.; Singh, A.; Seo, W.; Wang, M.; Ali, A.; Kaiser, T.; Price, N.L.; et al. Disulfiram Treatment Normalizes Body Weight in Obese Mice. Cell Metab. 2020, 32, 203–214.e4. [Google Scholar] [CrossRef] [PubMed]
- Bernier, M.; Harney, D.; Koay, Y.C.; Diaz, A.; Singh, A.; Wahl, D.; Pulpitel, T.; Ali, A.; Guiterrez, V.; Mitchell, S.J.; et al. Elucidating the mechanisms by which disulfiram protects against obesity and metabolic syndrome. NPJ Aging Mech. Dis. 2020, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Hothi, P.; Martins, T.J.; Chen, L.; Deleyrolle, L.; Yoon, J.G.; Reynolds, B.; Foltz, G. High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget 2012, 3, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allensworth, J.L.; Evans, M.K.; Bertucci, F.; Aldrich, A.J.; Festa, R.A.; Finetti, P.; Ueno, N.T.; Safi, R.; McDonnell, D.P.; Thiele, D.J.; et al. Disulfiram (DSF) acts as a copper ionophore to induce copper-dependent oxidative stress and mediate anti-tumor efficacy in inflammatory breast cancer. Mol. Oncol. 2015, 9, 1155–1168. [Google Scholar] [CrossRef] [Green Version]
- Falls-Hubert, K.C.; Butler, A.L.; Gui, K.; Anderson, M.; Li, M.; Stolwijk, J.M.; Rodman, S.N., III; Solst, S.R.; Tomanek-Chalkley, A.; Searby, C.C.; et al. Disulfiram causes selective hypoxic cancer cell toxicity and radio-chemo-sensitization via redox cycling of copper. Free Radic. Biol. Med. 2020, 150, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, L.; Pastore, A.; Rizzo, C.; Piemonte, F.; Rizzoni, G.; Emma, F. Impaired activity of the gamma-glutamyl cycle in nephropathic cystinosis fibroblasts. Pediatr. Res. 2006, 59, 332–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levtchenko, E.; de Graaf-Hess, A.; Wilmer, M.; van den Heuvel, L.; Monnens, L.; Blom, H. Altered status of glutathione and its metabolites in cystinotic cells. Nephrol. Dial. Transplant. 2005, 20, 1828–1832. [Google Scholar] [CrossRef] [Green Version]
- Cherqui, S.; Courtoy, P.J. The renal Fanconi syndrome in cystinosis: Pathogenic insights and therapeutic perspectives. Nat. Rev. Nephrol. 2017, 13, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, F.; Signorile, A.; Tamma, G.; Ranieri, M.; Emma, F.; De Rasmo, D. Impact of atypical mitochondrial cyclic-AMP level in nephropathic cystinosis. Cell Mol. Life Sci. 2018, 75, 3411–3422. [Google Scholar] [CrossRef]
- Galarreta, C.I.; Forbes, M.S.; Thornhill, B.A.; Antignac, C.; Gubler, M.C.; Nevo, N.; Murphy, M.P.; Chevalier, R.L. The swan-neck lesion: Proximal tubular adaptation to oxidative stress in nephropathic cystinosis. Am. J. Physiol. Renal. Physiol. 2015, 308, F1155–F1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Festa, B.P.; Chen, Z.; Berquez, M.; Debaix, H.; Tokonami, N.; Prange, J.A.; Hoek, G.V.; Alessio, C.; Raimondi, A.; Nevo, N.; et al. Impaired autophagy bridges lysosomal storage disease and epithelial dysfunction in the kidney. Nat. Commun. 2018, 9, 161. [Google Scholar] [CrossRef]
- Nobel, C.S.; Kimland, M.; Nicholson, D.W.; Orrenius, S.; Slater, A.F. Disulfiram is a potent inhibitor of proteases of the caspase family. Chem. Res. Toxicol. 1997, 10, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Nobel, C.S.; Burgess, D.H.; Zhivotovsky, B.; Burkitt, M.J.; Orrenius, S.; Slater, A.F. Mechanism of dithiocarbamate inhibition of apoptosis: Thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem. Res. Toxicol. 1997, 10, 636–643. [Google Scholar] [CrossRef] [PubMed]








| WT | KO | ||||
|---|---|---|---|---|---|
| Urine Tests | Measure Unit | Untreated | DSF 200 | Untreated | DSF 200 |
| Albumin | µg/mg Creatinine | 5.05 [3.49–12.1] | 7.66 [6.59–11.1] | 8.58 [5.02–9.79] | 20.7 [10.5–76.3] |
| Glucose | mg/mg Creatinine | 0.29 [0.26–1.02] | 0.34 [0.24–0.78] | 7.59 [4.41–16.2] § | 0.40 [0.28–1.66] * |
| LMWP | µg/mg Creatinine | 38.2 [13.8–39.5] | 37.7 [10.2–69.1] | 157 [75.1–447] § | 258 [79.6–474] |
| Calcium | mg/mg Creatinine | 0.12 [0.09–0.17] | 0.12 [0.09–0.18] | 0.21 [0.15–0.26] | 1.17 [0.52–3.36] * |
| Phosphate | mg/mg Creatinine | 0.60 [0.14–1.66] | 1.51 [0.15–2.88] | 0.66 [0.15–1.85] | 2.58 [0.99–6.43] |
| WT | KO | ||||
|---|---|---|---|---|---|
| Urine Tests | Measure Unit | Untreated | DSF 100 | Untreated | DSF 100 |
| Albumin | µg/mg Creatinine | 5.05 [4.26–6.40] | 11.5 [5.68–13.8] | 15.1 [14.4–36.2] § | 19.0 [17.9–36.8] * |
| Glucose | mg/mg Creatinine | 0.18 [0.08–0.84] | 0.42 [0.19–1.12] | 2.71 [1.34–3.85] § | 9.00 [4.21–29.4] * |
| LMWP | µg/mg Creatinine | 16.4 [5.30–26.6] | 35.4 [26.8–63.2] | 1939 [985–4353] § | 5758 [1434–13972] * |
| Calcium | mg/mg Creatinine | 0.30 [0.25–0.35] | 0.26 [0.22–0.35] | 0.32 [0.30–0.57] | 0.42 [0.21–0.54] |
| Phosphate | mg/mg Creatinine | 2.08 [0.70–2.77] | 1.74 [0.17–2.50] | 2.93 [1.47–3.56] | 2.62 [1.03–3.53] |
| Diuresis | ml | 1.20 [0.75–2.10] | 1.75 [1.62–2.00] | 3.05 [2.32–3.25] § | 3.00 [1.60–3.25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranta, A.; Elmonem, M.A.; Bellomo, F.; De Leo, E.; Boenzi, S.; Janssen, M.J.; Jamalpoor, A.; Cairoli, S.; Pastore, A.; De Stefanis, C.; et al. Benefits and Toxicity of Disulfiram in Preclinical Models of Nephropathic Cystinosis. Cells 2021, 10, 3294. https://doi.org/10.3390/cells10123294
Taranta A, Elmonem MA, Bellomo F, De Leo E, Boenzi S, Janssen MJ, Jamalpoor A, Cairoli S, Pastore A, De Stefanis C, et al. Benefits and Toxicity of Disulfiram in Preclinical Models of Nephropathic Cystinosis. Cells. 2021; 10(12):3294. https://doi.org/10.3390/cells10123294
Chicago/Turabian StyleTaranta, Anna, Mohamed A. Elmonem, Francesco Bellomo, Ester De Leo, Sara Boenzi, Manoe J. Janssen, Amer Jamalpoor, Sara Cairoli, Anna Pastore, Cristiano De Stefanis, and et al. 2021. "Benefits and Toxicity of Disulfiram in Preclinical Models of Nephropathic Cystinosis" Cells 10, no. 12: 3294. https://doi.org/10.3390/cells10123294
APA StyleTaranta, A., Elmonem, M. A., Bellomo, F., De Leo, E., Boenzi, S., Janssen, M. J., Jamalpoor, A., Cairoli, S., Pastore, A., De Stefanis, C., Colucci, M., Rega, L. R., Giovannoni, I., Francalanci, P., van den Heuvel, L. P., Dionisi-Vici, C., Goffredo, B. M., Masereeuw, R., Levtchenko, E., & Emma, F. (2021). Benefits and Toxicity of Disulfiram in Preclinical Models of Nephropathic Cystinosis. Cells, 10(12), 3294. https://doi.org/10.3390/cells10123294