Sperm Inspection for In Vitro Fertilization via Self-Assembled Microdroplet Formation and Quantitative Phase Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sperm Sample Preparation
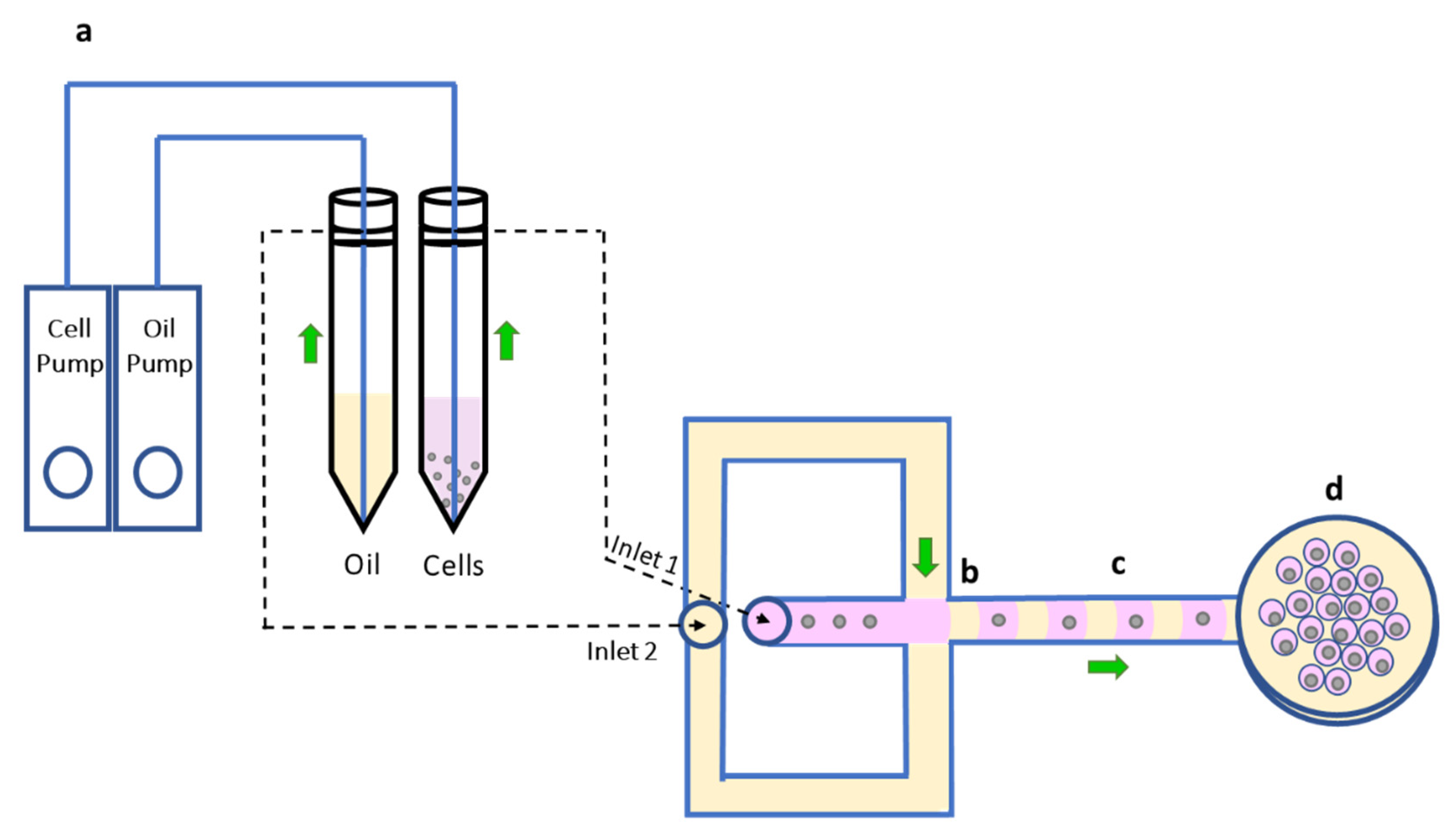
2.2. Disposable Microfluidic Device Architecture and the System Function
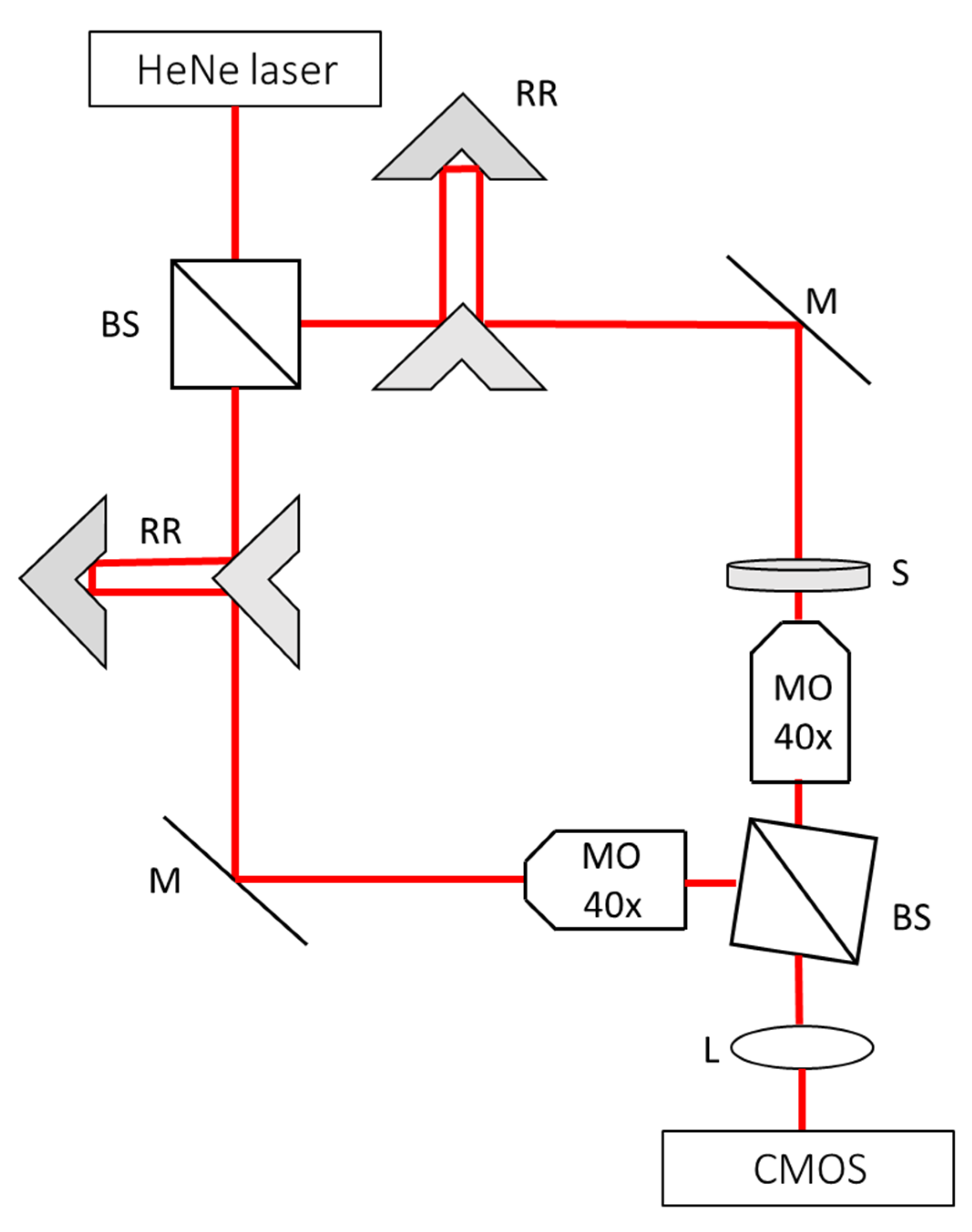
2.3. Quantitative Phase Imaging
2.4. Motility Analysis
2.5. Extracting the Sperm Cells from the Microdroplets
3. Results
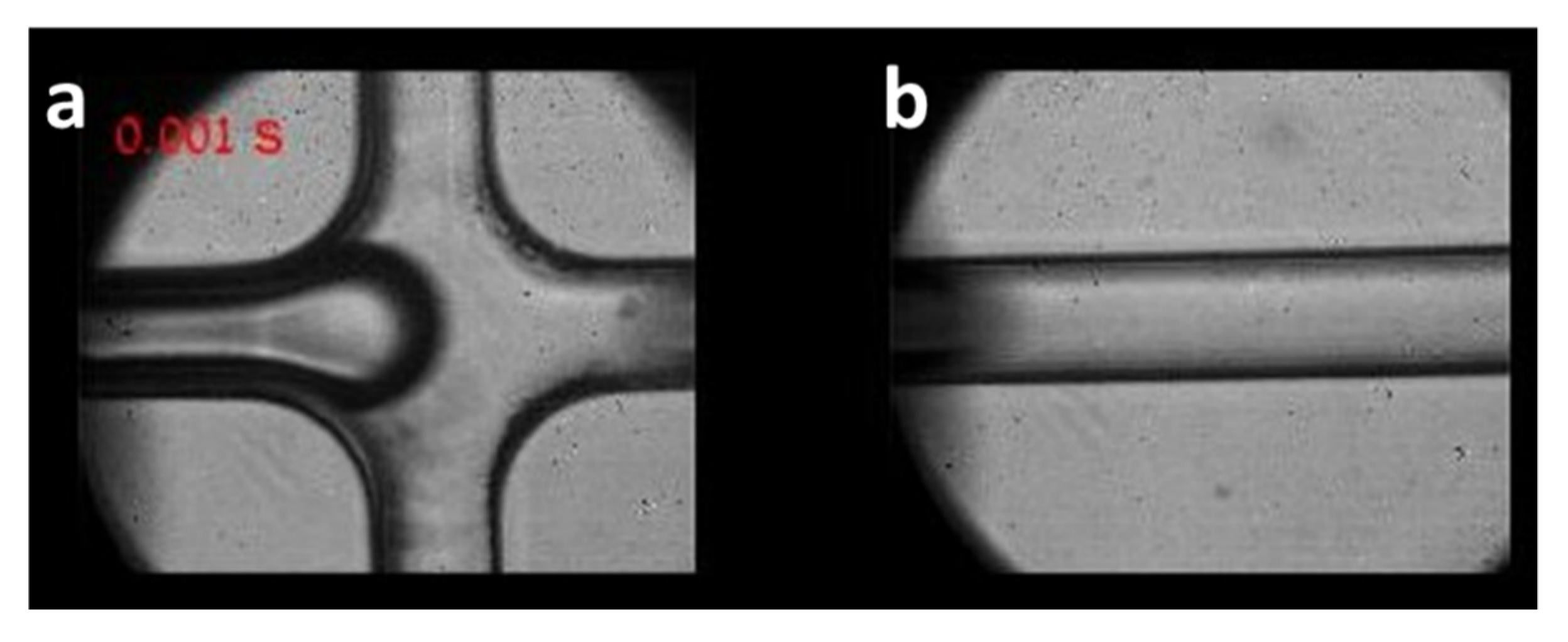
3.1. Trapping Sperm Cells inside Separated Microdroplets
3.2. Motility Analysis of the Cells
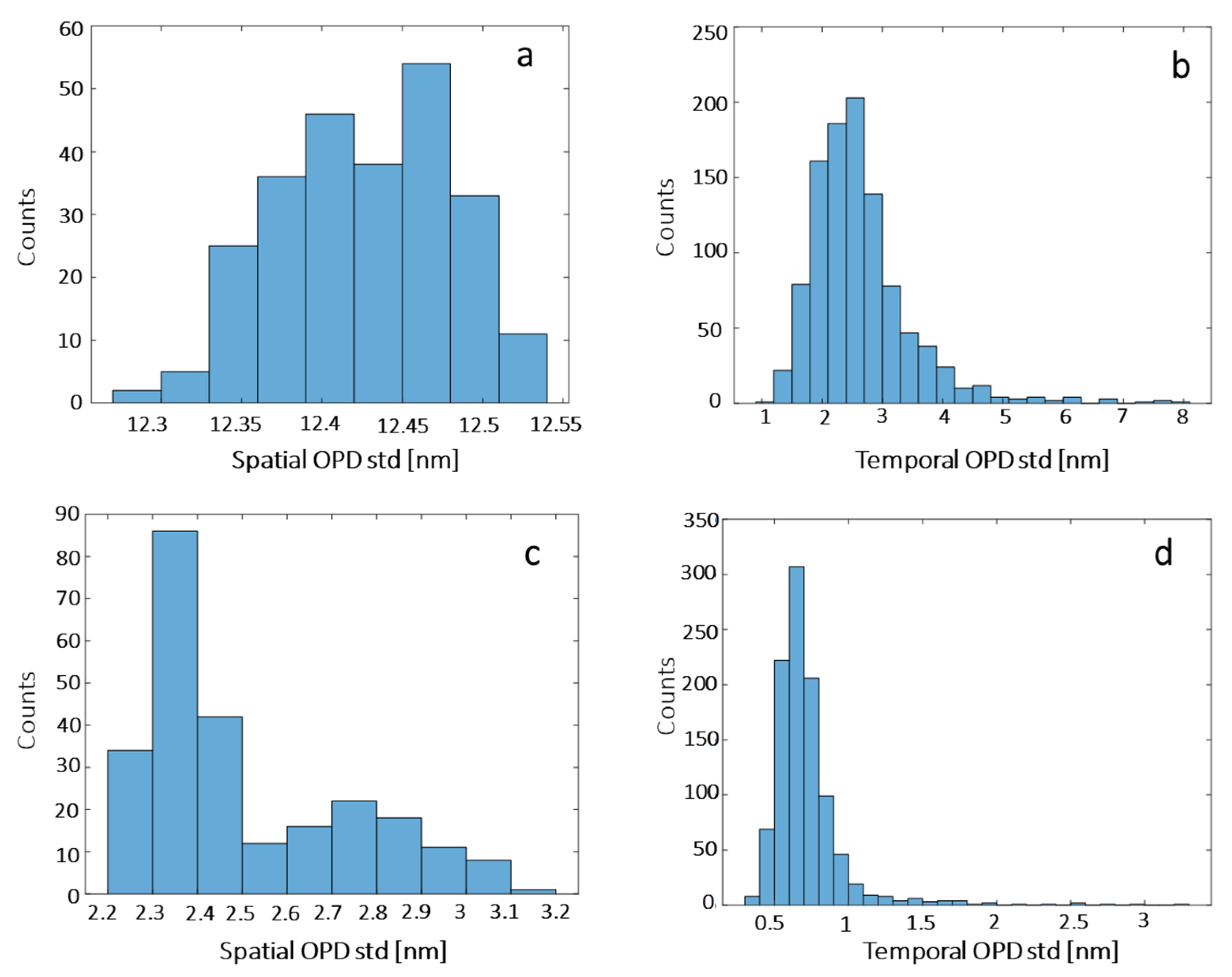
3.3. Noise Levels in the Quantitative Phase Imaging System
3.4. Morphological Analysis of the Cells by Quantitative Phase Imaging
3.5. Extraction of Sperm Cells from the Microdroplets
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2020, 113, 533–535. [Google Scholar] [CrossRef]
- Alhathal, N.; Maddirevula, S.; Coskun, S.; Alali, H.; Assoum, M.; Morris, T.; Deek, H.A.; Hamed, S.A.; Alsuhaibani, S.; Mirdawi, A.; et al. A genomics approach to male infertility. Genet. Med. 2020, 22, 1967–1975. [Google Scholar] [CrossRef]
- Donnelly, E.T.; Lewis, S.E.; McNally, J.A.; Thompson, W. In vitro fertilization and pregnancy rates: The influence of sperm motility and morphology on IVF outcome. Fertil. Steril. 1998, 70, 305–314. [Google Scholar] [CrossRef]
- Illiano, E.; Trama, F.; Zucchi, A.; Iannitti, R.G.; Fioretti, B.; Costantini, E. Resveratrol-Based Multivitamin Supplement Increases Sperm Concentration and Motility in Idiopathic Male Infertility: A Pilot Clinical Study. J. Clin. Med. 2020, 9, 4017. [Google Scholar] [CrossRef]
- Bonde, J.P.; Ernst, E.; Jensen, T.K.; Hjollund, N.H.; Kolstad, H.; Scheike, T.; Giwercman, A.; Skakkebæk, N.E.; Henriksen, T.B.; Olsen, J. Relation between semen quality and fertility: A population-based study of 430 first-pregnancy planners. Lancet 1998, 352, 1172–1177. [Google Scholar] [CrossRef]
- Edwards, R.G.; Steptoe, P.C. Current status of in-vitro fertilisation and implantation of human embryos. Lancet 1983, 322, 1265–1269. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Eravuchira, P.J.; Mirsky, S.K.; Barnea, I.; Levi, M.; Balberg, M.; Shaked, N.T. Individual sperm selection by microfluidics integrated with interferometric phase microscopy. Methods 2018, 136, 152–159. [Google Scholar] [CrossRef]
- Eugster, A.; Vingerhoets, A.J. Psychological aspects of in vitro fertilization: A review. Soc. Sci. Med. 1999, 48, 575–589. [Google Scholar] [CrossRef]
- Devroey, P.; Van Steirteghem, A. A review of ten years experience of ICSI. Hum. Reprod. Update 2004, 10, 19–28. [Google Scholar] [CrossRef]
- Barnes, F.L. Equipment and general technical aspects of micromanipulation of gametes and embryos. In Textbook of Assisted Reproductive Technologies: Laboratory and Clinical Perspectives, 3rd ed.; Gardner, D.K., Weissman, A., Howles, C.M., Shoham, Z., Eds.; Informa Healthcare: London, UK, 2009; pp. 163–170. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Haifler, M.; Girshovitz, P.; Band, G.; Dardikman, G.; Madjar, I.; Shaked, N.T. Interferometric phase microscopy for label-free morphological evaluation of sperm cells. Fertil. Steril. 2015, 104, 43–47. [Google Scholar] [CrossRef]
- Mirsky, S.K.; Barnea, I.; Levi, M.; Greenspan, H.; Shaked, N.T. Automated analysis of individual sperm cells using stain-free interferometric phase microscopy and machine learning. Cytom. Part A 2017, 91, 893–900. [Google Scholar] [CrossRef]
- Mirsky, S.; Barnea, I.; Shaked, N.T. Label-Free quantitative imaging of sperm for in vitro fertilization using interferometric phase microscopy. J. Fertil. Vitr.-IVF-Worldw. Reprod Med. Genet. Stem Cell Biol. 2016, 4, 1000190. [Google Scholar] [CrossRef]
- Balberg, M.; Levi, M.; Kalinowski, K.; Barnea, I.; Mirsky, S.K.; Shaked, N.T. Localized measurements of physical parameters within human sperm cells obtained with wide-field interferometry. J. Biophotonics 2017, 10, 1305–1314. [Google Scholar] [CrossRef]
- Kandel, M.E.; Rubessa, M.; He, Y.R.; Schreiber, S.; Meyers, S.; Naves, L.M.; Sermersheim, M.K.; Sell, S.G.; Szewczyk, M.J.; Sobh, N.; et al. Reproductive outcomes predicted by phase imaging with computational specificity of spermatozoon ultrastructure. Proc. Natl. Acad. Sci. USA 2020, 117, 18302–18309. [Google Scholar] [CrossRef] [PubMed]
- Butola, A.; Popova, D.; Prasad, D.K.; Ahmad, A.; Habib, A.; Tinguely, J.C.; Basnet, P.; Acharya, G.; Senthilkumaran, P.; Mehta D., S.; et al. High spatially sensitive quantitative phase imaging assisted with deep neural network for classification of human spermatozoa under stressed condition. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Mitome, M. Transport of intensity equation method and its applications. Microscopy 2021, 70, 69–74. [Google Scholar] [CrossRef]
- Dudaie, M.; Nissim, N.; Barnea, I.; Gerling, T.; Duschl, C.; Kirschbaum, M.; Shaked, N.T. Label-free discrimination and selection of cancer cells from blood during flow using holography-induced dielectrophoresis. J. Biophotonics 2020, 13, e202000151. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.; Gawad, S. The application of microfluidics in biology. In Microengineering in Biotechnology; Hughes, M.P., Hoettges, K.F., Eds.; Humana Press: New York, NY, USA, 2010; pp. 55–80. [Google Scholar]
- Tasoglu, S.; Safaee, H.; Zhang, X.; Kingsley, J.L.; Catalano, P.N.; Gurkan, U.A.; Nureddin, A.; Kayaalp, E.; Anchan, R.M.; Maas, R.L.; et al. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small 2013, 9, 3374–3384. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Schuster, T.G.; Zhu, X.; Chang, D.; Smith, G.D.; Takayama, S. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 2003, 75, 1671–1675. [Google Scholar] [CrossRef]
- Matuła, K.; Rivello, F.; Huck, W.T. Single-cell analysis using droplet microfluidics. Adv. Biosyst. 2020, 4, 1900188. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.K.; Ali, M.M.; Zhang, K.; Pone, E.J.; Zhao, W. Droplet microfluidics for single-molecule and single-cell analysis in cancer research, diagnosis and therapy. TrAC Trends Anal. Chem. 2014, 58, 145–153. [Google Scholar] [CrossRef]
- Girshovitz, P.; Shaked, N.T. Compact and portable low-coherence interferometer with off-axis geometry for quantitative phase microscopy and nanoscopy. Opt. Express 2013, 21, 5701–5714. [Google Scholar] [CrossRef]
- Raveshi, M.R.; Halim, M.S.; Agnihotri, S.N.; O’Bryan, M.K.; Neild, A.; Nosrati, R. Curvature in the reproductive tract alters sperm–surface interactions. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]





| Acrosome–Head Ratio [%] | Radii Ratio | Midpiece–Head Ratio | Vacuole Presence | Residual Cytoplasm Presence | |
|---|---|---|---|---|---|
| Cell a | 59.64 | 0.57 | 1.24 | No | No |
| Cell b | 47.3 | 0.56 | 1.11 | No | No |
| Cell c | 65.04 | 0.63 | 1.13 | No | No |
| Cell d | 31.01 | 0.7 | 1.16 | No | No |
| Cell e | 38.96 | 0.48 | 1.32 | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atzitz, Y.; Dudaie, M.; Barnea, I.; Shaked, N.T. Sperm Inspection for In Vitro Fertilization via Self-Assembled Microdroplet Formation and Quantitative Phase Microscopy. Cells 2021, 10, 3317. https://doi.org/10.3390/cells10123317
Atzitz Y, Dudaie M, Barnea I, Shaked NT. Sperm Inspection for In Vitro Fertilization via Self-Assembled Microdroplet Formation and Quantitative Phase Microscopy. Cells. 2021; 10(12):3317. https://doi.org/10.3390/cells10123317
Chicago/Turabian StyleAtzitz, Yuval, Matan Dudaie, Itay Barnea, and Natan T. Shaked. 2021. "Sperm Inspection for In Vitro Fertilization via Self-Assembled Microdroplet Formation and Quantitative Phase Microscopy" Cells 10, no. 12: 3317. https://doi.org/10.3390/cells10123317
APA StyleAtzitz, Y., Dudaie, M., Barnea, I., & Shaked, N. T. (2021). Sperm Inspection for In Vitro Fertilization via Self-Assembled Microdroplet Formation and Quantitative Phase Microscopy. Cells, 10(12), 3317. https://doi.org/10.3390/cells10123317







