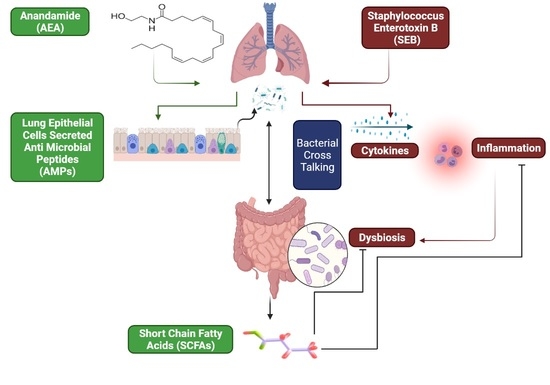
Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice Housing and Grouping
2.2. Induction of SEB-Mediated ARDS in Mice and Treatment with AEA
2.3. Evaluation of Lung Function in Mice
2.4. Collection of Serum and Broncho Alveolar Lavage Fluid (BALF)
2.5. Lung and Colon Histopathology
2.6. Capillary Leak Measurement
2.7. Gut Leakage Measurement
2.8. Isolation of Mesenteric Lymph Nodes (MLNs) and Flow Cytometric Analysis
2.9. Single-Cell RNA Sequencing (scRNA-seq) and Analysis
2.10. MiSeq Sequencing of the Gut–Lung Axis
2.11. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.12. Identification and Quantification of Short Chain Fatty Acids (SCFA)
2.13. Pretreatment with Butyrate and Treatment with Staphylococcus Enterotoxin B (SEB)
2.14. Statistical Analysis
3. Results
3.1. Anandamide Attenuates SEB-Mediated Inflammation in the Lungs
3.2. Effect of Anandamide on the Lung and Colon in SEB-Exposed Mice
3.3. Anandamide Attenuates SEB-Activated T Cells in the Mesenteric Lymph Nodes (MLNs)
3.4. scRNA-seq of Cells Isolated from the Lungs
3.5. The Effect of AEA on Microbial Profiles in the Lungs and the Gut
3.6. Role of Anandamide in the Regulation of Microbial Dysbiosis in the Gut
3.7. Effect of Anandamide on Short Chain Fatty Acids (SCFAs) in the Gut
3.8. The Role of Butyrate in the Amelioration of SEB-Mediated ARDS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huppert, L.A.; Matthay, M.A.; Ware, L.B. Pathogenesis of Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarkatti, P.; Miranda, K.; Nagarkatti, M. Use of Cannabinoids to Treat Acute Respiratory Distress Syndrome and Cytokine Storm Associated with Coronavirus Disease-2019. Front Pharmacol. 2020, 11, 589438. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Salton, F.; Fabiano, F. Acute respiratory distress syndrome. Eur. Respir. Rev. 2017, 26. [Google Scholar] [CrossRef] [Green Version]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [Green Version]
- Taub, D.D.; Rogers, T.J. Direct activation of murine T cells by staphylococcal enterotoxins. Cell Immunol. 1992, 140, 267–281. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Sultan, M.; Alghetaa, H.; Mohammed, A.; Abdulla, O.A.; Wisniewski, P.J.; Singh, N.; Nagarkatti, P.; Nagarkatti, M. The Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome by Downregulating miRNA that Target Inflammatory Pathways. Front. Pharmacol. 2021, 12, 644281. [Google Scholar] [CrossRef]
- Lang, C.; Behnke, H.; Bittersohl, J.; Eberhart, L.; Walthers, E.; Sommer, F.; Wulf, H.; Geldner, G. Special features of intensive care of toxic shock syndrome. Review and case report of a TSST-1 associated toxic-shock syndrome with adult respiratory distress syndrome and multiple organ failure from a staphylococcal panaritium. Anaesthesist 2003, 52, 805–813. [Google Scholar] [CrossRef]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal enterotoxins. Toxins 2010, 2, 2177–2197. [Google Scholar] [CrossRef] [Green Version]
- Verreault, D.; Ennis, J.; Whaley, K.; Killeen, S.Z.; Karauzum, H.; Aman, M.J.; Holtsberg, R.; Doyle-Meyers, L.; Didier, P.J.; Zeitlin, L.; et al. Effective Treatment of Staphylococcal Enterotoxin B Aerosol Intoxication in Rhesus Macaques by Using Two Parenterally Administered High-Affinity Monoclonal Antibodies. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.; Alghetaa, H.; Sultan, M.; Singh, N.P.; Nagarkatti, P.; Nagarkatti, M. Administration of Delta9-Tetrahydrocannabinol (THC) Post-Staphylococcal Enterotoxin B Exposure Protects Mice From Acute Respiratory Distress Syndrome and Toxicity. Front. Pharmacol. 2020, 11, 893. [Google Scholar] [CrossRef]
- Rao, R.; Nagarkatti, P.S.; Nagarkatti, M. Delta(9) Tetrahydrocannabinol attenuates Staphylococcal enterotoxin B-induced inflammatory lung injury and prevents mortality in mice by modulation of miR-17-92 cluster and induction of T-regulatory cells. Br. J. Pharmacol. 2015, 172, 1792–1806. [Google Scholar] [CrossRef] [Green Version]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Fut. Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors as therapeutic targets. Ann. Rev. Pharmacol. Toxicol. 2006, 46, 101–122. [Google Scholar] [CrossRef] [Green Version]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S.C. Cannabinoids, the endocannabinoid system, and pain: A review of preclinical studies. Pain 2021, 162, S5–S25. [Google Scholar] [CrossRef]
- Rahman, S.M.K.; Uyama, T.; Hussain, Z.; Ueda, N. Roles of Endocannabinoids and Endocannabinoid-like Molecules in Energy Homeostasis and Metabolic Regulation: A Nutritional Perspective. Ann. Rev. Nutr. 2021, 41, 177–202. [Google Scholar] [CrossRef]
- Ahmed, I.; Rehman, S.U.; Shahmohamadnejad, S.; Zia, M.A.; Ahmad, M.; Saeed, M.M.; Akram, Z.; Iqbal, H.M.N.; Liu, Q. Therapeutic Attributes of Endocannabinoid System against Neuro-Inflammatory Autoimmune Disorders. Molecules 2021, 26, 3389. [Google Scholar] [CrossRef]
- Jackson, A.R.; Hegde, V.L.; Nagarkatti, P.S.; Nagarkatti, M. Characterization of endocannabinoid-mediated induction of myeloid-derived suppressor cells involving mast cells and MCP-1. J. Leukoc. Biol. 2014, 95, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Osafo, N.; Yeboah, O.K.; Antwi, A.O. Endocannabinoid system and its modulation of brain, gut, joint and skin inflammation. Mol. Biol. Rep. 2021, 48, 3665–3680. [Google Scholar] [CrossRef]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. 2009, 60, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur. J. Immunol. 2016, 46, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Minichino, A.; Jackson, M.A.; Francesconi, M.; Steves, C.J.; Menni, C.; Burnet, P.W.J.; Lennox, B.R. Endocannabinoid system mediates the association between gut-microbial diversity and anhedonia/amotivation in a general population cohort. Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Heffernan, D.S.; Dossett, L.A.; Lightfoot, M.A.; Fremont, R.D.; Ware, L.B.; Sawyer, R.G.; May, A.K. Gender and acute respiratory distress syndrome in critically injured adults: A prospective study. J. Trauma 2011, 71, 878–883; discussion 883–875. [Google Scholar] [CrossRef] [Green Version]
- Rieder, S.A.; Nagarkatti, P.; Nagarkatti, M. CD1d-independent activation of invariant natural killer T cells by staphylococcal enterotoxin B through major histocompatibility complex class II/T cell receptor interaction results in acute lung injury. Infect. Immun. 2011, 79, 3141–3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, D.M.; Nagarkatti, M.; Nagarkatti, P.S. 3,39-Diindolylmethane Ameliorates Staphylococcal Enterotoxin B-Induced Acute Lung Injury through Alterations in the Expression of MicroRNA that Target Apoptosis and Cell-Cycle Arrest in Activated T Cells. J. Pharmacol. Exp. Ther. 2016, 357, 177–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.; Nagarkatti, P.; Nagarkatti, M. Role of miRNA in the regulation of inflammatory genes in staphylococcal enterotoxin B-induced acute inflammatory lung injury and mortality. Toxicol. Sci. 2015, 144, 284–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgun, E.; Caliskan, C.; Celik, H.A.; Ozutemiz, A.O.; Tuncyurek, M.; Aydin, H.H. Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J. Int. Med. Res. 2005, 33, 196–206. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M.; Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [Green Version]
- Rieder, S.A.; Nagarkatti, P.; Nagarkatti, M. Multiple anti-inflammatory pathways triggered by resveratrol lead to amelioration of staphylococcal enterotoxin B-induced lung injury. Br. J. Pharmacol. 2012, 167, 1244–1258. [Google Scholar] [CrossRef] [Green Version]
- Busbee, P.B.; Menzel, L.; Alrafas, H.R.; Dopkins, N.; Becker, W.; Miranda, K.; Tang, C.; Chatterjee, S.; Singh, U.; Nagarkatti, M.; et al. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22-dependent manner. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, O.A.; Neamah, W.; Sultan, M.; Alghetaa, H.K.; Singh, N.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P. The Ability of AhR Ligands to Attenuate Delayed Type Hypersensitivity Reaction Is Associated With Alterations in the Gut Microbiota. Front. Immunol. 2021, 12, 684727. [Google Scholar] [CrossRef]
- Mohammed, A.; Alghetaa, H.F.K.; Miranda, K.; Wilson, K.; Singh, N.P.; Cai, G.; Putluri, N.; Nagarkatti, P.; Nagarkatti, M. Delta9-Tetrahydrocannabinol Prevents Mortality from Acute Respiratory Distress Syndrome through the Induction of Apoptosis in Immune Cells, Leading to Cytokine Storm Suppression. Int. J. Mol. Sci. 2020, 21, 6244. [Google Scholar] [CrossRef]
- Alghetaa, H.; Mohammed, A.; Zhou, J.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Resveratrol-mediated attenuation of superantigen-driven acute respiratory distress syndrome is mediated by microbiota in the lungs and gut. Pharmacol. Res. 2021, 167, 105548. [Google Scholar] [CrossRef]
- Mohammed, A.; Alghetaa, H.K.; Zhou, J.; Chatterjee, S.; Nagarkatti, P.; Nagarkatti, M. Protective effects of Delta(9)—Tetrahydrocannabinol against enterotoxin-induced acute respiratory distress syndrome are mediated by modulation of microbiota. Br. J. Pharm. 2020, 177, 5078–5095. [Google Scholar] [CrossRef]
- Abdulla, O.A.; Neamah, W.; Sultan, M.; Chatterjee, S.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. AhR Ligands Differentially Regulate miRNA-132 Which Targets HMGB1 and to Control the Differentiation of Tregs and Th-17 Cells During Delayed-Type Hypersensitivity Response. Front. Immunol. 2021, 12, 635903. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef]
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef]
- Beisswenger, C.; Bals, R. Antimicrobial peptides in lung inflammation. Chem. Immunol. Allerg. 2005, 86, 55–71. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal. Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e51. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Gorshkova, E.A.; Polinova, A.I.; Drutskaya, M.S. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020, 53, 13–24. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Geitani, R.; Moubareck, C.A.; Xu, Z.; Karam Sarkis, D.; Touqui, L. Expression and Roles of Antimicrobial Peptides in Innate Defense of Airway Mucosa: Potential Implication in Cystic Fibrosis. Front. Immunol. 2020, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, G.; Jones, D.E.; Bevins, C.L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc. Natl. Acad. Sci. USA 1993, 90, 4596–4600. [Google Scholar] [CrossRef] [Green Version]
- Diamond, G.; Zasloff, M.; Eck, H.; Brasseur, M.; Maloy, W.L.; Bevins, C.L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: Peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. USA 1991, 88, 3952–3956. [Google Scholar] [CrossRef] [Green Version]
- Camper, N.; Glasgow, A.M.; Osbourn, M.; Quinn, D.J.; Small, D.M.; McLean, D.T.; Lundy, F.T.; Elborn, J.S.; McNally, P.; Ingram, R.J.; et al. A secretory leukocyte protease inhibitor variant with improved activity against lung infection. Mucosal. Immunol. 2016, 9, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Weldon, S.; McNally, P.; McElvaney, N.G.; Elborn, J.S.; McAuley, D.F.; Wartelle, J.; Belaaouaj, A.; Levine, R.L.; Taggart, C.C. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J. Immunol. 2009, 183, 8148–8156. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, D.; Wang, Y.; Liu, X.; Zhang, Y.; Qu, W.; Chen, K.; Francisco, N.M.; Feng, L.; Huang, X.; et al. Beta-Defensin 2 and 3 Promote Bacterial Clearance of Pseudomonas aeruginosa by Inhibiting Macrophage Autophagy through Downregulation of Early Growth Response Gene-1 and c-FOS. Front. Immunol. 2018, 9, 211. [Google Scholar] [CrossRef]
- Jaeger, N.; McDonough, R.T.; Rosen, A.L.; Hernandez-Leyva, A.; Wilson, N.G.; Lint, M.A.; Russler-Germain, E.V.; Chai, J.N.; Bacharier, L.B.; Hsieh, C.S.; et al. Airway Microbiota-Host Interactions Regulate Secretory Leukocyte Protease Inhibitor Levels and Influence Allergic Airway Inflammation. Cell Rep. 2020, 33, 108331. [Google Scholar] [CrossRef]
- Akinbi, H.T.; Epaud, R.; Bhatt, H.; Weaver, T.E. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J. Immunol. 2000, 165, 5760–5766. [Google Scholar] [CrossRef] [Green Version]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Galvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D.; Dal’Molin, C.G.; Palfreyman, R.W.; Nielsen, L.K.; Cooper, M.A.; et al. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Karnad, A.; Alvarez, S.; Berk, S.L. Enterobacter pneumonia. South Med. J. 1987, 80, 601–604. [Google Scholar] [CrossRef]
- von Baum, H.; Welte, T.; Marre, R.; Suttorp, N.; Ewig, S.; CAPNETZ Study Group. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: Diagnosis, incidence and predictors. Eur. Respir. J. 2010, 35, 598–605. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microb. 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [Green Version]
- Pope, J.L.; Bhat, A.A.; Sharma, A.; Ahmad, R.; Krishnan, M.; Washington, M.K.; Beauchamp, R.D.; Singh, A.B.; Dhawan, P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 2014, 63, 622–634. [Google Scholar] [CrossRef]
- Nawijn, M.C.; Hackett, T.L.; Postma, D.S.; van Oosterhout, A.J.; Heijink, I.H. E-cadherin: Gatekeeper of airway mucosa and allergic sensitization. Trends Immunol. 2011, 32, 248–255. [Google Scholar] [CrossRef]
- Post, S.; Heijink, I.H.; Hesse, L.; Koo, H.K.; Shaheen, F.; Fouadi, M.; Kuchibhotla, V.N.S.; Lambrecht, B.N.; Van Oosterhout, A.J.M.; Hackett, T.L.; et al. Characterization of a lung epithelium specific E-cadherin knock-out model: Implications for obstructive lung pathology. Sci. Rep. 2018, 8, 13275. [Google Scholar] [CrossRef] [Green Version]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieers, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Silver, A.; Gangopadhyay, A.; Gawarkiewicz, G.; Silva, E.N.S.; Clark, J. Interannual and seasonal asymmetries in Gulf Stream Ring Formations from 1980 to 2019. Sci. Rep. 2021, 11, 2207. [Google Scholar] [CrossRef]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 2), S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.Y.; Zhang, Z.; Feng, Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front. Microbiol. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Lei, Y.; Tang, L.; Hu, S.; Yang, M.; Wu, L.; Yang, S.; Tang, B. Gut Microbiota: The Emerging Link to Lung Homeostasis and Disease. J. Bacteriol. 2021, 203. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Long, C.; Fanning, K.V.; Zou, C. Studying Effects of Cigarette Smoke on Pseudomonas Infection in Lung Epithelial Cells. J. Vis. Exp. 2020. [Google Scholar] [CrossRef]
- Patterson, L.L.; Byerly, C.D.; McBride, J.W. Anaplasmataceae: Dichotomous Autophagic Interplay for Infection. Front. Immunol. 2021, 12, 642771. [Google Scholar] [CrossRef]
- Skennerton, C.T.; Haroon, M.F.; Briegel, A.; Shi, J.; Jensen, G.J.; Tyson, G.W.; Orphan, V.J. Phylogenomic analysis of Candidatus ’Izimaplasma’ species: Free-living representatives from a Tenericutes clade found in methane seeps. ISME J. 2016, 10, 2679–2692. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Feng, Y.; Wei, L.; Zong, Z. Genome-Based Taxonomy of Brevundimonas with Reporting Brevundimonas huaxiensis sp. nov. Microbiol. Spectr. 2021, 9, e00111-21. [Google Scholar] [CrossRef]
- do Nascimento, L.E.; Amaral, R.R.; Ferreira, R.; Trindade, D.V.S.; do Nascimento, R.E.; da Costa, T.S.; Souto, R.N.P. Ants (Hymenoptera: Formicidae) as Potential Mechanical Vectors of Pathogenic Bacteria in a Public Hospital in the Eastern Amazon, Brazil. J. Med. Entomol. 2020, 57, 1619–1626. [Google Scholar] [CrossRef]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef] [Green Version]
- Chai, L.J.; Lu, Z.M.; Zhang, X.J.; Ma, J.; Xu, P.X.; Qian, W.; Xiao, C.; Wang, S.T.; Shen, C.H.; Shi, J.S.; et al. Zooming in on Butyrate-Producing Clostridial Consortia in the Fermented Grains of Baijiu via Gene Sequence-Guided Microbial Isolation. Front. Microbiol. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Buettner, M.; Bode, U. Lymph node dissection--understanding the immunological function of lymph nodes. Clin. Exp. Immunol. 2012, 169, 205–212. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Sun, Q.; Sun, H.; Liu, N.; Liu, Q.; Ma, J.; Wang, P.; Hu, C.; Wu, J.; et al. Mesenteric Lymph Duct Drainage Attenuates Lung Inflammatory Injury and Inhibits Endothelial Cell Apoptosis in Septic Rats. BioMed Res. Int. 2020, 2020, 3049302. [Google Scholar] [CrossRef]
- Jin, C.; Chen, J.; Gu, J.; Zhang, W. Gut-lymph-lung pathway mediates sepsis-induced acute lung injury. Chin. Med. J. Engl. 2020, 133, 2212–2218. [Google Scholar] [CrossRef]
- Guida, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Piscitelli, F.; De Filippis, F.; Paino, S.; Ricciardi, F.; Siniscalco, D.; Marabese, I.; et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain. Behav. Immun. 2020, 85, 128–141. [Google Scholar] [CrossRef]
- Becker, W.; Alrafas, H.R.; Busbee, P.B.; Walla, M.D.; Wilson, K.; Miranda, K.; Cai, G.; Putluri, V.; Putluri, N.; Nagarkatti, M.; et al. Cannabinoid Receptor Activation on Haematopoietic Cells and Enterocytes Protects against Colitis. J. Crohns Colitis 2021, 15, 1032–1048. [Google Scholar] [CrossRef]
- Alrafas, H.R.; Busbee, P.B.; Chitrala, K.N.; Nagarkatti, M.; Nagarkatti, P. Alterations in the Gut Microbiome and Suppression of Histone Deacetylases by Resveratrol Are Associated with Attenuation of Colonic Inflammation and Protection Against Colorectal Cancer. J. Clin. Med. 2020, 9, 1796. [Google Scholar] [CrossRef]










| Name of the Gene | Primer | Sequence |
|---|---|---|
| Tracheal Antimicrobial Peptide 1(TAP1) | Forward Reverse | 5-GGA CTT GCC TTG TTC CGA GAG-3 5-GCT GCC ACA TAA CTG ATA GCG-3 |
| Tracheal Antimicrobial Peptide2 (TAP2) | Forward Reverse | 5-CTC CCA CTT TTA GCA GTC CCC-3 5-CTG GCG ATG GCT TTA CTT-3 |
| Secretory Leukocyte Peptidase Inhibitor (SLPI) | Forward Reverse | 5-TAC GGC ATT GTG GCT TCT CAA-3 5-TAC GGC ATT GTG GCT TCT CAA-3 |
| Lysozyme 2 | Forward Reverse | 5-ATG GAA TGG CTG GCT ACT ATG-G 3 5-ACC AGT ATC GGC TAT TGA TCT GA-3 |
| Claudin 1 (CLDN1) | Forward Reverse | 5-GGG GAC AAC ATC GTG ACC G-3 5-AGG AGT CGA AGA CTT TGC ACT-3 |
| Epithelial Cadherin (ECDH) | Forward Reverse | 5-CAG GTC TCC TCA TGG CTT TGC -3 5-CTT CCG AAA AGA AGG CTG TCC-3 |
| Murine Beta Defensine 2 (MBD2) | Forward Reverse | 5-AGA ACA AGG GTA AAC CAG ACC T-3 5-ACT TCA CCT TAT TGC TCG GGT-3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, M.; Wilson, K.; Abdulla, O.A.; Busbee, P.B.; Hall, A.; Carter, T.; Singh, N.; Chatterjee, S.; Nagarkatti, P.; Nagarkatti, M. Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis. Cells 2021, 10, 3305. https://doi.org/10.3390/cells10123305
Sultan M, Wilson K, Abdulla OA, Busbee PB, Hall A, Carter T, Singh N, Chatterjee S, Nagarkatti P, Nagarkatti M. Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis. Cells. 2021; 10(12):3305. https://doi.org/10.3390/cells10123305
Chicago/Turabian StyleSultan, Muthanna, Kiesha Wilson, Osama A. Abdulla, Philip Brandon Busbee, Alina Hall, Taylor Carter, Narendra Singh, Saurabh Chatterjee, Prakash Nagarkatti, and Mitzi Nagarkatti. 2021. "Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis" Cells 10, no. 12: 3305. https://doi.org/10.3390/cells10123305
APA StyleSultan, M., Wilson, K., Abdulla, O. A., Busbee, P. B., Hall, A., Carter, T., Singh, N., Chatterjee, S., Nagarkatti, P., & Nagarkatti, M. (2021). Endocannabinoid Anandamide Attenuates Acute Respiratory Distress Syndrome through Modulation of Microbiome in the Gut-Lung Axis. Cells, 10(12), 3305. https://doi.org/10.3390/cells10123305