Blood Immunoproteasome Activity Is Regulated by Sex, Age and in Chronic Inflammatory Diseases: A First Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Collection and PBMC Isolation
2.3. Protein Extraction
2.4. Activity-Based Probe Labeling
2.5. Gel Run
2.6. Gel Analysis
2.7. Data Normalization
2.8. Statistical Analyses
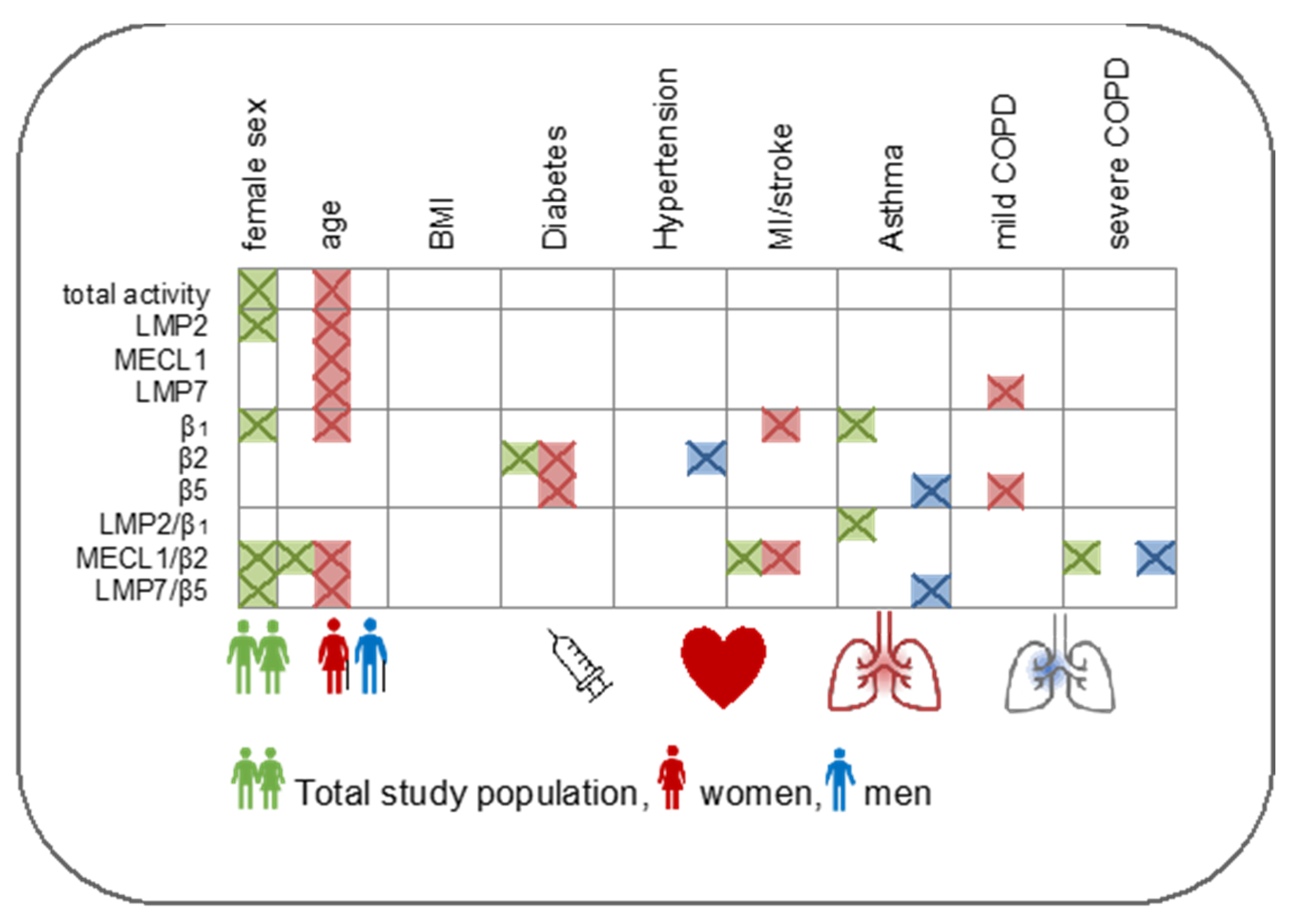
3. Results
3.1. Large Scale Immunoproteasome Activity Profiling in Blood Cells Using Activity-Based Probes
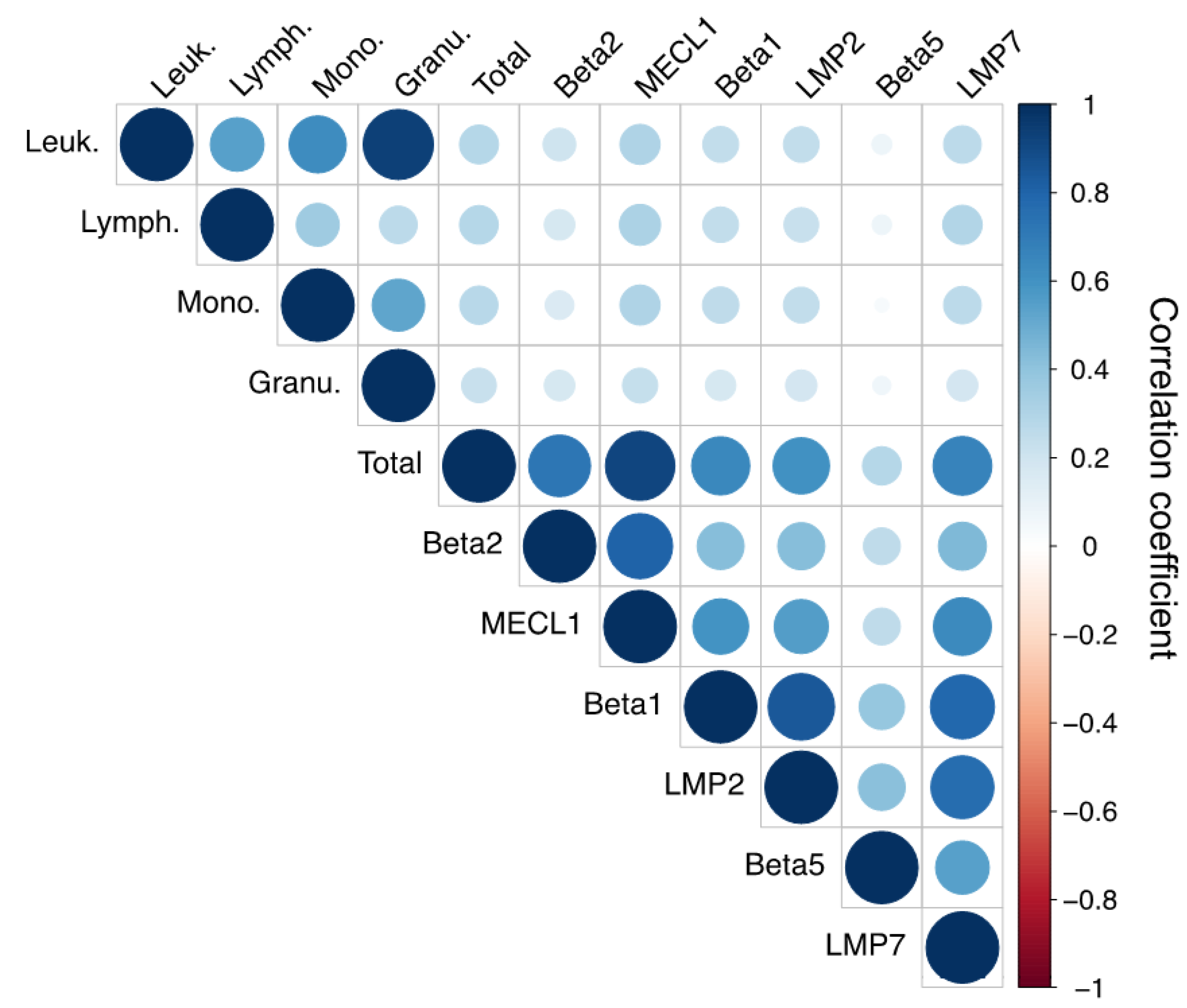
3.2. Immunoproteasome Activity Correlates with Leukocyte Cell Count
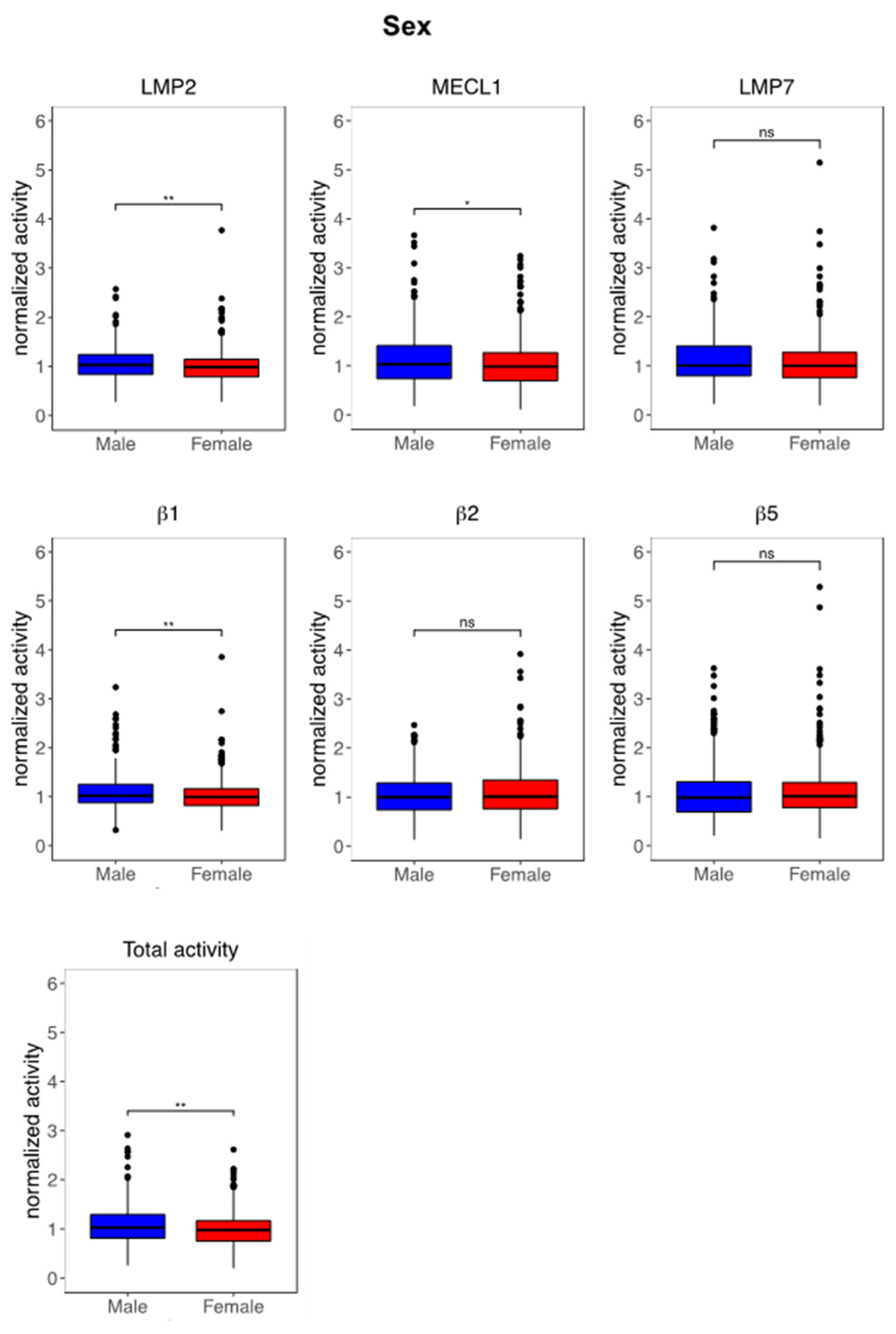
3.3. Immunoproteasome Activity Is Regulated by Sex and Age
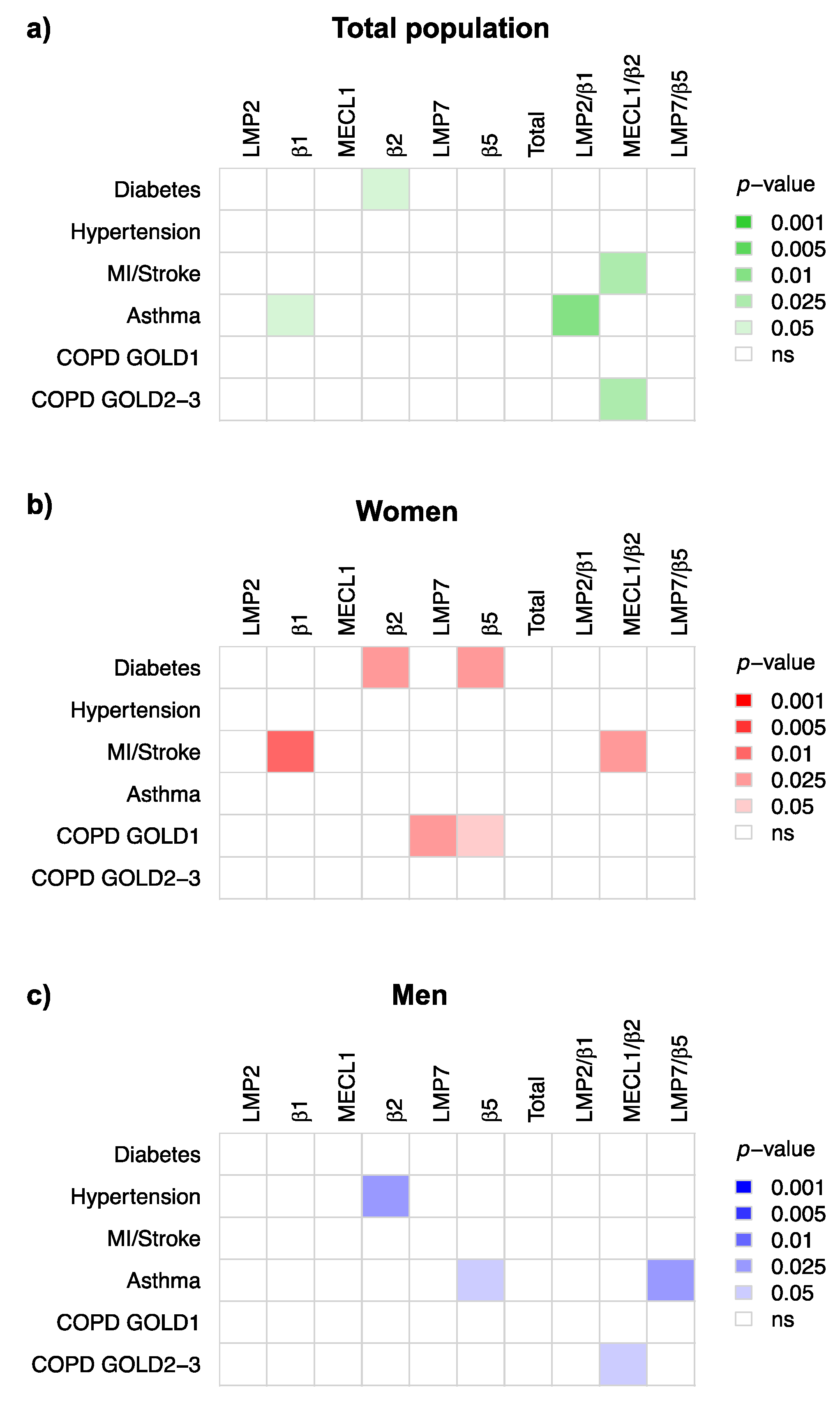
3.4. Immunoproteasome Activity Is Differentially Activated in Chronic Inflammatory Diseases
4. Discussion
4.1. Impact of Sex on Immunoproteasome Function
4.2. Age-Related Regulation of the Immunoproteasome
4.3. Immunoproteasome Activation in Chronic Inflammatory Diseases
4.4. Intermediate Immunoproteasomes in Peripheral Immune Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval Statement
References
- Löwer, M.; Bukur, T.; Castle, J.C.; Boegel, S.; Sahin, U.; Sorn, P.; Löwer, M.; Bukur, T.; Sorn, P.; Castle, J.C.; et al. HLA and Proteasome Expression Body Map. BMC Med. Genom. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Schmidt, C.; Berger, T.; Groettrup, M.; Basler, M. Immunoproteasome Inhibition Impairs T and B Cell Activation by Restraining ERK Signaling and Proteostasis. Front. Immunol. 2018, 9, 2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammerl, I.E.; Meiners, S. Proteasome Function Shapes Innate and Adaptive Immune Responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 311, L328–L336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sijts, E.J.A.M.; Kloetzel, P.M. The Role of the Proteasome in the Generation of MHC Class I Ligands and Immune Responses. Cell. Mol. Life Sci. 2011, 68, 1491–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kincaid, E.Z.; Che, J.W.; York, I.; Escobar, H.; Reyes-Vargas, E.; Delgado, J.C.; Welsh, R.M.; Karow, M.L.; Murphy, A.J.; Valenzuela, D.M.; et al. Mice Completely Lacking Immunoproteasomes Show Major Changes in Antigen Presentation. Nat. Immunol. 2012, 13, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley, S.E.; Zanker, D.; Dolan, B.P.; David, A.; Hickman, H.D.; Embry, A.C.; Skon, C.N.; Grebe, K.M.; Griffin, T.A.; Chen, W.; et al. Unexpected Role for the Immunoproteasome Subunit LMP2 in Antiviral Humoral and Innate Immune Responses. J. Immunol. 2010, 184, 4115–4122. [Google Scholar] [CrossRef] [Green Version]
- Kalim, K.W.; Basler, M.; Kirk, C.J.; Groettrup, M. Immunoproteasome Subunit LMP7 Deficiency and Inhibition Suppresses Th1 and Th17 but Enhances Regulatory T Cell Differentiation. J. Immunol. 2012, 189, 4182–4193. [Google Scholar] [CrossRef]
- Li, J.; Basler, M.; Alvarez, G.; Brunner, T.; Kirk, C.J.; Groettrup, M. Immunoproteasome Inhibition Prevents Chronic Antibody-Mediated Allograft Rejection in Renal Transplantation. Kidney Int. 2018, 93, 670–680. [Google Scholar] [CrossRef] [Green Version]
- Moebius, J.; van den Broek, M.; Groettrup, M.; Basler, M. Immunoproteasomes Are Essential for Survival and Expansion of T Cells in Virus-Infected Mice. Eur. J. Immunol. 2010, 40, 3439–3449. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Kammerl, I.E.; Vosyka, O.; Baumann, T.; Yu, Y.; Wu, Y.; Irmler, M.; Overkleeft, H.S.; Beckers, J.; Eickelberg, O.; et al. Immunoproteasome Dysfunction Augments Alternative Polarization of Alveolar Macrophages. Cell Death Differ. 2016, 23, 1026–1037. [Google Scholar] [CrossRef] [Green Version]
- de Verteuil, D.; Muratore-Schroeder, T.L.; Granados, D.P.; Fortier, M.-H.; Hardy, M.-P.; Bramoullé, A.; Caron, E.; Vincent, K.; Mader, S.; Lemieux, S.; et al. Deletion of Immunoproteasome Subunits Imprints on the Transcriptome and Has a Broad Impact on Peptides Presented by Major Histocompatibility Complex I Molecules. Mol. Cell. Proteom. 2010, 9, 2034–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Studencka-Turski, M.; Çetin, G.; Junker, H.; Ebstein, F.; Krüger, E. Molecular Insight Into the IRE1α-Mediated Type I Interferon Response Induced by Proteasome Impairment in Myeloid Cells of the Brain. Front. Immunol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Seifert, U.; Bialy, L.P.; Ebstein, F.; Bech-Otschir, D.; Voigt, A.; Schröter, F.; Prozorovski, T.; Lange, N.; Steffen, J.; Rieger, M.; et al. Immunoproteasomes Preserve Protein Homeostasis upon Interferon-Induced Oxidative Stress. Cell 2010, 142, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Raynes, R.; Pomatto, L.C.D.; Davies, K.J.A. Degradation of Oxidized Proteins by the Proteasome: Distinguishing between the 20S, 26S, and Immunoproteasome Proteolytic Pathways. Mol. Asp. Med. 2016, 50, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.S.; Kim, K.H.; Tschida, B.; Sachs, Z.; Noble-Orcutt, K.E.; Moriarity, B.S.; Ai, T.; Ding, R.; Williams, J.; Chen, L.; et al. MTORC1 Coordinates Protein Synthesis and Immunoproteasome Formation via PRAS40 to Prevent Accumulation of Protein Stress. Mol. Cell 2016, 61, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Abi Habib, J.; De Plaen, E.; Stroobant, V.; Zivkovic, D.; Bousquet, M.P.; Guillaume, B.; Wahni, K.; Messens, J.; Busse, A.; Vigneron, N.; et al. Efficiency of the Four Proteasome Subtypes to Degrade Ubiquitinated or Oxidized Proteins. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Dahlmann, B. Mammalian Proteasome Subtypes: Their Diversity in Structure and Function. Arch. Biochem. Biophys. 2016, 591, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Gohlke, S.; Kloss, A.; Tsokos, M.; Textoris-Taube, K.; Keller, C.; Kloetzel, P.-M.; Dahlmann, B. Adult Human Liver Contains Intermediate-Type Proteasomes with Different Enzymatic Properties. Ann. Hepatol. 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Morozov, A.V.; Karpov, V.L. Biological Consequences of Structural and Functional Proteasome Diversity. Heliyon 2018, 4, e00894. [Google Scholar] [CrossRef] [Green Version]
- Vigneron, N.; Van den Eynde, B.J. Proteasome Subtypes and the Processing of Tumor Antigens: Increasing Antigenic Diversity. Curr. Opin. Immunol. 2012, 24, 84–91. [Google Scholar] [CrossRef]
- Krause, S.; Kuckelkorn, U.; Dörner, T.; Burmester, G.R.; Feist, E.; Kloetzel, P.M. Immunoproteasome Subunit LMP2 Expression Is Deregulated in Sjögren’s Syndrome but Not in Other Autoimmune Disorders. Ann. Rheum. Dis. 2006, 65, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, K.; Martinez-Gamboa, L.; Spengler, L.; Krause, S.; Smiljanovic, B.; Bonin, M.; Bhattarai, S.; Grützkau, A.; Burmester, G.-R.; Häupl, T.; et al. Upregulation of Immunoproteasome Subunits in Myositis Indicates Active Inflammation with Involvement of Antigen Presenting Cells, CD8 T-Cells and IFNγ. PLoS ONE 2014, 9, e104048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppo, R.; Camilla, R.; Alfarano, A.; Balegno, S.; Mancuso, D.; Peruzzi, L.; Amore, A.; Dal Canton, A.; Sepe, V.; Tovo, P. Upregulation of the Immunoproteasome in Peripheral Blood Mononuclear Cells of Patients with IgA Nephropathy. Kidney Int. 2009, 75, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peruzzi, L.; Coppo, R.; Cocchi, E.; Loiacono, E.; Bergallo, M.; Bodria, M.; Vergano, L.; Krutova, A.; Russo, M.L.; Amore, A.; et al. The Switch from Proteasome to Immunoproteasome Is Increased in Circulating Cells of Patients with Fast Progressive Immunoglobulin A Nephropathy and Associated with Defective CD46 Expression. Nephrol. Dial. Transplant. 2020. [Google Scholar] [CrossRef]
- Kammerl, I.E.; Hardy, S.; Flexeder, C.; Urmann, A.; Peierl, J.; Wang, Y.; Vosyka, O.; Frankenberger, M.; Milger, K.; Behr, J.; et al. Activation of Immune Cell Proteasomes in Peripheral Blood of Smokers and COPD Patients - Implications for Therapy. Eur Respir J. 2021, 2101798. [Google Scholar] [CrossRef]
- Kammerl, I.E.; Dann, A.; Mossina, A.; Brech, D.; Lukas, C.; Vosyka, O.; Nathan, P.; Conlon, T.M.; Wagner, D.E.; Overkleeft, H.S.; et al. Impairment of Immunoproteasome Function by Cigarette Smoke and in COPD. Am. J. Respir. Crit. Care Med. 2016. [Google Scholar] [CrossRef]
- Baker, T.A.; Bach Iv, H.H.; Gamelli, R.L.; Love, R.B.; Majetschak, M. Proteasomes in Lungs From Organ Donors and Patients With End-Stage Pulmonary Diseases. Physiol. Res. 2014, 63, 311–319. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Keller, I.E.; John, G.; Kohse, K.; Yildirim, A.Ö.O.; Eickelberg, O.; Meiners, S. Acute Cigarette Smoke Exposure Impairs Proteasome Function in the Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L814–L823. [Google Scholar] [CrossRef]
- Kammerl, I.E.; Caniard, A.; Merl-Pham, J.; Ben-Nissan, G.; Mayr, C.H.; Mossina, A.; Geerlof, A.; Eickelberg, O.; Hauck, S.M.; Sharon, M.; et al. Dissecting the Molecular Effects of Cigarette Smoke on Proteasome Function. J. Proteom. 2019, 193, 1–9. [Google Scholar] [CrossRef]
- Somborac-Bacura, A.; van der Toorn, M.; Franciosi, L.; Slebos, D.-J.J.; Zanic-Grubisic, T.; Bischoff, R.; van Oosterhout, A.J.M. Cigarette Smoke Induces Endoplasmic Reticulum Stress Response and Proteasomal Dysfunction in Human Alveolar Epithelial Cells. Exp. Physiol. 2013, 98, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Drews, O.; Taegtmeyer, H. Targeting the Ubiquitin-Proteasome System in Heart Disease: The Basis for New Therapeutic Strategies. Antioxid. Redox Signal. 2014, 21, 2322–2343. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Lerman, A.; Herrmann, J. Dysfunction of the Ubiquitin-Proteasome System in Atherosclerotic Cardiovascular Disease. Am. J. Cardiovasc. Dis. 2015, 5, 83–100. [Google Scholar] [PubMed]
- Xie, X.; Wang, H.-X.; Li, N.; Deng, Y.-W.; Bi, H.-L.; Zhang, Y.-L.; Xia, Y.-L.; Li, H.-H. Selective Inhibition of the Immunoproteasome Β5i Prevents PTEN Degradation and Attenuates Cardiac Hypertrophy. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xie, Y.; Lin, Q.; Yang, X.; An, X.; Xia, Y.; Du, J.; Wang, F.; Li, H. Immunoproteasome Subunit Β5i Regulates Diet-induced Atherosclerosis through Altering MERTK-mediated Efferocytosis in Apoe Knockout Mice. J. Pathol. 2020, 250, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Bi, H.-L.L.; Lai, S.; Zhang, Y.-L.L.; Li, N.; Cao, H.-J.J.; Han, L.; Wang, H.-X.X.; Li, H.-H.H. The Immunoproteasome Catalytic Β5i Subunit Regulates Cardiac Hypertrophy by Targeting the Autophagy Protein ATG5 for Degradation. Sci. Adv. 2019, 5, eaau0495. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, S.; Bai, J.; Yang, X.-L.; Zhang, Y.-L.; Che, Y.-L.; Li, H.-H.; Yang, Y.-Z. Novel Role for the Immunoproteasome Subunit PSMB10 in Angiotensin II–Induced Atrial Fibrillation in Mice. Hypertension 2018, 71, 866–876. [Google Scholar] [CrossRef]
- Bockstahler, M.; Fischer, A.; Goetzke, C.C.; Neumaier, H.L.; Sauter, M.; Kespohl, M.; Müller, A.M.; Meckes, C.; Salbach, C.; Schenk, M.; et al. Heart-Specific Immune Responses in an Animal Model of Autoimmune- Related Myocarditis Mitigated by an Immunoproteasome Inhibitor and Genetic Ablation. Circulation 2020, 141, 1885–1902. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.-J.; Fang, J.; Zhang, Y.-L.; Zou, L.-X.; Han, X.; Yang, J.; Yan, X.; Li, P.; Wang, H.-X.; Guo, S.-B.; et al. Genetic Ablation and Pharmacological Inhibition of Immunosubunit Β5i Attenuates Cardiac Remodeling in Deoxycorticosterone-Acetate (DOCA)-Salt Hypertensive Mice. J. Mol. Cell. Cardiol. 2019, 137, 34–45. [Google Scholar] [CrossRef]
- Broca, C.; Varin, E.; Armanet, M.; Tourrel-Cuzin, C.; Bosco, D.; Dalle, S.; Wojtusciszyn, A. Proteasome Dysfunction Mediates High Glucose-Induced Apoptosis in Rodent Beta Cells and Human Islets. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Bugliani, M.; Liechti, R.; Cheon, H.; Suleiman, M.; Marselli, L.; Kirkpatrick, C.; Filipponi, F.; Boggi, U.; Xenarios, I.; Syed, F.; et al. Microarray Analysis of Isolated Human Islet Transcriptome in Type 2 Diabetes and the Role of the Ubiquitin-Proteasome System in Pancreatic Beta Cell Dysfunction. Mol. Cell. Endocrinol. 2013, 367, 1–10. [Google Scholar] [CrossRef]
- Casas, S.; Gomis, R.; Gribble, F.; Altirriba, J.; Knuutila, S.; Novials, A. Impairment of the Ubiquitin-Proteasome Pathway Is a Downstream Endoplasmic Reticulum Stress Response Induced By Extracellular Human Islet Amyloid Polypeptide and Contributes To Pancreatic. Diabetes 2007, 56, 2284–2294. [Google Scholar] [CrossRef] [Green Version]
- Khilji, M.S.; Verstappen, D.; Dahlby, T.; Burstein Prause, M.C.; Pihl, C.; Bresson, S.E.; Bryde, T.H.; Keller Andersen, P.A.; Klindt, K.; Zivkovic, D.; et al. The Intermediate Proteasome Is Constitutively Expressed in Pancreatic Beta Cells and Upregulated by Stimulatory, Low Concentrations of Interleukin 1 β. PLoS ONE 2020, 15, 1–21. [Google Scholar] [CrossRef]
- Lundh, M.; Bugliani, M.; Dahlby, T.; Chou, D.H.C.; Wagner, B.; Ghiasi, S.M.; De Tata, V.; Chen, Z.; Lund, M.N.; Davies, M.J.; et al. The Immunoproteasome Is Induced by Cytokines and Regulates Apoptosis in Human Islets. J. Endocrinol. 2017, 233, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W.; Bekker, C.P.J.; Gröne, A.; Lie, B.A.; Sijts, A.J. Proteasome Immunosubunits Protect against the Development of CD8 T Cell-Mediated Autoimmune Diseases. J. Immunol. 2011, 187, 2302–2309. [Google Scholar] [CrossRef] [Green Version]
- Breczko, W.; Lemancewicz, D.; Dzięcioł, J.; Kłoczko, J.; Bołkun, Ł. High Immunoproteasome Concentration in the Plasma of Patients with Newly Diagnosed Multiple Myeloma Treated with Bortezomib Is Predictive of Longer OS. Adv. Med. Sci. 2021, 66, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz-Krzeminska, I.; de Ramón, C.; Corchete, L.A.; Krzeminski, P.; Rojas, E.A.; Isidro, I.; García-Sanz, R.; Martínez-López, J.; Oriol, A.; Bladé, J.; et al. Quantitative Expression of Ikaros, IRF4, and PSMD10 Proteins Predicts Survival in VRD-Treated Patients with Multiple Myeloma. Blood Adv. 2020, 4, 6023–6033. [Google Scholar] [CrossRef] [PubMed]
- Woodle, E.S.; Tremblay, S.; Brailey, P.; Girnita, A.; Alloway, R.R.; Aronow, B.; Dasgupta, N.; Ebstein, F.; Kloetzel, P.; Lee, M.J.; et al. Proteasomal Adaptations Underlying Carfilzomib-resistance in Human Bone Marrow Plasma Cells. Am. J. Transplant. 2020, 20, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Berryman, K.; Buhimschi, C.S.; Zhao, G.; Axe, M.; Locke, M.; Buhimschi, I.A. Proteasome Levels and Activity in Pregnancies Complicated by Severe Preeclampsia and Hemolysis, Elevated Liver Enzymes, and Thrombocytopenia (HELLP) Syndrome. Hypertension 2019, 73, 1308–1318. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Fu, M.; Lei, H.; Cheng, Q.; Zhang, X. Plasma Immunoproteasome Predicts Early Hemorrhagic Transformation in Acute Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 49–56. [Google Scholar] [CrossRef]
- Chen, X.Y.; Fu, M.; Wan, S.F.; Zhang, X.; Wang, Y.Z. Association between Plasma Immunoproteasome and 90-Day Prognosis after First-Ever Ischemic Stroke. Neural Regen. Res. 2021, 16, 790–795. [Google Scholar] [CrossRef]
- Matuszczak, E.; Weremijewicz, A.; Komarowska, M.; Sankiewicz, A.; Markowska, D.; Debek, W.; Gorodkiewicz, E.; Milewski, R.; Hermanowicz, A. Immunoproteasome in the Plasma of Pediatric Patients With Moderate and Major Burns, and Its Correlation With Proteasome and UCHL1 Measured by SPR Imaging Biosensors. J. Burn Care Res. 2018, 39, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Sixt, S.U.; Dahlmann, B. Extracellular, Circulating Proteasomes and Ubiquitin - Incidence and Relevance. Biochim. Et Biophys. Acta 2008, 1782, 817–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holle, R.; Happich, M.; Löwel, H.; Wichmann, H.E.; null for the MONICA/KORA Study Group. KORA—A Research Platform for Population Based Health Research. Das Gesundh. 2005, 67, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, H.-E.; Gieger, C.; Illig, T. KORA-gen - Resource for Population Genetics, Controls and a Broad Spectrum of Disease Phenotypes. Gesundheitswesen 2005, 67, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karrasch, S.; Flexeder, C.; Behr, J.; Holle, R.; Huber, R.M.; Jörres, R.A.; Nowak, D.; Peters, A.; Wichmann, H.-E.; Heinrich, J.; et al. Spirometric Reference Values for Advanced Age from a South German Population. Respir. Int. Rev. Thorac. Dis. 2013, 85, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-Ethnic Reference Values for Spirometry for the 3–95-Yr Age Range: The Global Lung Function 2012 Equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- de Bruin, G.; Xin, B.T.; Kraus, M.; van der Stelt, M.; van der Marel, G.A.; Kisselev, A.F.; Driessen, C.; Florea, B.I.; Overkleeft, H.S. A Set of Activity-Based Probes to Visualize Human (Immuno)Proteasome Activities. Angew. Chem. Int. Ed. 2016, 55, 4199–4203. [Google Scholar] [CrossRef]
- Keller, I.E.; Vosyka, O.; Takenaka, S.; Kloß, A.; Dahlmann, B.; Willems, L.I.; Verdoes, M.; Overkleeft, H.S.; Marcos, E.; Adnot, S.; et al. Regulation of Immunoproteasome Function in the Lung. Sci. Rep. 2015, 5, 10230. [Google Scholar] [CrossRef] [Green Version]
- Hewings, D.S.; Flygare, J.A.; Wertz, I.E.; Bogyo, M. Activity-Based Probes for the Multicatalytic Proteasome. FEBS J. 2017, 284, 1540–1554. [Google Scholar] [CrossRef] [Green Version]
- Krämer, U.; Illig, T.; Grune, T.; Krutmann, J.; Esser, C. Strong Associations of Psoriasis with Antigen Processing LMP and Transport Genes TAP Differ by Gender and Phenotype. Genes Immun. 2007, 8, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Mishto, M.; Bellavista, E.; Ligorio, C.; Textoris-Taube, K.; Santoro, A.; Giordano, M.; D’Alfonso, S.; Listì, F.; Nacmias, B.; Cellini, E.; et al. Immunoproteasome LMP2 60HH Variant Alters MBP Epitope Generation and Reduces the Risk to Develop Multiple Sclerosis in Italian Female Population. PLoS ONE 2010, 5, e9287. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2021, 11, 604000. [Google Scholar] [CrossRef] [PubMed]
- Bongen, E.; Lucian, H.; Khatri, A.; Fragiadakis, G.K.; Bjornson, Z.B.; Nolan, G.P.; Utz, P.J.; Khatri, P. Sex Differences in the Blood Transcriptome Identify Robust Changes in Immune Cell Proportions with Aging and Influenza Infection. Cell Rep. 2019, 29, 1961–1973.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, E.C.; Shah, N.; Gomez, M.; Casalena, G.; Zhao, D.; Kenny, T.C.; Guariglia, S.R.; Manfredi, G.; Germain, D. Proteasome Mapping Reveals Sexual Dimorphism in Tissue-Specific Sensitivity to Protein Aggregations. EMBO Rep. 2020, e48978. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Mishra, M.; Salemi, M.R.; Phinney, B.S.; Newens, J.L.; Gomes, A.V. Gender-Specific Changes in Energy Metabolism and Protein Degradation as Major Pathways Affected in Livers of Mice Treated with Ibuprofen. Sci Rep. 2020, 10, 3386. [Google Scholar] [CrossRef] [PubMed]
- Caniard, A.; Ballweg, K.; Lukas, C.; Yildirim, A.Ö.; Eickelberg, O.; Meiners, S. Proteasome Function Is Not Impaired in Healthy Aging of the Lung. Aging 2015, 7, 776–792. [Google Scholar] [CrossRef] [Green Version]
- Breusing, N.; Grune, T. Regulation of Proteasome-Mediated Protein Degradation during Oxidative Stress and Aging. Biol. Chem. 2008, 389, 203–209. [Google Scholar] [CrossRef]
- Dasuri, K.; Zhang, L.; Ebenezer, P.; Liu, Y.; Fernandez-Kim, S.O.; Keller, J.N. Aging and Dietary Restriction Alter Proteasome Biogenesis and Composition in the Brain and Liver. Mech. Ageing Dev. 2009, 130, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.N.; Hanni, K.B.; Markesbery, W.R. Possible Involvement of Proteasome Inhibition in Aging: Implications for Oxidative Stress. Mech. Ageing Dev. 2000, 113, 61–70. [Google Scholar] [CrossRef]
- Bellavista, E.; Martucci, M.; Vasuri, F.; Santoro, A.; Mishto, M.; Kloss, A.; Capizzi, E.; Degiovanni, A.; Lanzarini, C.; Remondini, D.; et al. Lifelong Maintenance of Composition, Function and Cellular/Subcellular Distribution of Proteasomes in Human Liver. Mech. Ageing Dev. 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ethen, C.M.; Hussong, S.A.; Reilly, C.; Feng, X.; Olsen, T.W.; Ferrington, D.A. Transformation of the Proteasome with Age-Related Macular Degeneration. FEBS Lett. 2007, 581, 885–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasacka, I.; Piotrowska, Ż.; Niezgoda, M.; Lewandowska, A.; Łebkowski, W. Ageing-Related Changes in the Levels of β-Catenin, CacyBP/SIP, Galectin-3 and Immunoproteasome Subunit LMP7 in the Heart of Men. PLoS ONE 2020, 15, e0229462. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.O.; Sarup, P.; Loeschcke, V.; Rattan, S.I.S. Age-Related and Sex-Specific Differences in Proteasome Activity in Individual Drosophila Flies from Wild Type, Longevity-Selected and Stress Resistant Strains. Biogerontology 2012. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Wong, S.; Carney, C.; Shen, B.; Tower, J.; Davies, K.J.A. The Age- and Sex-Specific Decline of the 20s Proteasome and the Nrf2/CncC Signal Transduction Pathway in Adaption and Resistance to Oxidative Stress in Drosophila Melanogaster. Aging 2017, 9, 1153–1185. [Google Scholar] [CrossRef] [Green Version]
- Nikolich-Žugich, J. The Twilight of Immunity: Emerging Concepts in Aging of the Immune System. Nat. Immunol 2018, 19, 10–19. [Google Scholar] [CrossRef]
- Rieckmann, J.C.; Geiger, R.; Hornburg, D.; Wolf, T.; Kveler, K.; Jarrossay, D.; Sallusto, F.; Shen-Orr, S.S.; Lanzavecchia, A.; Mann, M.; et al. Social Network Architecture of Human Immune Cells Unveiled by Quantitative Proteomics. Nat. Immunol. 2017, 18, 583–593. [Google Scholar] [CrossRef]
- Volkov, A.; Hagner, S.; Löser, S.; Alnahas, S.; Raifer, H.; Hellhund, A.; Garn, H.; Steinhoff, U. Β5i Subunit Deficiency of the Immunoproteasome Leads to Reduced Th2 Response in OVA Induced Acute Asthma. PLoS ONE 2013, 8, e60565. [Google Scholar] [CrossRef]
- De, M.; Jayarapu, K.; Elenich, L.; Monaco, J.J.; Colbert, R.A.; Griffin, T.A. Β2 Subunit Propeptides Influence Cooperative Proteasome Assembly. J. Biol. Chem. 2003, 278, 6153–6159. [Google Scholar] [CrossRef] [Green Version]
- Griffin, T.A.; Nandi, D.; Cruz, M.; Fehling, H.J.; Kaer, L.V.; Monaco, J.J.; Colbert, R.A. Immunoproteasome Assembly: Cooperative Incorporation of Interferon Gamma (IFN-Gamma)-Inducible Subunits. J. Exp. Med. 1998, 187, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaume, B.; Chapiro, J.; Stroobant, V.; Colau, D.; Van Holle, B.; Parvizi, G.; Bousquet-Dubouch, M.-P.; Théate, I.; Parmentier, N.; Van den Eynde, B.J. Two Abundant Proteasome Subtypes That Uniquely Process Some Antigens Presented by HLA Class I Molecules. Proc. Natl. Acad. Sci. USA 2010, 107, 18599–18604. [Google Scholar] [CrossRef] [Green Version]
- Fehling, H.J.; Swat, W.; Laplace, C.; Kühn, R.; Rajewsky, K.; Müller, U.; von Boehmer, H. MHC Class I Expression in Mice Lacking the Proteasome Subunit LMP-7. Science 1994, 265, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.C.; Sanders, M.T.; Monaco, J.J.; Doherty, P.C.; Turner, S.J.; Chen, W. Immunoproteasome Subunit Deficiencies Impact Differentially on Two Immunodominant Influefnt of Antigen Presenting Cells, CD8 T-Cells and IFNnza Virus-Specific CD8+ T Cell Responses. J. Immunol. 2006, 177, 7680–7688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwell, C.E.; Monaco, J.J.; Qureshi, N. A Critical Role for the Inducible Proteasomal Subunits LMP7 and MECL1 in Cytokine Production by Activated Murine Splenocytes. Pharmacology 2012, 89, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kaer, L.; Ashton-Rickardt, P.G.; Eichelberger, M.; Gaczynska, M.; Nagashima, K.; Rock, K.L.; Goldberg, A.L.; Doherty, P.C.; Tonegawa, S.; Van Kaer, L.; et al. Altered Peptidase and Viral-Specific T Cell Response in LMP2 Mutant Mice. Immunity 1994, 1, 533–541. [Google Scholar] [CrossRef]
- Rock, K.L.; Gramm, C.; Rothstein, L.; Clark, K.; Stein, R.; Dick, L.; Hwang, D.; Goldberg, A.L. Inhibitors of the Proteasome Block the Degradation of Most Cell Proteins and the Generation of Peptides Presented on MHC Class I Molecules. Cell 1994, 78, 761–771. [Google Scholar] [CrossRef]


| Parameters | Mean (SD) or % | N or n/N |
|---|---|---|
| Age, years | 57.9 (5.7) | 873 |
| Sex | ||
| male | 47.0 | 410/873 |
| female | 53.0 | 463/873 |
| BMI, kg/m² | 27.9 (5.0) | 873 |
| FEV1, L | 3.0 (0.8) | 872 |
| FVC, L | 4.0 (1.0) | 872 |
| FEV1/FVC, % | 75.3 (7.4) | 872 |
| COPD | ||
| no COPD | 81.0 | 706/872 |
| GOLD 1 | 11.7 | 102/872 |
| GOLD 2 or 3 | 7.3 | 64/872 |
| CVD | ||
| no | 63.9 | 557/872 |
| yes | 36.1 | 315/872 |
| Hypertension | ||
| no | 65.1 | 568/872 |
| yes | 34.9 | 304/872 |
| Myocardial infarction | ||
| no | 97.7 | 853/873 |
| yes | 2.3 | 20/873 |
| Stroke | ||
| no | 98.7 | 862/873 |
| yes | 1.3 | 11/873 |
| Diabetes | ||
| no | 93.6 | 817/873 |
| yes | 6.4 | 56/873 |
| Asthma DD ever | ||
| no | 90.8 | 793/873 |
| yes | 9.2 | 80/873 |
| Smoking | ||
| current | 18.2 | 159/873 |
| former | 44.3 | 387/873 |
| never | 37.5 | 327/873 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kammerl, I.E.; Flexeder, C.; Karrasch, S.; Thorand, B.; Heier, M.; Peters, A.; Schulz, H.; Meiners, S. Blood Immunoproteasome Activity Is Regulated by Sex, Age and in Chronic Inflammatory Diseases: A First Population-Based Study. Cells 2021, 10, 3336. https://doi.org/10.3390/cells10123336
Kammerl IE, Flexeder C, Karrasch S, Thorand B, Heier M, Peters A, Schulz H, Meiners S. Blood Immunoproteasome Activity Is Regulated by Sex, Age and in Chronic Inflammatory Diseases: A First Population-Based Study. Cells. 2021; 10(12):3336. https://doi.org/10.3390/cells10123336
Chicago/Turabian StyleKammerl, Ilona Elisabeth, Claudia Flexeder, Stefan Karrasch, Barbara Thorand, Margit Heier, Annette Peters, Holger Schulz, and Silke Meiners. 2021. "Blood Immunoproteasome Activity Is Regulated by Sex, Age and in Chronic Inflammatory Diseases: A First Population-Based Study" Cells 10, no. 12: 3336. https://doi.org/10.3390/cells10123336
APA StyleKammerl, I. E., Flexeder, C., Karrasch, S., Thorand, B., Heier, M., Peters, A., Schulz, H., & Meiners, S. (2021). Blood Immunoproteasome Activity Is Regulated by Sex, Age and in Chronic Inflammatory Diseases: A First Population-Based Study. Cells, 10(12), 3336. https://doi.org/10.3390/cells10123336






