Targeting CAMKK2 and SOC Channels as a Novel Therapeutic Approach for Sensitizing Acute Promyelocytic Leukemia Cells to All-Trans Retinoic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
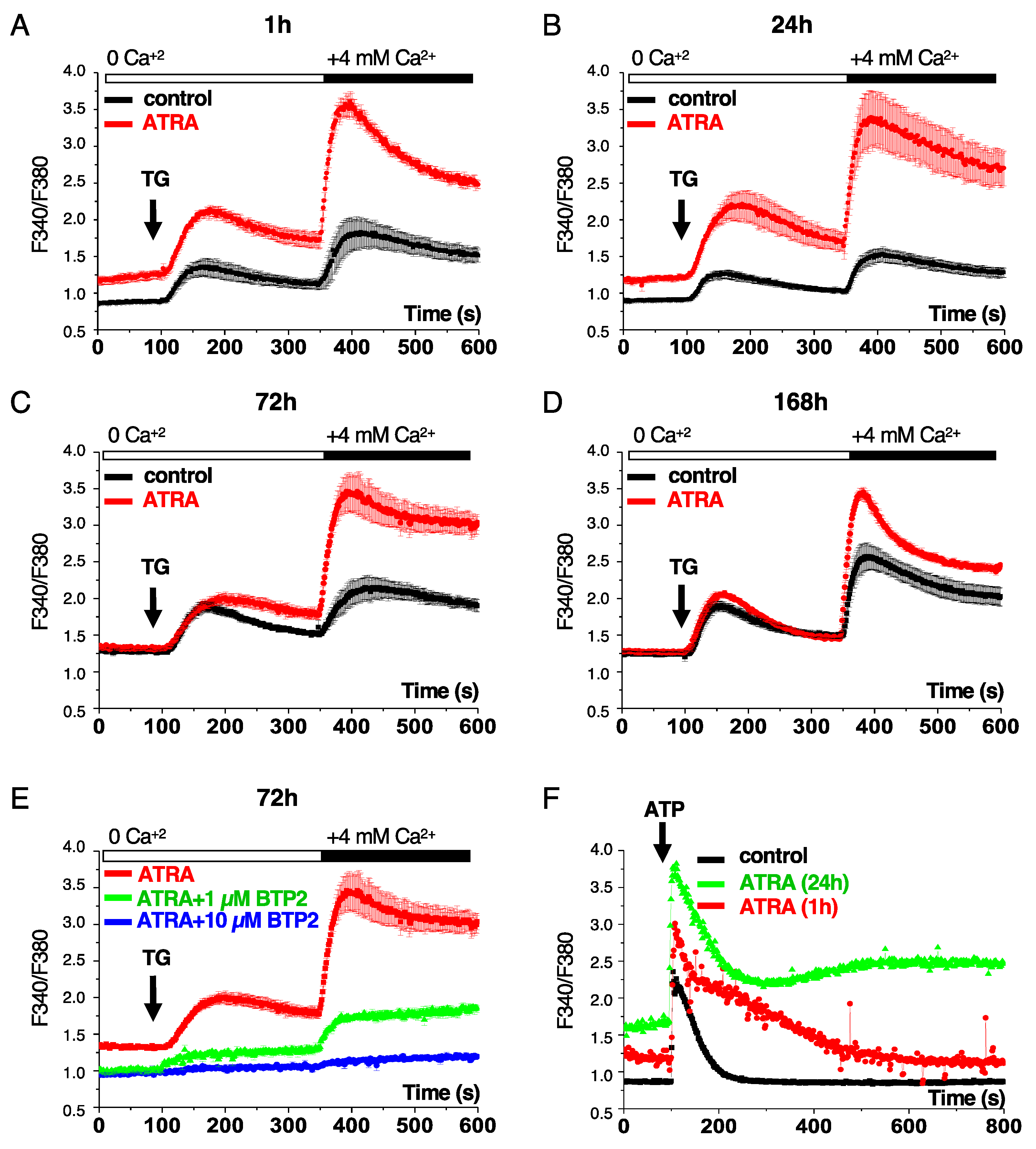
2.2. Measurement of Intracellular Ca2+ Concentration
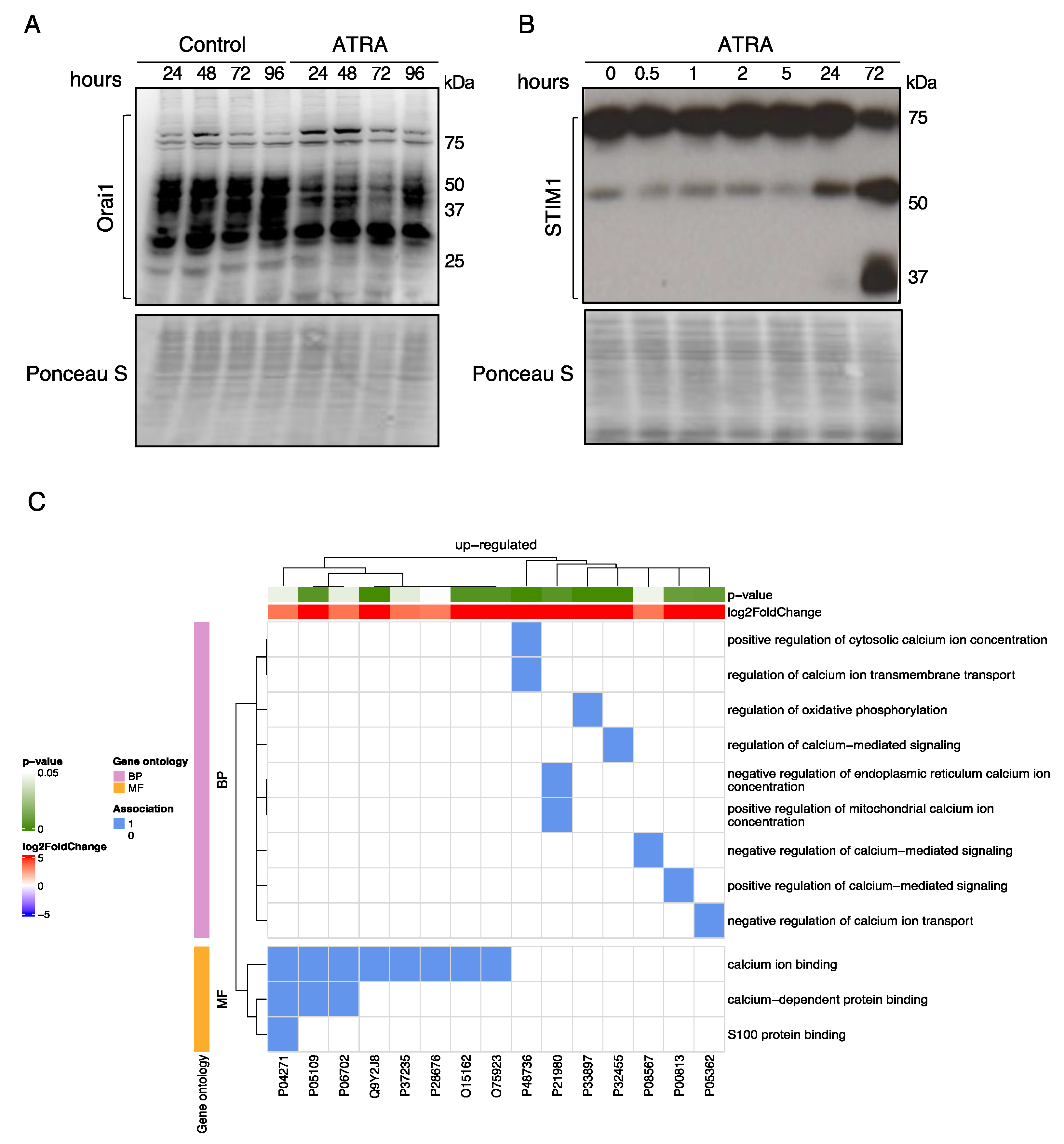
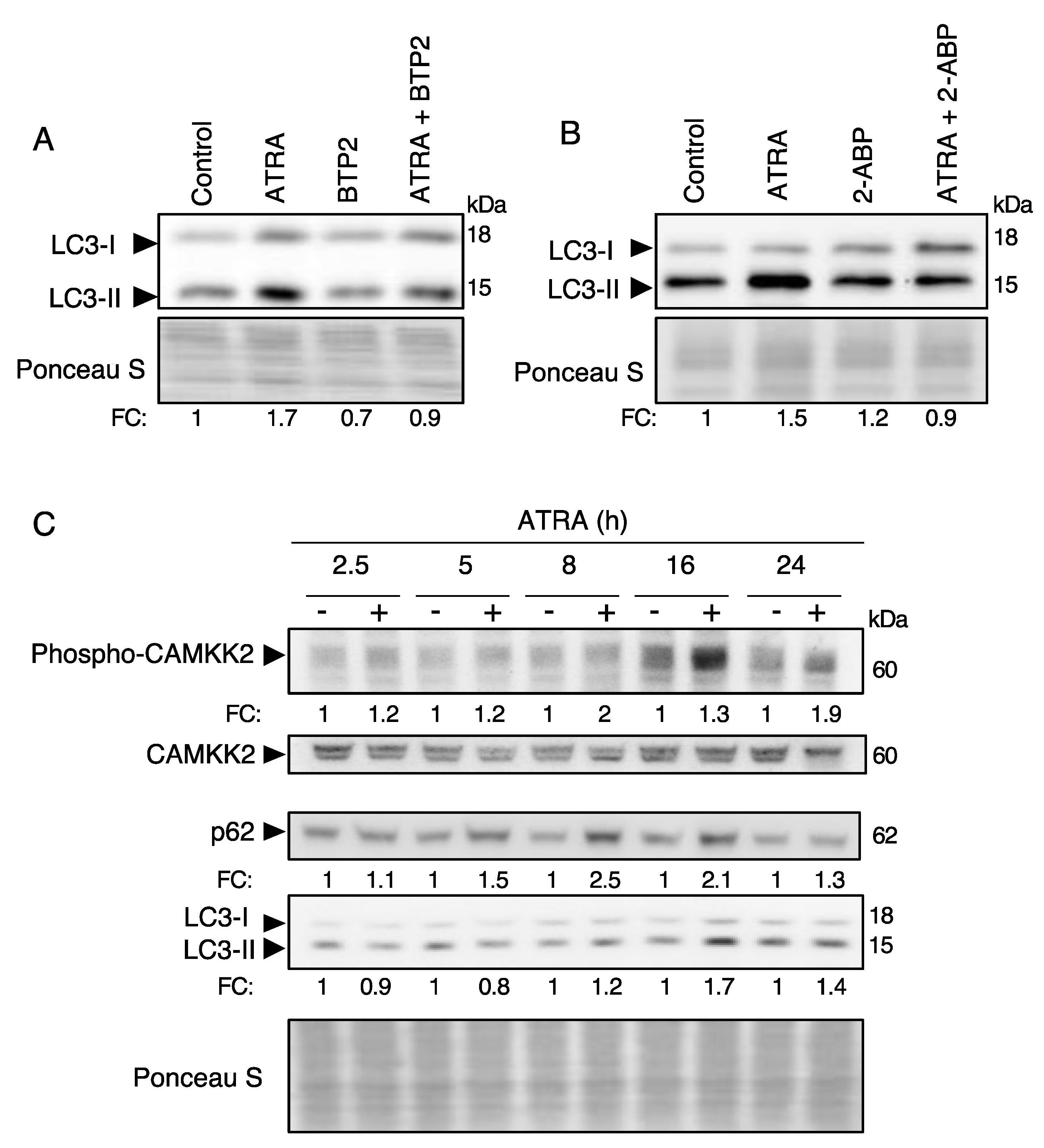
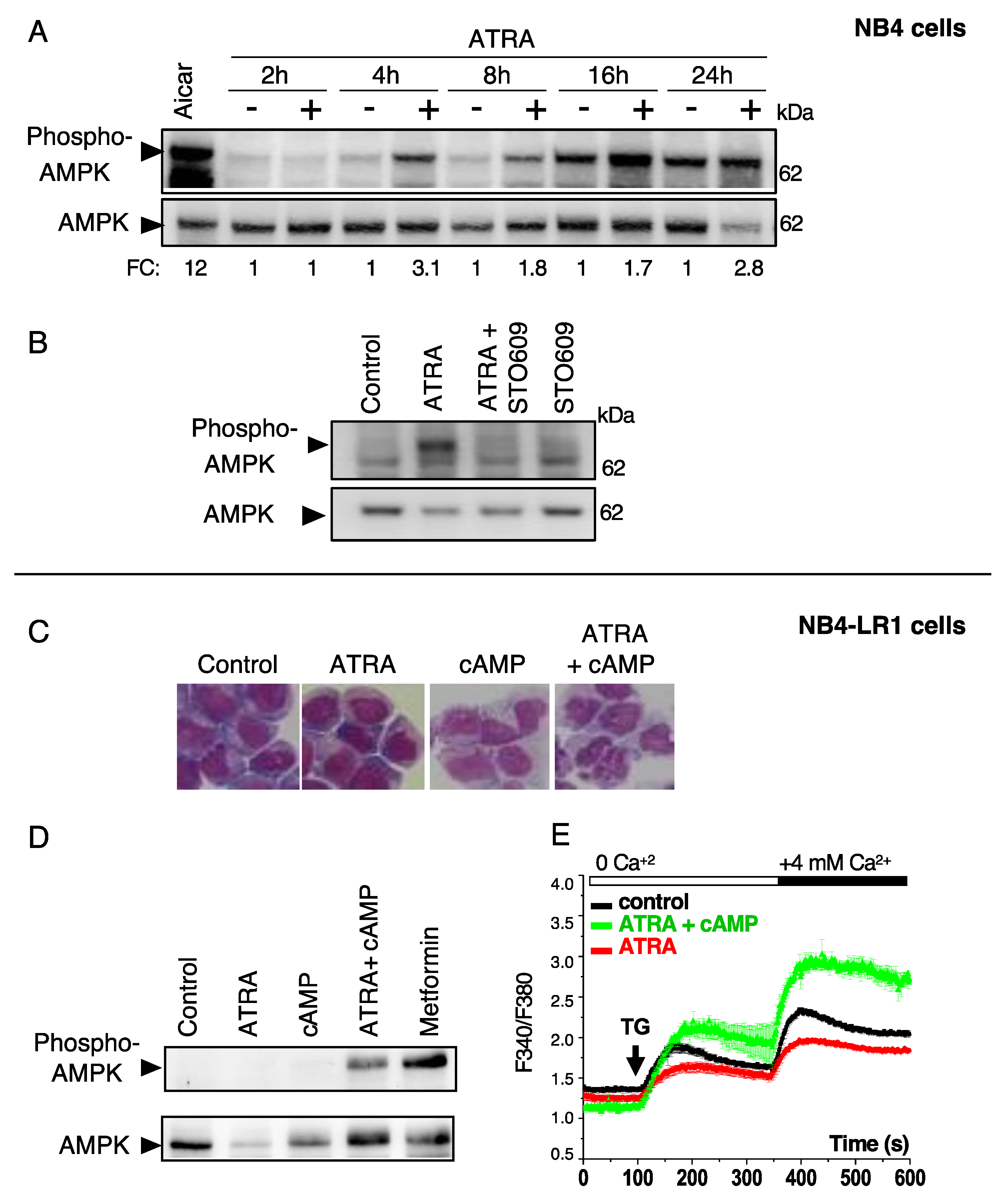
2.3. Immunoblot Assay
2.4. Label-Free Quantitative Proteomics
2.5. Bioinformatic Analysis of Proteomic Data
2.6. Cell Death Measurement
2.7. Evaluation of Granulocytic Differentiation
2.8. Statistical Analysis
3. Results
3.1. ATRA Activates the Capacitative Ca2+ Entry into Acute Promyelocytic Leukemia Cells
3.2. Regulation of Ca2+-Regulatory Proteins by ATRA
3.3. ATRA Induces Autophagy through a Ca2+-Dependent Mechanism
3.4. ATRA Promotes AMPK Activation through a Ca2+ -Dependent Mechanism in Differentiation-Sensitive NB4 Cells but Not in Differentiation-Resistant NB4-LR1 Cells
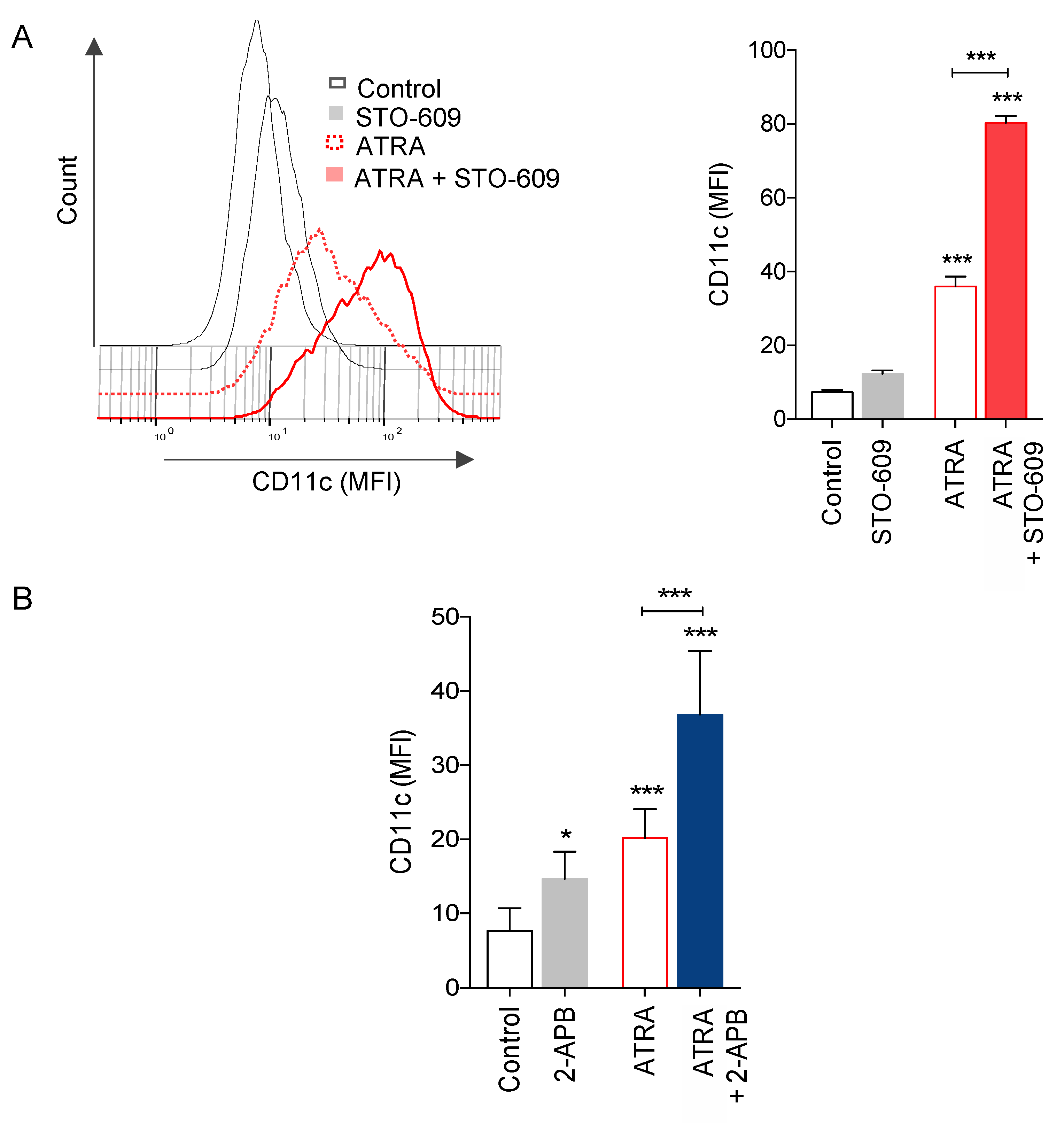
3.5. Role of Ca2+ Responses on ATRA-Induced Granulocytic Differentiation of APL Cells
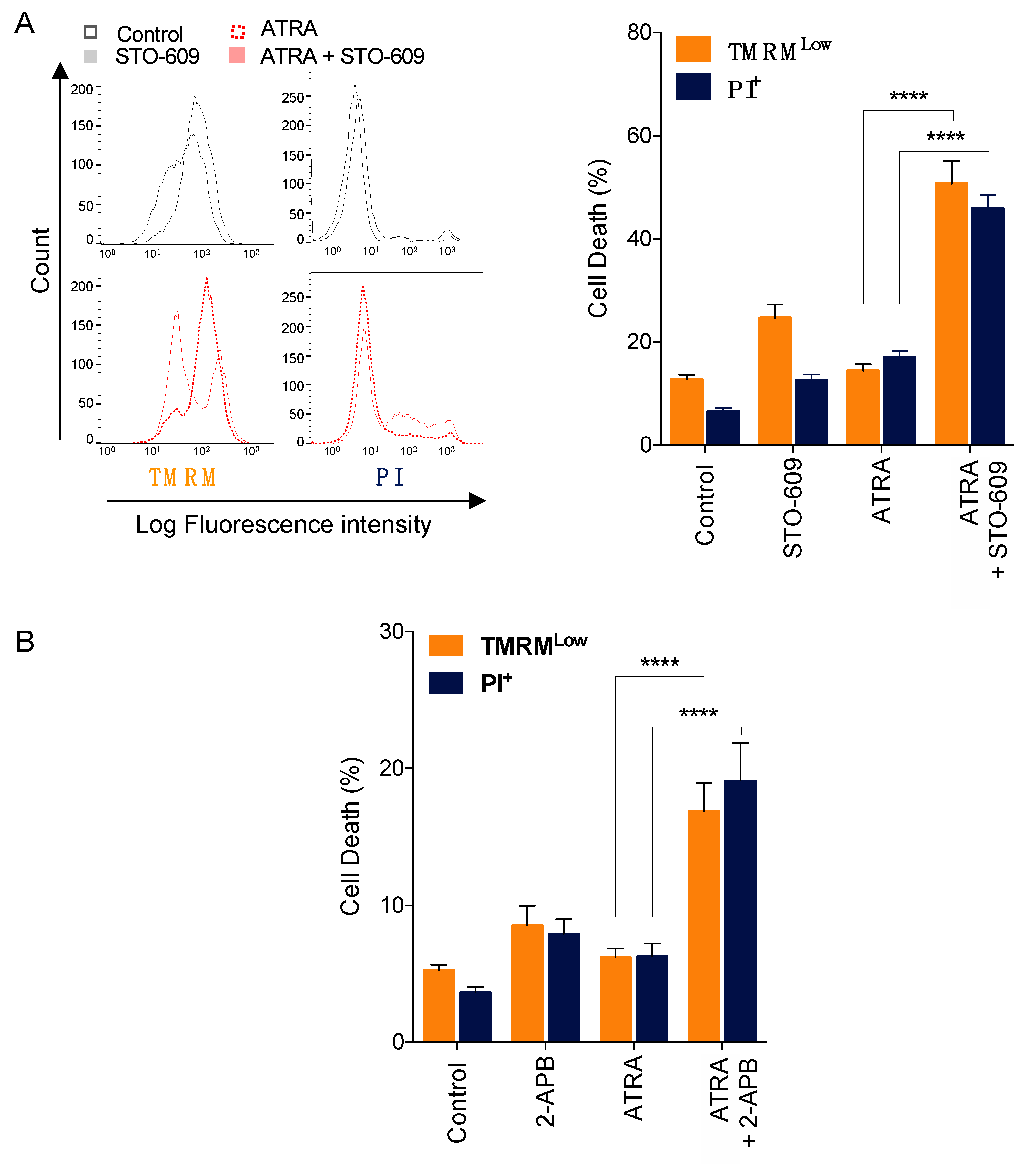
3.6. Impact of Calcium Responses on ATRA-Induced APL Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-APB | 2-aminoethoxydiphenyl borate; |
| AMPK | AMP-activated protein kinase; |
| APL | acute promyelocytic leukemia; |
| ATRA | all-trans retinoic acid; |
| ATG | Autophagy-related gene; |
| BAPTA-AM | 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester); |
| BTP2 | N-(4-(3,5-bis(tri uoromethyl)-1H-pyrazol-1-yl)phenyl)-4-methyl-1,2,3-thiadiazole-5-carboxamide; |
| Ca2+ | Calcium ions; |
| CAMKK2 | calcium/calmodulin-dependent protein kinase kinase 2; |
| 8-CPT-cAMP | 8-(4-chlorophenylthio), 5’-cyclic adenosine monophosphate; |
| CRAC | calcium released activated channel; LC3-II, microtubule-associated protein 1 light chain 3-II; |
| ER | endoplasmic reticulum; |
| GO | gene ontology; |
| MGG | May-Grunwald Giemsa; |
| MFI | Median fluorescence intensity; |
| PI | propidium iodide; |
| ORAI1 | ORAI calcium release-activated calcium modulator 1; |
| SERCA | sarco-/endoplasmic reticulum Ca2+-ATPase; |
| SOC | Store-operated calcium (SOC); |
| SOCE | store operated calcium entry; |
| sh | small hairpin; |
| SQSTM1 | sequestosome 1; |
| STIM1 | Stromal Interaction Molecule 1; |
| TG | thapsigargin. |
| TMRM | tetramethylrhodamine methyl ester; |
| MTORC1 | mammalian target of rapamycin complex 1; |
References
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parys, J.B.; Bultynck, G. Ca2+ signaling and cell death: Focus on the role of Ca2+ signals in the regulation of cell death & survival processes in health, disease and therapy. Cell Calcium 2018, 70, 1–2. [Google Scholar] [CrossRef]
- Parys, J.B.; Bultynck, G. Calcium signaling in health, disease and therapy. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.B. Store-operated CRAC channels: Function in health and disease. Nat. Rev. Drug Discov. 2010, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [Green Version]
- Racioppi, L.; Means, A.R. Calcium/calmodulin-dependent protein kinase kinase 2: Roles in signaling and pathophysiology. J. Biol. Chem. 2012, 287, 31658–31665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Cahalan, M.D. STIMulating store-operated Ca(2+) entry. Nat. Cell Biol. 2009, 11, 669–677. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Codogno, P. The mechanism and physiological function of macroautophagy. J. Innate Immun. 2013, 5, 427–433. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological functions of autophagy genes: A disease perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [Green Version]
- Kondratskyi, A.; Kondratska, K.; Skryma, R.; Klionsky, D.J.; Prevarskaya, N. Ion channels in the regulation of autophagy. Autophagy 2018, 14, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, B.; Zhao, M.; Song, D.; Wu, K.; Yi, L.; Li, W.; Li, X.; Wang, K.; Chen, J. Induction of autophagy and suppression of Type I IFN secretion by CSFV. Autophagy 2021, 17, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Gulati, P.; Gaspers, L.D.; Dann, S.G.; Joaquin, M.; Nobukuni, T.; Natt, F.; Kozma, S.C.; Thomas, A.P.; Thomas, G. Amino acids activate MTOR complex 1 via Ca2+/CaM Signaling to HVps34. Cell Metab. 2008, 7, 456–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.E.; Ye, Y.C.; Chen, S.R.; Chai, J.R.; Lu, J.X.; Zhoa, L.; Gu, L.J.; Wang, Z.Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988, 72, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Moosavi, M.A.; Djavaheri-Mergny, M. Autophagy: New insights into mechanisms of action and resistance of treatment in acute promyelocytic leukemia. Int. J. Mol. Sci. 2019, 20, 3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djavaheri-Mergny, M.; Giuriato, S.; Tschan, M.P.; Humbert, M. Therapeutic modulation of autophagy in leukaemia and lymphoma. Cells 2019, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Trocoli, A.; Mathieu, J.; Priault, M.; Reiffers, J.; Souquere, S.; Pierron, G.; Besançon, F.; Djavaheri-Mergny, M. ATRA-induced upregulation of beclin 1 prolongs the life span of differentiated acute promyelocytic leukemia cells. Autophagy 2011, 7, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigger, D.; Proikas-Cezanne, T.; Tschan, M.P. WIPI-dependent autophagy during neutrophil differentiation of NB4 acute promyelocytic leukemia cells. Cell Death Dis. 2014, 5, e1315. [Google Scholar] [CrossRef] [Green Version]
- Trocoli, A.; Bensadoun, P.; Richard, E.; Labrunie, G.; Merhi, F.; Schläfli, A.M.; Brigger, D.; Souquere, S.; Pierron, G.; Pasquet, J.-M.; et al. P62/SQSTM1 upregulation constitutes a survival mechanism that occurs during granulocytic differentiation of acute myeloid leukemia cells. Cell Death Differ. 2014, 21, 1852–1861. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Britschgi, A.; Schläfli, A.M.; Humbert, M.; Shan-Krauer, D.; Batliner, J.; Federzoni, E.A.; Ernst, M.; Torbett, B.E.; Yousefi, S.; et al. Low autophagy (ATG) gene expression is associated with an immature AML blast cell phenotype and can be restored during AML differentiation therapy. Oxid. Med. Cell Longev. 2018, 2018, 1482795. [Google Scholar] [CrossRef]
- Orfali, N.; O’Donovan, T.R.; Nyhan, M.J.; Britschgi, A.; Tschan, M.P.; Cahill, M.R.; Mongan, N.P.; Gudas, L.J.; McKenna, S.L. Induction of autophagy is a key component of all-trans-retinoic acid-induced differentiation in leukemia cells and a potential target for pharmacological modulation. Exp. Hematol. 2015, 43, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Orfali, N.; O’Donovan, T.R.; Cahill, M.R.; Benjamin, D.; Nanus, D.M.; McKenna, S.L.; Gudas, L.J.; Mongan, N.P. All-trans retinoic acid (ATRA)-induced TFEB expression is required for myeloid differentiation in acute promyelocytic leukemia (APL). Eur. J. Haematol. 2020, 104, 236–250. [Google Scholar] [CrossRef]
- Lanotte, M.; Martin-Thouvenin, V.; Najman, S.; Balerini, P.; Valensi, F.; Berger, R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991, 77, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Khadra, N.; Bresson-Bepoldin, L.; Penna, A.; Chaigne-Delalande, B.; Ségui, B.; Levade, T.; Vacher, A.-M.; Reiffers, J.; Ducret, T.; Moreau, J.-F.; et al. CD95 triggers Orai1-mediated localized Ca2+ entry, regulates recruitment of protein kinase C (PKC) β2, and prevents death-inducing signaling complex formation. Proc. Natl. Acad. Sci. USA 2011, 108, 19072–19077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djavaheri-Mergny, M.; Amelotti, M.; Mathieu, J.; Besançon, F.; Bauvy, C.; Souquère, S.; Pierron, G.; Codogno, P. NF-κB activation represses tumor necrosis factor-alpha-induced autophagy. J. Biol. Chem. 2006, 281, 30373–30382. [Google Scholar] [CrossRef] [Green Version]
- Beauvarlet, J.; Bensadoun, P.; Darbo, E.; Labrunie, G.; Rousseau, B.; Richard, E.; Draskovic, I.; Londono-Vallejo, A.; Dupuy, J.-W.; Nath Das, R.; et al. Modulation of the ATM/autophagy pathway by a G-quadruplex ligand tips the balance between senescence and apoptosis in cancer cells. Nucleic Acids Res. 2019, 47, 2739–2756. [Google Scholar] [CrossRef]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
- Tian, C.; Du, L.; Zhou, Y.; Li, M. Store-operated CRAC channel inhibitors: Opportunities and challenges. Future Med. Chem. 2016, 8, 817–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörr, K.; Kilch, T.; Kappel, S.; Alansary, D.; Schwär, G.; Niemeyer, B.A.; Peinelt, C. Cell type-specific glycosylation of Orai1 modulates store-operated Ca2+ entry. Sci. Signal. 2016, 9, ra25. [Google Scholar] [CrossRef] [PubMed]
- Prins, D.; Michalak, M. STIM1 is cleaved by calpain. FEBS Lett. 2015, 589, 3294–3301. [Google Scholar] [CrossRef] [Green Version]
- Hörtenhuber, M.; Toledo, E.M.; Smedler, E.; Arenas, E.; Malmersjö, S.; Louhivuori, L.; Uhlén, P. Mapping genes for calcium signaling and their associated human genetic disorders. Bioinformatics 2017, 33, 2547–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høyer-Hansen, M.; Bastholm, L.; Szyniarowski, P.; Campanella, M.; Szabadkai, G.; Farkas, T.; Bianchi, K.; Fehrenbacher, N.; Elling, F.; Rizzuto, R.; et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 2007, 25, 193–205. [Google Scholar] [CrossRef]
- Ghislat, G.; Patron, M.; Rizzuto, R.; Knecht, E. Withdrawal of essential amino acids increases autophagy by a pathway involving Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β). J. Biol. Chem. 2012, 287, 38625–38636. [Google Scholar] [CrossRef] [Green Version]
- Crawford, S.E.; Hyser, J.M.; Utama, B.; Estes, M.K. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. Proc. Natl. Acad. Sci. USA 2012, 109, E3405–E3413. [Google Scholar] [CrossRef] [Green Version]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviram, A.; Rephaeli, A.; Shaklai, M. The role of increased calcium influx rate in receptor mediated function of differentiating HL-60 cells. Cell Calcium 1990, 11, 269–274. [Google Scholar] [CrossRef]
- Rephaeli, A.; Aviram, A.; Rabizadeh, E.; Englender, T.; Shaklai, M. The role of calcium in differentiation of leukemic cell lines. Cancer Biochem. Biophys. 1990, 11, 119–125. [Google Scholar] [PubMed]
- Chapekar, M.S.; Hartman, K.D.; Knode, M.C.; Glazer, R.I. Synergistic effect of retinoic acid and calcium ionophore A23187 on differentiation, c-Myc expression, and membrane tyrosine kinase activity in human promyelocytic leukemia cell line HL-60. Mol. Pharmacol. 1987, 31, 140–145. [Google Scholar]
- Launay, S.; Giannì, M.; Kovàcs, T.; Bredoux, R.; Bruel, A.; Gélébart, P.; Zassadowski, F.; Chomienne, C.; Enouf, J.; Papp, B. Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood 1999, 93, 4395–4405. [Google Scholar] [CrossRef]
- Launay, S.; Giannì, M.; Diomede, L.; Machesky, L.M.; Enouf, J.; Papp, B. Enhancement of ATRA-induced cell differentiation by inhibition of calcium accumulation into the endoplasmic reticulum: Cross-talk between RAR alpha and calcium-dependent signaling. Blood 2003, 101, 3220–3228. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yu, J.; Li, D.; Yu, S.; Ke, J.; Wang, L.; Wang, Y.; Qiu, Y.; Gao, X.; Zhang, J.; et al. Store-operated calcium entry-activated autophagy protects EPC proliferation via the CAMKK2-MTOR pathway in Ox-LDL exposure. Autophagy 2017, 13, 82–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondratskyi, A.; Yassine, M.; Slomianny, C.; Kondratska, K.; Gordienko, D.; Dewailly, E.; Lehen’kyi, V.; Skryma, R.; Prevarskaya, N. Identification of ML-9 as a lysosomotropic agent targeting autophagy and cell death. Cell Death Dis. 2014, 5, e1193. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the MTOR pathway. Mol. Carcinog. 2016, 55, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.-D.; Xia, X.; Lv, X.-F.; Yu, B.-X.; Yuan, J.-N.; Mai, X.-Y.; Shang, J.-Y.; Zhou, J.-G.; Liang, S.-J.; Pang, R.-P. Inhibition of Orai1-mediated Ca2+ entry enhances chemosensitivity of HepG2 hepatocarcinoma cells to 5-fluorouracil. J. Cell Mol. Med. 2017, 21, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chun, J.; Pan, C.; Kumar, A.; Zhang, G.; Ha, Y.; Li, D.; Alesi, G.N.; Kang, Y.; Zhou, L.; et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer. Mol. Cell 2018, 69, 87–99.e7. [Google Scholar] [CrossRef] [Green Version]
- Racioppi, L.; Nelson, E.R.; Huang, W.; Mukherjee, D.; Lawrence, S.A.; Lento, W.; Masci, A.M.; Jiao, Y.; Park, S.; York, B.; et al. CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nat. Commun. 2019, 10, 2450. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yi, X.; Wu, Z.; Guo, S.; Dai, W.; Wang, H.; Shi, Q.; Zeng, K.; Guo, W.; Li, C. CAMKK2 defines ferroptosis sensitivity of melanoma cells by regulating AMPK–NRF2 pathway. J. Invest. Dermatol. 2021. [CrossRef]
- Ye, C.; Zhang, D.; Zhao, L.; Li, Y.; Yao, X.; Wang, H.; Zhang, S.; Liu, W.; Cao, H.; Yu, S.; et al. CaMKK2 suppresses muscle regeneration through the inhibition of myoblast proliferation and differentiation. Int. J. Mol. Sci. 2016, 17, 1695. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Ribar, T.J.; Means, A.R. The Ca2+/calmodulin-dependent protein kinase kinase, CaMKK2, inhibits preadipocyte differentiation. Endocrinology 2011, 152, 3668–3679. [Google Scholar] [CrossRef] [Green Version]
- Obba, S.; Hizir, Z.; Boyer, L.; Selimoglu-Buet, D.; Pfeifer, A.; Michel, G.; Hamouda, M.-A.; Gonçalvès, D.; Cerezo, M.; Marchetti, S.; et al. The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. Autophagy 2015, 11, 1114–1129. [Google Scholar] [CrossRef]
- Teng, E.C.; Racioppi, L.; Means, A.R. A cell-intrinsic role for CaMKK2 in granulocyte lineage commitment and differentiation. J. Leukoc. Biol. 2011, 90, 897–909. [Google Scholar] [CrossRef]
- Soboloff, J.; Zhang, Y.; Minden, M.; Berger, S.A. Sensitivity of myeloid leukemia cells to calcium influx blockade: Application to bone marrow purging. Exp. Hematol. 2002, 30, 1219–1226. [Google Scholar] [CrossRef]
- Kondratska, K.; Kondratskyi, A.; Yassine, M.; Lemonnier, L.; Lepage, G.; Morabito, A.; Skryma, R.; Prevarskaya, N. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim. Biophys. Acta 2014, 1843, 2263–2269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacher, P.; Vacher, A.-M.; Pineau, R.; Latour, S.; Soubeyran, I.; Pangault, C.; Tarte, K.; Soubeyran, P.; Ducret, T.; Bresson-Bepoldin, L. Localized store-operated calcium influx represses CD95-dependent apoptotic effects of rituximab in non-Hodgkin B lymphomas. J. Immunol. 2015, 195, 2207–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, B.J.; Irrinki, K.M.; Mallilankaraman, K.; Lien, Y.-C.; Wang, Y.; Bhanumathy, C.D.; Subbiah, R.; Ritchie, M.F.; Soboloff, J.; Baba, Y.; et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 2010, 190, 391–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, P.-F.; Liu, Z.-H.; Li, N.; Cheng, W.-J.; Guo, S.-W. Knockdown of STIM1 improves neuronal survival after traumatic neuronal injury through regulating MGluR1-dependent Ca(2+) signaling in mouse cortical neurons. Cell Mol. Neurobiol. 2015, 35, 283–292. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Csordas, A.; Sun, Z.; Jarnuczak, A.; Perez-Riverol, Y.; Ternent, T.; Campbell, D.S.; Bernal-Llinares, M.; Okuda, S.; Kawano, S.; et al. The ProteomeXchange consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017, 45, D1100–D1106. [Google Scholar] [CrossRef]
| Accession | Description | Log2FC | p-Value |
|---|---|---|---|
| P32455 | Guanylate-binding protein 1 | 9.96 | 0 |
| P33897 | ATP-binding cassette sub-family D member 1 | 9.96 | 0 |
| P48736 | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | 9.96 | 0 |
| Q9Y2J8 | Protein-arginine deiminase type-2 | 9.96 | 0 |
| O15162 | Phospholipid scramblase 1 | 7.1 | 0.0034 |
| O75923 | Dysferlin | 6.93 | 0.0039 |
| P05109 | Protein S100-A8 | 6.78 | 0.0043 |
| P21980 | Protein-glutamine gamma-glutamyltransferase 2 | 6.73 | 0.0045 |
| P05362 | Intercellular adhesion molecule 1 | 6.05 | 0.0072 |
| P00813 | Adenosine deaminase | 5.82 | 0.0084 |
| P37235 | Hippocalcin-like protein 1 | 3.52 | 0.0420 |
| P06702 | Protein S100-A9 | 3.48 | 0.0430 |
| P04271 | Protein S100-B | 3.44 | 0.0442 |
| P08567 | Pleckstrin | 3.41 | 0.0451 |
| P28676 | Grancalcin | 3.29 | 0.0493 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merhi, F.; Alvarez-Valadez, K.; Trepiana, J.; Lescoat, C.; Groppi, A.; Dupuy, J.-W.; Soubeyran, P.; Kroemer, G.; Vacher, P.; Djavaheri-Mergny, M. Targeting CAMKK2 and SOC Channels as a Novel Therapeutic Approach for Sensitizing Acute Promyelocytic Leukemia Cells to All-Trans Retinoic Acid. Cells 2021, 10, 3364. https://doi.org/10.3390/cells10123364
Merhi F, Alvarez-Valadez K, Trepiana J, Lescoat C, Groppi A, Dupuy J-W, Soubeyran P, Kroemer G, Vacher P, Djavaheri-Mergny M. Targeting CAMKK2 and SOC Channels as a Novel Therapeutic Approach for Sensitizing Acute Promyelocytic Leukemia Cells to All-Trans Retinoic Acid. Cells. 2021; 10(12):3364. https://doi.org/10.3390/cells10123364
Chicago/Turabian StyleMerhi, Faten, Karla Alvarez-Valadez, Jenifer Trepiana, Claire Lescoat, Alexis Groppi, Jean-William Dupuy, Pierre Soubeyran, Guido Kroemer, Pierre Vacher, and Mojgan Djavaheri-Mergny. 2021. "Targeting CAMKK2 and SOC Channels as a Novel Therapeutic Approach for Sensitizing Acute Promyelocytic Leukemia Cells to All-Trans Retinoic Acid" Cells 10, no. 12: 3364. https://doi.org/10.3390/cells10123364
APA StyleMerhi, F., Alvarez-Valadez, K., Trepiana, J., Lescoat, C., Groppi, A., Dupuy, J. -W., Soubeyran, P., Kroemer, G., Vacher, P., & Djavaheri-Mergny, M. (2021). Targeting CAMKK2 and SOC Channels as a Novel Therapeutic Approach for Sensitizing Acute Promyelocytic Leukemia Cells to All-Trans Retinoic Acid. Cells, 10(12), 3364. https://doi.org/10.3390/cells10123364








