Guidelines for a Morphometric Analysis of Prokaryotic and Eukaryotic Cells by Scanning Electron Microscopy
Abstract
:1. Introduction
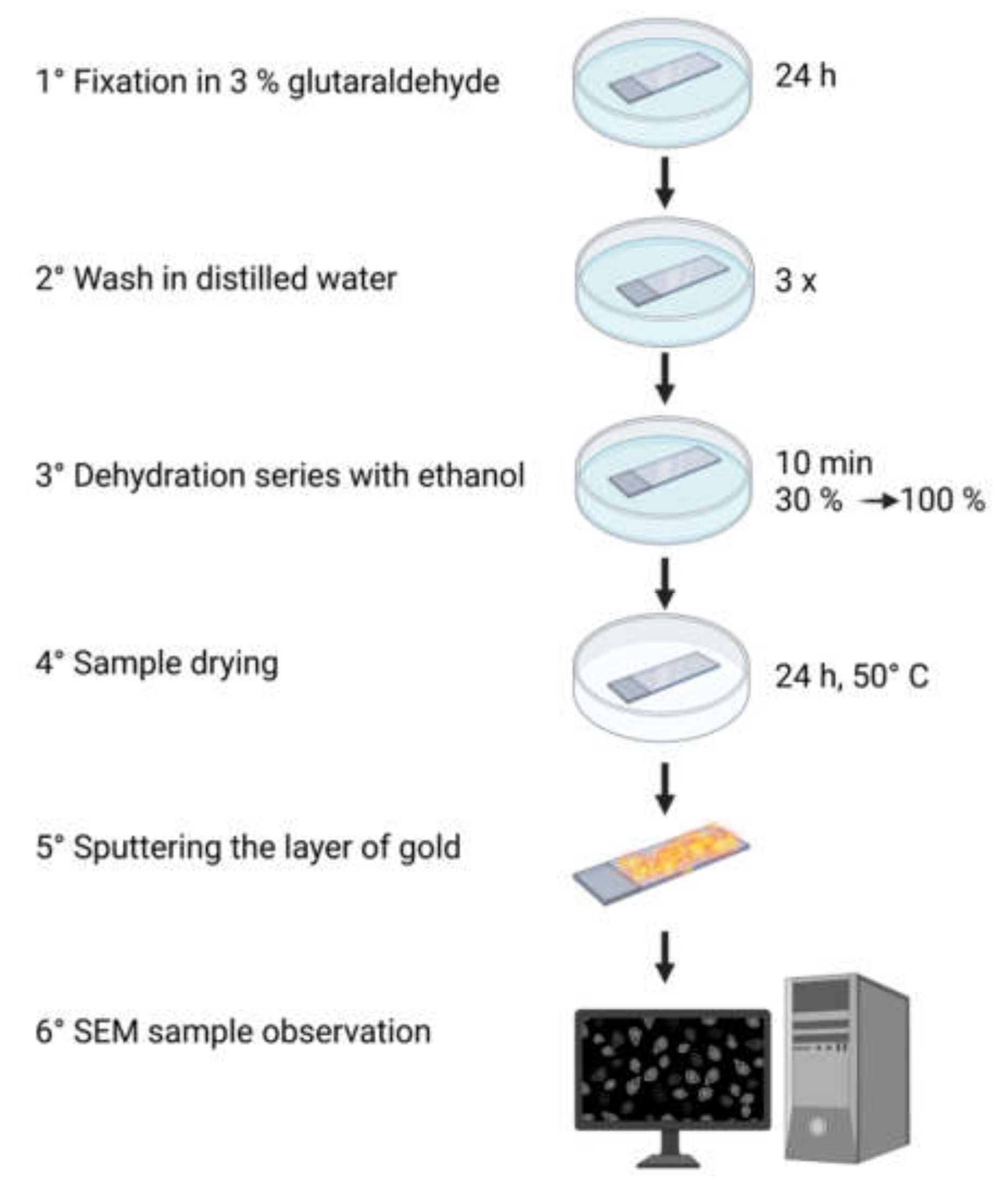
2. Sample Preparation
2.1. Fixation
2.2. Dehydration
2.3. Sputter-Coating
2.4. Optimised Protocol for Sample Preparation
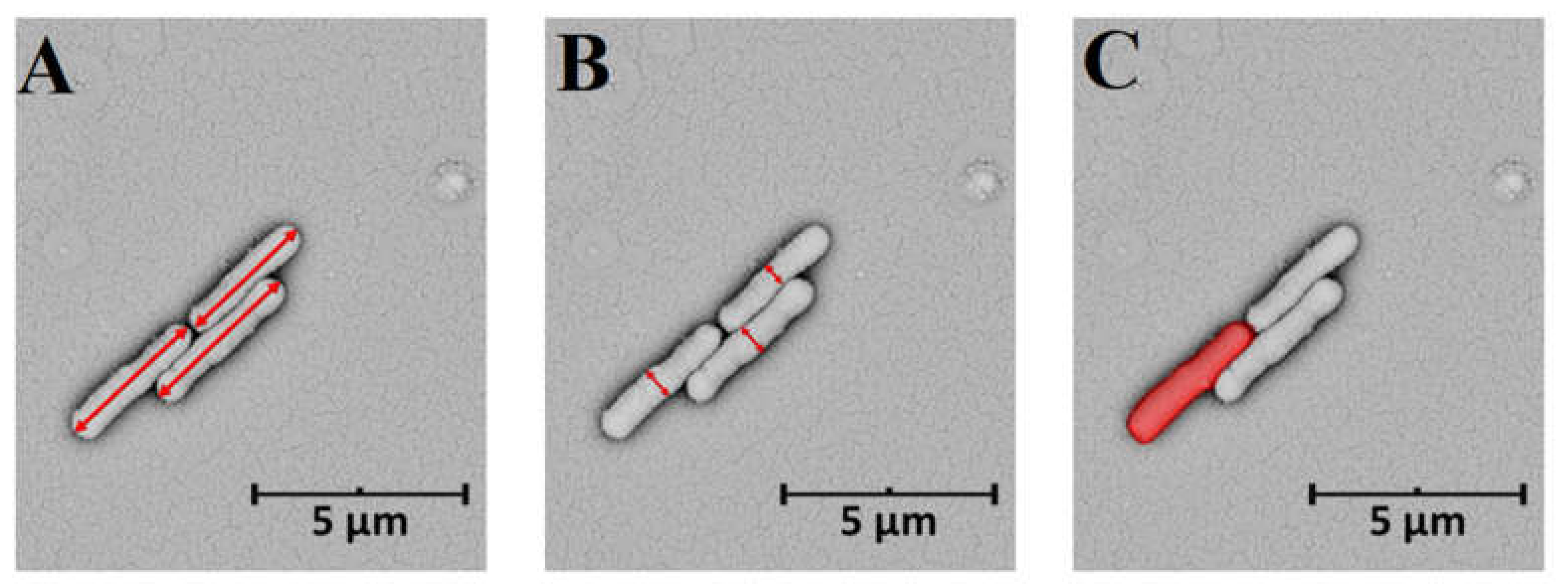
3. Morphometric Analysis of Model Prokaryotic Cells: Escherichia coli
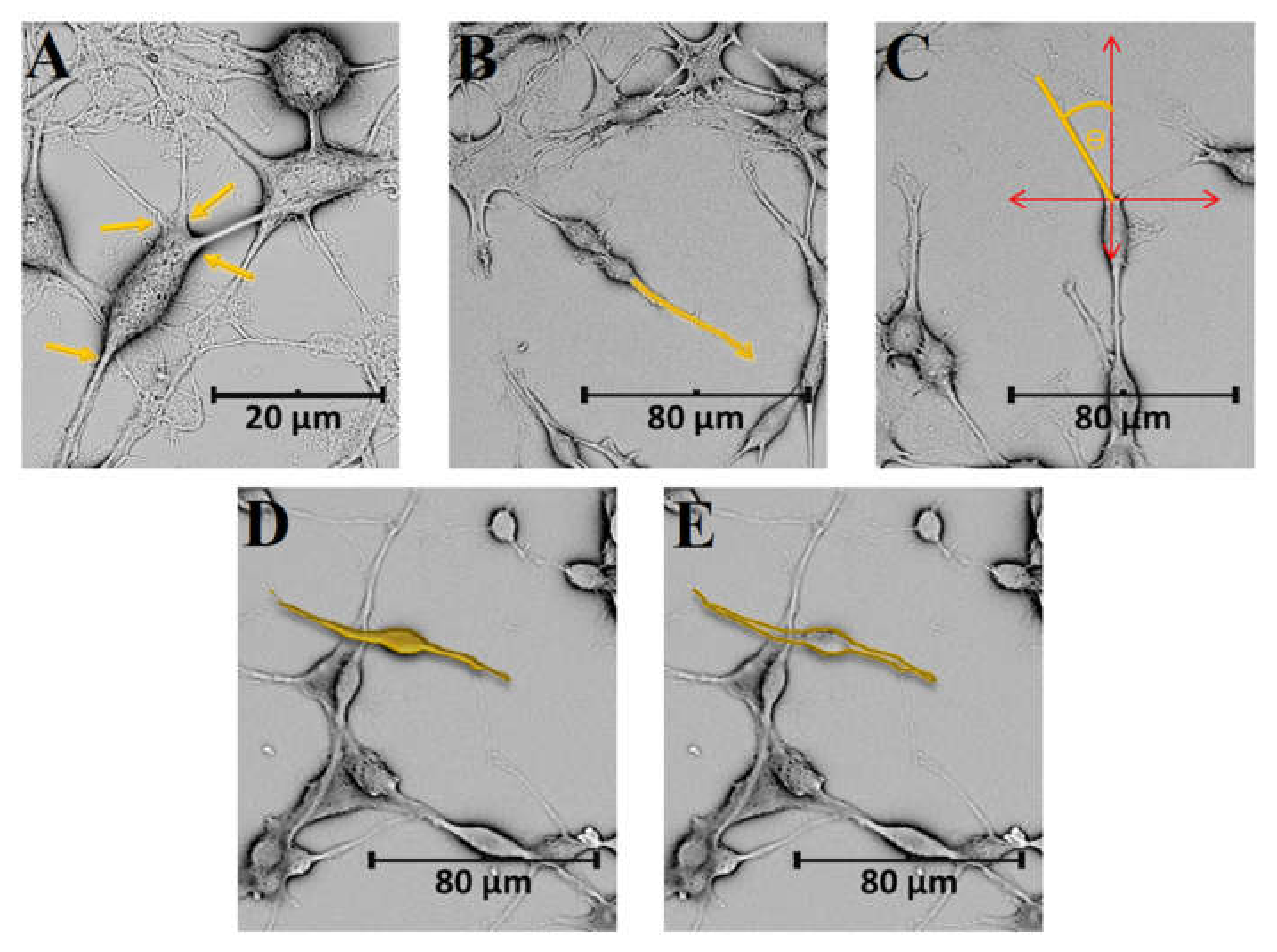
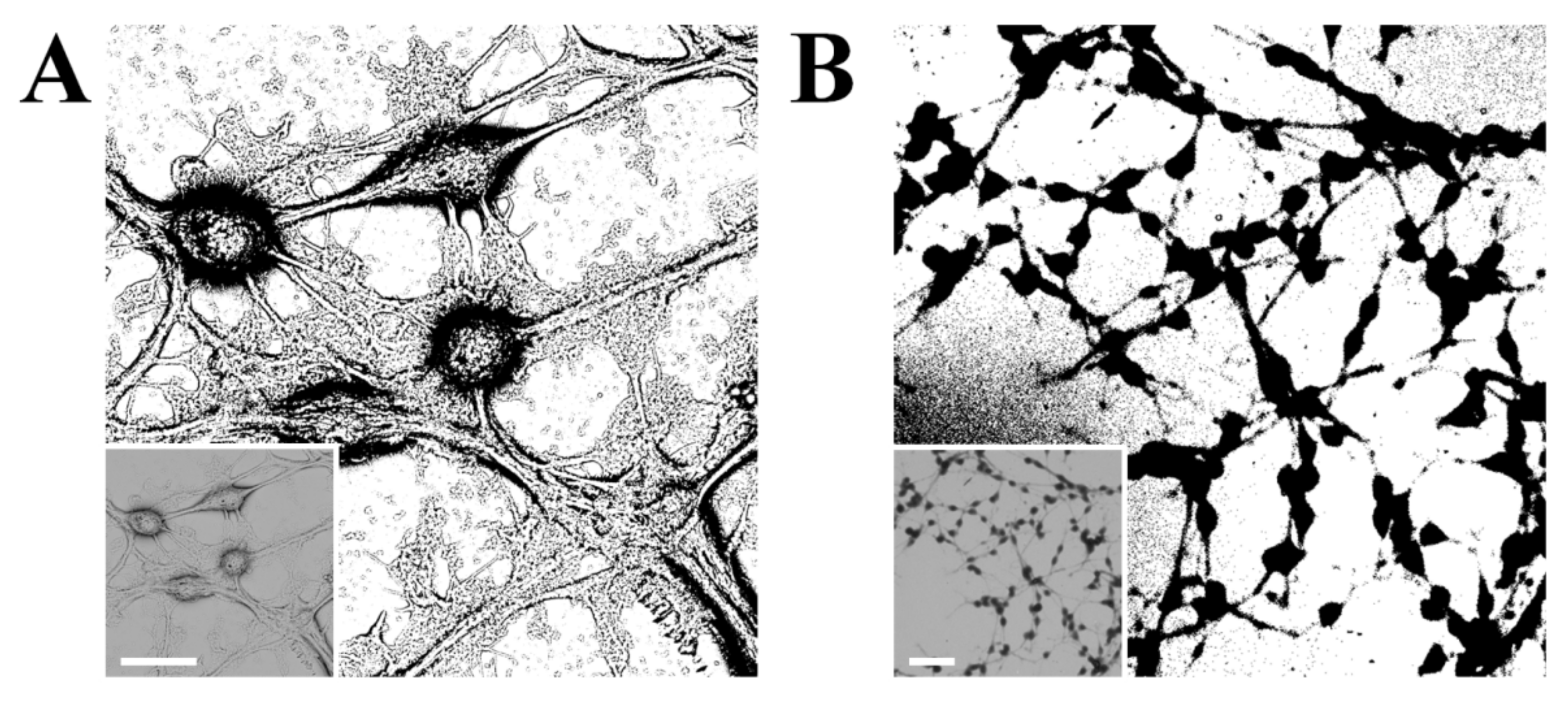
4. Morphometric Analysis of Model Eukaryotic Cells: B35 Neuroblastoma Cells
4.1. Conventional Morphometric Descriptors
4.2. Fractal Analysis
4.3. Textural Descriptors
5. Conclusions
Funding
Conflicts of Interest
References
- Vihinen, H.; Belevich, I.; Jokitalo, E. Three dimensional electron microscopy of cellular organelles by serial block face SEM and ET. Microsc. Anal. 2013, 27, 7–10. [Google Scholar]
- Smith, K.C.A.; Wells, O.C.; McMullan, D. The fiftieth anniversary of the first applications of the scanning electron microscope in materials research. Phys. Procedia 2008, 1, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Dokland, T.; Hutmacher, D.W.; Ng, M.M.-L.; Schantz, J.-T. Techniques in Microscopy for Biomedical Applications; World Scientific: Singapore, 2006; Volume 2. [Google Scholar] [CrossRef]
- Goldstein, A.; Soroka, Y.; Frušić-Zlotkin, M.; Popov, I.; Kohen, R. High resolution SEM imaging of gold nanoparticles in cells and tissues. J. Microsc. 2014, 256, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Schatten, H. Low voltage high-resolution SEM (LVHRSEM) for biological structural and molecular analysis. Micron 2011, 42, 175–185. [Google Scholar] [CrossRef]
- Idalia, V.-M.N.; Bernardo, F. Escherichia coli as a Model Organism and Its Application in Biotechnology. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; IntechOpen: London, UK, 2017; ISBN 978-953-51-3330-8. [Google Scholar]
- Otey, C.A.; Boukhelifa, M.; Maness, P. B35 neuroblastoma cells: An easily transfected, cultured cell model of central nervous system neurons. Methods Cell Biol. 2003, 2003, 287–304. [Google Scholar] [CrossRef]
- Rahmah Aid, S.; Nur Anis Awadah Nik Zain, N.; Nadhirah Mohd Rashid, N.; Hara, H.; Shameli, K.; Koji, I. A Study on Biological Sample Preparation for High Resolution Imaging of Scanning Electron Microscope. J. Phys. Conf. Ser. 2020, 1447, 012034. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. Microbiol. 2012, 25, 2B.2.1–2B.2.47. [Google Scholar] [CrossRef]
- Singh, H.; Bishen, K.A.; Garg, D.; Sukhija, H.; Sharma, D.; Tomar, U. Fixation and Fixatives: Roles and Functions—A Short Review. Dent. J. Adv. Stud. 2019, 7, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Hopwood, D. Theoretical and practical aspects of glutaraldehyde fixation. Histochem. J. 1972, 4, 267–303. [Google Scholar] [CrossRef]
- Hopwood, D. Some aspects of fixation with glutaraldehyde. A biochemical and histochemical comparison of the effects of formaldehyde and glutaraldehyde fixation on various enzymes and glycogen, with a note on penetration of glutaraldehyde into liver. J. Anat. 1967, 101, 83–92. [Google Scholar]
- Zhang, Y.; Huang, T.; Jorgens, D.M.; Nickerson, A.; Lin, L.-J.; Pelz, J.; Gray, J.W.; López, C.S.; Nan, X. Quantitating morphological changes in biological samples during scanning electron microscopy sample preparation with correlative super-resolution microscopy. PLoS ONE 2017, 12, e0176839. [Google Scholar] [CrossRef] [Green Version]
- Wisse, E.; Braet, F.; Duimel, H.; Vreuls, C.; Koek, G.; Damink, S.W.O.; van den Broek, M.A.; De Geest, B.; Dejong, C.H.; Tateno, C.; et al. Fixation methods for electron microscopy of human and other liver. World J. Gastroenterol. 2010, 16, 2851. [Google Scholar] [CrossRef]
- Dassanayake, R.P.; Falkenberg, S.M.; Stasko, J.A.; Shircliff, A.L.; Lippolis, J.D.; Briggs, R.E. Identification of a reliable fixative solution to preserve the complex architecture of bacterial biofilms for scanning electron microscopy evaluation. PLoS ONE 2020, 15, e0233973. [Google Scholar] [CrossRef]
- Fischer Scientific Osmium(VIII)-Tetroxide, 99.9+% MSDS. Available online: https://fscimage.fishersci.com/msds/96359.htm (accessed on 16 August 2021).
- Mhatre, S.; Singh, N.K.; Wood, J.M.; Parker, C.W.; Pukall, R.; Verbarg, S.; Tindall, B.J.; Neumann-Schaal, M.; Venkateswaran, K. Description of Chloramphenicol Resistant Kineococcus rubinsiae sp. nov. Isolated From a Spacecraft Assembly Facility. Front. Microbiol. 2020, 11, 1957. [Google Scholar] [CrossRef]
- Fischer Scientific Paraformaldehyde MSDS. Available online: https://fscimage.fishersci.com/msds/18000.htm (accessed on 16 August 2021).
- Cancer Diagnostics Inc Methacarn MSDS. Available online: https://www.msdsdigital.com/methacarn-msds (accessed on 16 August 2021).
- Gusnard, D.; Kirschner, R.H. Cell and organelle shrinkage during preparation for scanning electron microscopy: Effects of fixation, dehydration and critical point drying. J. Microsc. 1977, 110, 51–57. [Google Scholar] [CrossRef]
- Braet, F.; De Zanger, R.; Wisse, E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: A study on hepatic endothelial cells. J. Microsc. 1997, 186, 84–87. [Google Scholar] [CrossRef]
- Moran, P.; Coats, B. Biological Sample Preparation for SEM Imaging of Porcine Retina. Micros. Today 2012, 20, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Nikara, S.; Ahmadi, E.; Nia, A.A. Effects of different preparation techniques on the microstructural features of biological materials for scanning electron microscopy. J. Agric. Food Res. 2020, 2, 100036. [Google Scholar] [CrossRef]
- Chissoe, W.F.; Vezey, E.L.; Skvarla, J.J. Hexamethyldisilazane as a Drying Agent for Pollen Scanning Electron Microscopy. Biotech. Histochem. 2009, 69, 192–198. [Google Scholar] [CrossRef]
- Hazrin-Chong, N.H.; Manefield, M. An alternative SEM drying method using hexamethyldisilazane (HMDS) for microbial cell attachment studies on sub-bituminous coal. J. Microbiol. Methods 2012, 90, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W.; Warner, M.G.; Pittman, J.W.; Dehoff, K.J.; Wietsma, T.W.; Zhang, C.; Oostrom, M. Silane modification of glass and silica surfaces to obtain equally oil-wet surfaces in glass-covered silicon micromodel applications. Water Resour. Res. 2013, 49, 4724–4729. [Google Scholar] [CrossRef]
- Sigma Aldrich Hexamethyldisilazane MSDS. Available online: https://www.sigmaaldrich.com/RS/en/substance/hexamethyldisilazane16139999973 (accessed on 16 August 2021).
- Tai, S.S.W.; Tang, X.M. Manipulating biological samples for environmental scanning electron microscopy observation. Scanning 2001, 23, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, L.; Moisiadis, P.; Van Meerbeek, B.; Quirynen, M.; Lambrechts, P. Microscopic observation of bacteria: Review highlighting the use of environmental SEM. Int. Endod. J. 2005, 38, 775–788. [Google Scholar] [CrossRef] [Green Version]
- Muscariello, L.; Rosso, F.; Marino, G.; Giordano, A.; Barbarisi, M.; Cafiero, G.; Barbarisi, A. A critical overview of ESEM applications in the biological field. J. Cell. Physiol. 2005, 205, 328–334. [Google Scholar] [CrossRef]
- Bennett, P.C.; Engel, A.S.; Roberts, J.A. Counting and Imaging Bacteria on Mineral Surfaces. In Methods for Study of Microbe—Mineral Interactions; Clay Minerals Society: Chantilly, VA, USA, 2006; Volume 14, pp. 37–77. ISBN 188120815X. [Google Scholar]
- Korpa, A.; Trettin, R. The influence of different drying methods on cement paste microstructures as reflected by gas adsorption: Comparison between freeze-drying (F-drying), D-drying, P-drying and oven-drying methods. Cem. Concr. Res. 2006, 36, 634–649. [Google Scholar] [CrossRef]
- Lee, J.T.Y.; Chow, K.L. SEM sample preparation for cells on 3D scaffolds by freeze-drying and HMDS. Scanning 2012, 34, 12–25. [Google Scholar] [CrossRef]
- Velasco, D.; Benito, L.; Fernández-Gutiérrez, M.; San Román, J.; Elvira, C. Preparation in supercritical CO2 of porous poly(methyl methacrylate)-poly(l-lactic acid) (PMMA-PLA) scaffolds incorporating ibuprofen. J. Supercrit. Fluids 2010, 54, 335–341. [Google Scholar] [CrossRef]
- Rai, B.; Lin, J.L.; Lim, Z.X.H.; Guldberg, R.E.; Hutmacher, D.W.; Cool, S.M. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL–TCP scaffolds. Biomaterials 2010, 31, 7960–7970. [Google Scholar] [CrossRef]
- Golding, C.G.; Lamboo, L.L.; Beniac, D.R.; Booth, T.F. The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci. Rep. 2016, 6, 26516. [Google Scholar] [CrossRef] [Green Version]
- Heu, R.; Shahbazmohamadi, S.; Yorston, J.; Capeder, P. Target Material Selection for Sputter Coating of SEM Samples. Micros. Today 2019, 27, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Fichtman, B.; Shaulov, L.; Harel, A. Imaging Metazoan Nuclear Pore Complexes by Field Emission Scanning Electron Microscopy. Methods Cell Biol. 2014, 122, 41–58. [Google Scholar] [CrossRef]
- Hover, T.; Maya, T.; Ron, S.; Sandovsky, H.; Shadkchan, Y.; Kijner, N.; Mitiagin, Y.; Fichtman, B.; Harel, A.; Shanks, R.M.Q.; et al. Mechanisms of Bacterial (Serratia marcescens) Attachment to, Migration along, and Killing of Fungal Hyphae. Appl. Environ. Microbiol. 2016, 82, 2585–2594. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, M.W.; Allen, T.D. High resolution scanning electron microscopy of the nuclear envelope: Demonstration of a new, regular, fibrous lattice attached to the baskets of the nucleoplasmic face of the nuclear pores. J. Cell Biol. 1992, 119, 1429–1440. [Google Scholar] [CrossRef] [Green Version]
- Bray, D. Critical Point Drying of Biological Specimens for Scanning Electron Microscopy. Supercrit. Fluid Methods Protoc. 2003, 235–243. [Google Scholar] [CrossRef]
- Czerwińska-Główka, D.; Przystaś, W.; Zabłocka-Godlewska, E.; Student, S.; Cwalina, B.; Łapkowski, M.; Krukiewicz, K. Bacterial Surface Colonization of Sputter-Coated Platinum Films. Materials (Basel). 2020, 13, 2674. [Google Scholar] [CrossRef]
- Czerwińska-Główka, D.; Przystaś, W.; Zabłocka-Godlewska, E.; Student, S.; Cwalina, B.; Łapkowski, M.; Krukiewicz, K. Electrically-responsive antimicrobial coatings based on a tetracycline-loaded poly(3,4-ethylenedioxythiophene) matrix. Mater. Sci. Eng. C 2021, 123, 112017. [Google Scholar] [CrossRef]
- Skorupa, M.; Więcławska, D.; Czerwińska-Główka, D.; Skonieczna, M.; Krukiewicz, K. Dopant-dependent electrical and biological functionality of pedot in bioelectronics. Polymers 2021, 13, 1948. [Google Scholar] [CrossRef]
- Guzev, V.S.; Byzov, B.A. Morphometric analysis of bacteria associated with soil millipedes. Microbiology 2006, 75, 219–225. [Google Scholar] [CrossRef]
- Gomes, L.C.; Mergulhão, F.J. SEM analysis of surface impact on biofilm antibiotic treatment. Scanning 2017, 2017, 2960194. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W. High-resolution imaging of the microbial cell surface. J. Microbiol. 2016, 54, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of adherent micro-organisms using different techniques. J. Med. Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhu, M. High Osmolarity Modulates Bacterial Cell Size through Reducing Initiation Volume in Escherichia coli. mSphere 2018, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, K.D. The Selective Value of Bacterial Shape. Microbiol. Mol. Biol. Rev. 2006, 70, 660–703. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.; Young, K.D. FtsZ Collaborates with Penicillin Binding Proteins To Generate Bacterial Cell Shape in Escherichia coli. J. Bacteriol. 2004, 186, 6768–6774. [Google Scholar] [CrossRef] [Green Version]
- Ursell, T.; Lee, T.K.; Shiomi, D.; Shi, H.; Tropini, C.; Monds, R.D.; Colavin, A.; Billings, G.; Bhaya-Grossman, I.; Broxton, M.; et al. Rapid, precise quantification of bacterial cellular dimensions across a genomic-scale knockout library. BMC Biol. 2017, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Ho, P.-Y.; Jiang, M.; Tang, B.; Liu, W.; Li, D.; Yu, X.; Kleckner, N.E.; Amir, A.; Liu, C. Interrogating the Escherichia coli cell cycle by cell dimension perturbations. Proc. Natl. Acad. Sci. USA 2016, 113, 15000–15005. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.K.; Theriot, J.A. Surface Area to Volume Ratio: A Natural Variable for Bacterial Morphogenesis. Trends Microbiol. 2018, 26, 815–832. [Google Scholar] [CrossRef] [Green Version]
- Ojkic, N.; Serbanescu, D.; Banerjee, S. Surface-to-volume scaling and aspect ratio preservation in rod-shaped bacteria. Elife 2019, 8, e47033. [Google Scholar] [CrossRef]
- Paluch, E.; Heisenberg, C.-P. Biology and Physics of Cell Shape Changes in Development. Curr. Biol. 2009, 19, R790–R799. [Google Scholar] [CrossRef] [Green Version]
- Abdel Wahab, S.I.; Abdul, A.B.; Alzubairi, A.S.; Mohamed Elhassan, M.; Mohan, S. In vitro ultramorphological assessment of apoptosis induced by Zerumbone on (HeLa). J. Biomed. Biotechnol. 2009, 2009, 769568. [Google Scholar] [CrossRef]
- Toth, S.R. Cell Cycle-related Morphological Changes of Feline Lymphoid Cells as Revealed by Electron Microscopy. Cancer Res. 1981, 41, 4727–4736. [Google Scholar]
- Pasqualato, A.; Palombo, A.; Cucina, A.; Mariggiò, M.A.; Galli, L.; Passaro, D.; Dinicola, S.; Proietti, S.; D’Anselmi, F.; Coluccia, P.; et al. Quantitative shape analysis of chemoresistant colon cancer cells: Correlation between morphotype and phenotype. Exp. Cell Res. 2012, 318, 835–846. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, M.; Wu, G.; Yao, C.; Zhang, J. Recent advances in morphological cell image analysis. Comput. Math. Methods Med. 2012, 2012, 101536. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, P.; Das, S.; Krishnan, M.M.R.; Chakraborty, C.; Chaudhury, K.; Mandal, M. Effect of AEE788 and/or Celecoxib on colon cancer cell morphology using advanced microscopic techniques. Micron 2010, 41, 247–256. [Google Scholar] [CrossRef]
- Timashev, P.S.; Vedunova, M.V.; Guseva, P.D.; Ponimaskin, E.; Deiwick, A.; Mishchenko, T.; Mitroshina, E.V.; Koroleva, A.V.; Pimashkin, A.S.; Mukhina, I.V.; et al. 3D in vitro platform produced by two-photon polymerization for the analysis of neural network formation and function. Biomed. Phys. Eng. Express 2016, 2, 035001. [Google Scholar] [CrossRef]
- Lobo, J.; See, E.Y.S.; Biggs, M.; Pandit, A. An insight into morphometric descriptors of cell shape that pertain to regenerative medicine. J. Tissue Eng. Regen. Med. 2016, 539–553. [Google Scholar] [CrossRef]
- Pincus, Z.; Theriot, J.A. Comparison of quantitative methods for cell-shape analysis. J. Microsc. 2007, 227, 140–156. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Huang, W.-Y.; Chen, L.-H.; Liang, N.-W.; Wang, H.-C.; Lu, J.; Wang, X.; Wang, T.-W. Neural tissue engineering: The influence of scaffold surface topography and extracellular matrix microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Bardia, A.; Lakkireddy, C.; Paspala, S.A.B.; Khan, A.A. Bioengineering Human Neurological Constructs Using Decellularized Meningeal Scaffolds for Application in Spinal Cord Injury. Front. Bioeng. Biotechnol. 2018, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lynch, K.; Skalli, O.; Sabri, F. Growing Neural PC-12 Cell on Crosslinked Silica Aerogels Increases Neurite Extension in the Presence of an Electric Field. J. Funct. Biomater. 2018, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zychowicz, M.; Mehn, D.; Ruiz, A.; Frontczak-Baniewicz, M.; Rossi, F.; Buzanska, L. Patterning of human cord blood-derived stem cells on single cell posts and lines: Implications for neural commitment. Acta Neurobiol. Exp. (Wars). 2012, 72, 325–336. [Google Scholar] [PubMed]
- Simitzi, C.; Ranella, A.; Stratakis, E. Controlling the morphology and outgrowth of nerve and neuroglial cells: The effect of surface topography. Acta Biomater. 2017, 51, 21–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, J.S.; Chng, C.-P.; Moe, A.A.K.; Tann, J.Y.; Goh, E.L.K.; Chiam, K.-H.; Yim, E.K.F. Extending neurites sense the depth of the underlying topography during neuronal differentiation and contact guidance. Biomaterials 2014, 35, 7750–7761. [Google Scholar] [CrossRef]
- Park, M.; Oh, E.; Seo, J.; Kim, M.-H.; Cho, H.; Choi, J.Y.; Lee, H.; Choi, I.S. Control over Neurite Directionality and Neurite Elongation on Anisotropic Micropillar Arrays. Small 2016, 12, 1148–1152. [Google Scholar] [CrossRef]
- Das, K.P.; Freudenrich, T.M.; Mundy, W.R. Assessment of PC12 cell differentiation and neurite growth: A comparison of morphological and neurochemical measures. Neurotoxicol. Teratol. 2004, 26, 397–406. [Google Scholar] [CrossRef]
- Chen-Yang, Y.; Feng, Z.V.; Chen, W.S.; Keratithamkul, K.; Stoick, M.; Kapala, B.; Johnson, E.; Huang, A.-C.; Chin, T.Y.; Yang, M.-L. Degradation of the electrospun silica nanofiber in a biological medium for primary hippocampal neuron–effect of surface modification. Int. J. Nanomedicine 2016, 11, 729. [Google Scholar] [CrossRef] [Green Version]
- Su, W.-T.; Liao, Y.-F.; Wu, T.-W.; Wang, B.-J.; Shih, Y.-Y. Microgrooved patterns enhanced PC12 cell growth, orientation, neurite elongation, and neuritogenesis. J. Biomed. Mater. Res. Part. A 2013, 101A, 185–194. [Google Scholar] [CrossRef]
- Haq, F.; Anandan, V.; Keith, C.; Zhang, G. Neurite development in PC12 cells cultured on nanopillars and nanopores with sizes comparable with fi lopodia. Int. J. Nanomedicine 2007, 2, 107–115. [Google Scholar] [CrossRef]
- Repić, T.; Madirazza, K.; Bektur, E.; Sapunar, D. Characterization of dorsal root ganglion neurons cultured on silicon micro-pillar substrates. Sci. Rep. 2016, 6, 39560. [Google Scholar] [CrossRef] [Green Version]
- Radotić, V.; Braeken, D.; Drviš, P.; Mattotti, M.; Kovačić, D. Advantageous environment of micro-patterned, high-density complementary metal–oxide–semiconductor electrode array for spiral ganglion neurons cultured in vitro. Sci. Rep. 2018, 8, 7446. [Google Scholar] [CrossRef] [Green Version]
- Rocchi, M.B.L.; Sisti, D.; Albertini, M.C.; Teodori, L. Current trends in shape and texture analysis in neurology: Aspects of the morphological substrate of volume and wiring transmission. Brain Res. Rev. 2007, 55, 97–107. [Google Scholar] [CrossRef]
- Tonazzini, I.; Masciullo, C.; Savi, E.; Sonato, A.; Romanato, F.; Cecchini, M. Neuronal contact guidance and YAP signaling on ultra-small nanogratings. Sci. Rep. 2020, 10, 3742. [Google Scholar] [CrossRef] [Green Version]
- Sołtys, Z.; Ziaja, M.; Pawliński, R.; Setkowicz, Z.; Janeczko, K. Morphology of reactive microglia in the cerebral cortex. Fractal analysis and complementary quantitative methods. J. Neurosci. Res. 2001, 63, 90–97. [Google Scholar] [CrossRef]
- Fernández-Arjona, M. del M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Perez-Pouchoulen, M.; VanRyzin, J.W.; McCarthy, M.M. Morphological and Phagocytic Profile of Microglia in the Developing Rat Cerebellum. eNeuro 2015, 2, 36–51. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.H.; Rowland, C.; Harland, B.; Moslehi, S.; Montgomery, R.D.; Schobert, K.; Watterson, W.J.; Dalrymple-Alford, J.; Taylor, R.P. How neurons exploit fractal geometry to optimize their network connectivity. Sci. Reports 2021 111 2021, 11, 1–13. [Google Scholar] [CrossRef]
- John, A.M.; Elfanagely, O.; Ayala, C.A.; Cohen, M.; Prestigiacomo, C.J. The utility of fractal analysis in clinical neuroscience. Rev. Neurosci. 2015, 26, 633–645. [Google Scholar] [CrossRef]
- Mandelbrot, B. Fractals: Form, chance, and dimension; W.H. Freeman: San Francisco, CA, USA, 1977; ISBN 9780716704737. [Google Scholar]
- Jakubowicz-Gil, J.; Rzeski, W.; Zdzisinska, B.; Dobrowolski, P.; Gawron, A. Cell death and neuronal arborization upon quercetin treatment in rat neurons. Acta Neurobiol. Exp. 2008, 68, 139–146. [Google Scholar]
- Katsaloulis, P.; Verganelakis, D.A.; Provata, A. Fractal dimension and lacunarity of tractography images of the human brain. Fractals 2009, 17, 181–189. [Google Scholar] [CrossRef]
- Wang, S.; Tanzi, R.E.; Li, A. Quantitative Analysis of Neuronal Dendritic Arborization Complexity in Drosophila. JoVE (J. Vis. Exp. 2019, 2019, e57139. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.G.; Marks, W.B.; Lange, G.D.; Sheriff, W.H.; Neale, E.A. A fractal analysis of cell images. J. Neurosci. Methods 1989, 27, 173–180. [Google Scholar] [CrossRef]
- Takeda, T.; Ishikawa, A.; Ohtomo, K.; Kobayashi, Y.; Matsuoka, T. Fractal dimension of dendritic tree of cerebellar Purkinje cell during onto- and phylogenetic development. Neurosci. Res. 1992, 13, 19–31. [Google Scholar] [CrossRef]
- Mufson, E.J.; Mahady, L.; Waters, D.; Counts, S.E.; Perez, S.E.; DeKosky, S.T.; Ginsberg, S.D.; Ikonomovic, M.D.; Scheff, S.W.; Binder, L.I. Hippocampal plasticity during the progression of Alzheimer’s disease. Neuroscience 2015, 309, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Nichita, M.V.; Paun, M.A.; Paun, V.A.; Paun, V.P. Fractal analysis of brain glial cells. Fractal dimension and lacunarity. UPB Sci. Bull. Ser. A Appl. Math. Phys. 2019, 81, 273–284. [Google Scholar]
- Armatas, G.S.; Kolonia, K.M.; Pomonis, P.J. Morphometry of porous solids: Lacunarity, fractal dimensions, connectivity, and some topological similarities with neurons. Langmuir 2002, 18, 10421–10429. [Google Scholar] [CrossRef]
- Bordescu, D.; Paun, M.A.; Paun, V.A.; Paun, V.P. Fractal analysis of neuroimagistics. Lacunarity degree, a precious indicator in the detection of Alzheimer’s disease. UPB Sci. Bull. Ser. A Appl. Math. Phys. 2018, 80, 309–320. [Google Scholar]
- Shapiro, L.G.; Stockman, G. . Computer Vision; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Haralick, R.M. Statistical and Structural Approaches to Texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar]
- Wu, P.; Manjunath, B.S.; Newsam, S.; Shin, H.D. A texture descriptor for browsing and similarity retrieval. Signal. Process. Image Commun. 2000, 1–2, 33–43. [Google Scholar] [CrossRef]
- Przybył, K.; Koszela, K.; Adamski, F.; Samborska, K.; Walkowiak, K.; Polarczyk, M. Deep and Machine Learning Using SEM, FTIR, and Texture Analysis to Detect Polysaccharide in Raspberry Powders. Sensors 2021, Vol. 21, Page 5823 2021, 21, 5823. [Google Scholar] [CrossRef]
- Tsutsui, K.; Terasaki, H.; Uto, K.; Maemura, T.; Hiramatsu, S.; Hayashi, K.; Moriguchi, K.; Morito, S. A methodology of steel microstructure recognition using SEM images by machine learning based on textural analysis. Mater. Today Commun. 2020, 25, 101514. [Google Scholar] [CrossRef]
- Zhang, H.; He, C.; Yu, M.; Fu, J. Texture feature extraction and classification of SEM images of wheat straw/polypropylene composites in accelerated aging test. Adv. Mater. Sci. Eng. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jusman, Y.; Ng, S.-C.; Hasikin, K.; Kurnia, R.; Osman, N.A.A.; Teoh, K.H. A system for detection of cervical precancerous in field emission scanning electron microscope images using texture features. J. Innov. Opt. Health Sci. 2017, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Haralick, R.M.; Dinstein, I.; Shanmugam, K. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef] [Green Version]

| Fixing Agent | Fixing Conditions | Fixing Time | Rinsing Conditions | Major Risks | Ref. |
|---|---|---|---|---|---|
| Osmium tetroxide | 1% OsO4 in dH2O or 0.1 M CAC | 30–60 min | 1 × 2 min with 0.1 M CAC or dH2O, and 2 × 2 min with dH2O | Causes eye and skin burns. Causes digestive and respiratory tract burns. Aspiration hazard if swallowed. Can enter lungs and cause damage. May cause adverse reproductive effects. Target organs: kidneys. | [9,15,16,17] |
| 0.5% OsO4 and 0.8% K4Fe(CN)6 in dH2O or 0.1M CAC (reduced osmium) | 30–60 min | 1 × 2 min with 0.1 M CAC or dH2O, and 2 × 2 min with dH2O | Causes eye and skin burns. Causes digestive and respiratory tract burns. Aspiration hazard if swallowed. Can enter lungs and cause damage. May cause adverse reproductive effects. Target organs: kidneys. | [9,15,16,17] | |
| Glutaraldehyde | 1.5–4% in 0.1 M CAC or PB, pH 6.8–7.4 | 20–60 min for animal cells, 1–48 h for bacterial cells | 3 × 2 min with 0.1–0.2 M CAC or PB | Causes eye and skin burns. Causes digestive and respiratory tract burns. May cause allergic respiratory and skin reaction. Harmful if swallowed, inhaled, or absorbed through the skin. Aspiration hazard if swallowed. Can enter lungs and cause damage. Dangerous for the environment. Target organs: central nervous system, lungs, respiratory system, eyes, skin. | [9,14,15,16,18] |
| Paraformaldehyde | 4% in 0.1 M CAC or PB, pH 6.8–7.4 | 30–60 min for animal cells, 48 h for bacterial cells | 4 × 5 min with 0.1 M CAC or PB | Harmful if swallowed. Causes skin irritation. May cause an allergic skin reaction. Causes serious eye damage. Harmful if inhaled. May cause respiratory irritation. Suspected of causing cancer. Target organs: respiratory system. | [9,16,19] |
| Methacarn | methanol/chloroform/acetic acid 6:3:1 | 48 h for bacterial cells | 4 × 5 min with 0.1 M CAC | May cause irritation to the eyes, nose, throat, headache, dizziness, nausea. Target organs: eyes, skin, respiratory system, central nervous system, gastrointestinal tract. | [16,20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwińska-Główka, D.; Krukiewicz, K. Guidelines for a Morphometric Analysis of Prokaryotic and Eukaryotic Cells by Scanning Electron Microscopy. Cells 2021, 10, 3304. https://doi.org/10.3390/cells10123304
Czerwińska-Główka D, Krukiewicz K. Guidelines for a Morphometric Analysis of Prokaryotic and Eukaryotic Cells by Scanning Electron Microscopy. Cells. 2021; 10(12):3304. https://doi.org/10.3390/cells10123304
Chicago/Turabian StyleCzerwińska-Główka, Dominika, and Katarzyna Krukiewicz. 2021. "Guidelines for a Morphometric Analysis of Prokaryotic and Eukaryotic Cells by Scanning Electron Microscopy" Cells 10, no. 12: 3304. https://doi.org/10.3390/cells10123304