Co-Expression of Nogo-A in Dopaminergic Neurons of the Human Substantia Nigra Pars Compacta Is Reduced in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of the Human Substantia Nigra Pars Compacta TMA
2.2. Immunohistochemistry
2.3. Cell Count Analysis
2.4. Statistical Analysis
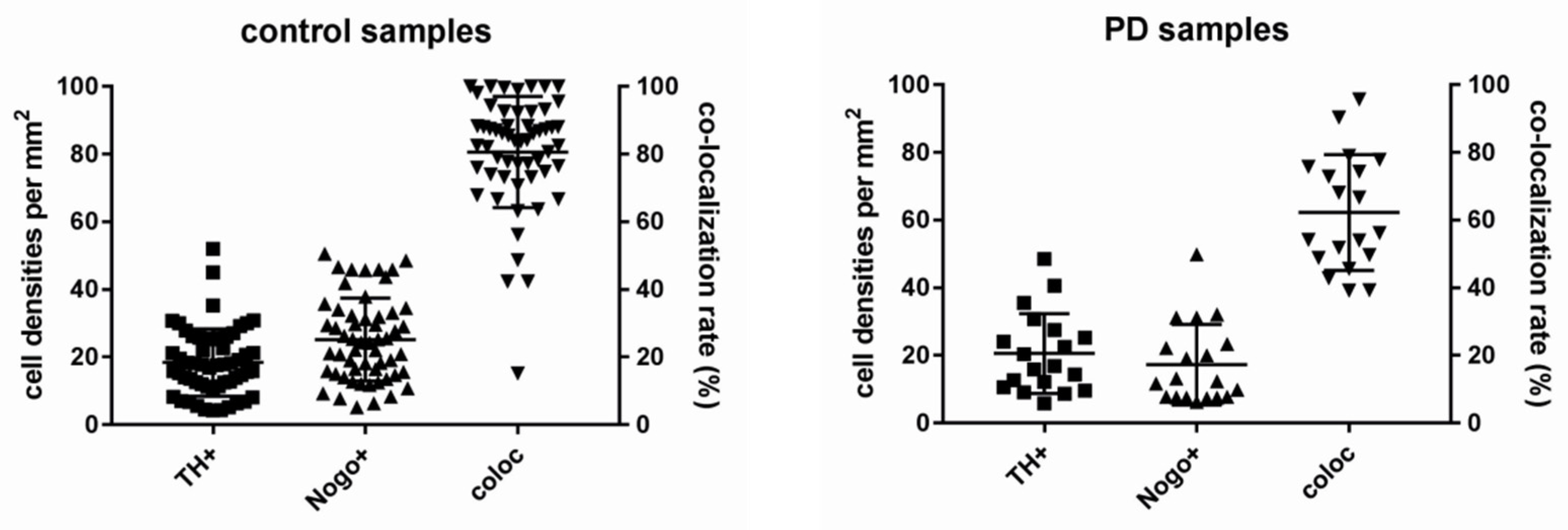
3. Results
3.1. Nogo-A Is Expressed in DAneurons Human SNc
3.2. Co-Localization Rates Increase with Age in the Non-Diseased Brains
3.3. Lower Numbers of TH-ir Neurons in Normal Aging
3.4. Co-Localization Rates in PD Decrease Depending on Age
3.5. Age Is Associated with Lower Numbers of TH-ir and Nogo-A-ir Neurons in PD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Immunohistochemistry for α-Synuclein

| Sample Number | Age |
|---|---|
| 1 | 32 |
| 2 | 46 |
| 3 | 50 |
| 4 | 52 |
| 5 | 55 |
| 6 | 58 |
| 7 | 59 |
| 8 | 64 |
| 9 | 64 |
| 10 | 64 |
| 11 | 67 |
| 12 | 70 |
| 13 | 73 |
| 14 | 74 |
| 15 | 74 |
| 16 | 74 |
| 17 | 75 |
| 18 | 79 |
| 19 | 79 |
| 20 | 83 |
| 21 | 84 |
| 22 | 85 |
| 23 | 91 |
| Sample Number | Age |
|---|---|
| 1 | 41 |
| 2 | 47 |
| 3 | 47 |
| 4 | 49 |
| 5 | 54 |
| 6 | 54 |
| 7 | 54 |
| 8 | 58 |
| 9 | 60 |
| 10 | 60 |
| 11 | 64 |
| 12 | 64 |
| 13 | 64 |
| 14 | 66 |
| 15 | 66 |
| 16 | 67 |
| 17 | 70 |
| 18 | 71 |
| 19 | 71 |
| 20 | 71 |
| 21 | 71 |
| 22 | 72 |
| 23 | 74 |
| 24 | 74 |
| 25 | 75 |
| 26 | 75 |
| 27 | 76 |
| 28 | 78 |
| 29 | 78 |
| 30 | 81 |
| 31 | 81 |
| 32 | 87 |
| 33 | 88 |
| Sample Number | Gender | Age |
|---|---|---|
| 1 | Female | 69 |
| 2 | Female | 75 |
| 3 | Female | 76 |
| 4 | Female | 80 |
| 5 | Female | 81 |
| 6 | Female | 83 |
| 7 | Male | 68 |
| 8 | Male | 72 |
| 9 | Male | 74 |
| 10 | Male | 78 |
| 11 | Male | 78 |
| 12 | Male | 79 |
| 13 | Male | 79 |
| 14 | Male | 81 |
| 15 | Male | 81 |
| 16 | Male | 81 |
| 17 | Male | 82 |
| 18 | Male | 83 |
| 19 | Male | 90 |
Appendix B. Immunohistochemistry for Establishment of Best Staining Method

Appendix C. A Subpopulation of Neurons in the SNc Express Nogo-A Only

Appendix D. Cell Count Approach

References
- GBD 2016 Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Bogetofte, H.; Alamyar, A.; Blaabjerg, M.; Meyer, M. Levodopa Therapy for Parkinson’s Disease: History, Current Status and Perspectives. CNS Neurol. Disord. Drug Targets 2020, 19, 572–583. [Google Scholar] [CrossRef]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Mouatt-Prigent, A.; Agid, Y.; Hirsch, E.C. Does the calcium binding protein calretinin protect dopaminergic neurons against degeneration in Parkinson’s disease? Brain Res. 1994, 668, 62–70. [Google Scholar] [CrossRef]
- Rodríguez, M.; Barroso-Chinea, P.; Abdala, P.; Obeso, J.; González-Hernández, T. Dopamine cell degeneration induced by intraventricular administration of 6-hydroxydopamine in the rat: Similarities with cell loss in parkinson’s disease. Exp. Neurol. 2001, 169, 163–181. [Google Scholar] [CrossRef]
- González-Hernández, T.; Barroso-Chinea, P.; Rodríguez, M. Response of the GABAergic and dopaminergic mesostriatal projections to the lesion of the contralateral dopaminergic mesostriatal pathway in the rat. Mov. Disord. 2004, 19, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Anglade, P.; Vyas, S.; Hirsch, E.C.; Agid, Y. Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol. Histopathol. 1997, 12, 603–610. [Google Scholar]
- Hawkes, C.H. The prodromal phase of sporadic Parkinson’s disease: Does it exist and if so how long is it? Mov. Disord. 2008, 23, 1799–1807. [Google Scholar] [CrossRef]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999, 122, 1437–1448. [Google Scholar] [CrossRef]
- Schwab, M.E. Nogo and axon regeneration. Curr. Opin. Neurobiol. 2004, 14, 118–124. [Google Scholar] [CrossRef]
- Wälchli, T.; Pernet, V.; Weinmann, O.; Shiu, J.Y.; Guzik-Kornacka, A.; Decrey, G.; Yüksel, D.; Schneider, H.; Vogel, J.; Ingber, D.E.; et al. Nogo-A is a negative regulator of CNS angiogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, E1943–E1952. [Google Scholar] [CrossRef]
- Schwab, M.E. Functions of Nogo proteins and their receptors in the nervous system. Nat. Rev. Neurosci. 2010, 11, 799–811. [Google Scholar] [CrossRef]
- Willi, R.; Aloy, E.M.; Yee, B.K.; Feldon, J.; Schwab, M.E. Behavioral characterization of mice lacking the neurite outgrowth inhibitor Nogo-A. Genes Brain Behav. 2009, 8, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Di Santo, S.; Widmer, H.R. Nogo-A Neutralization Improves Graft Function in a Rat Model of Parkinson’s Disease. Front. Cell. Neurosci. 2016, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Pollini, D.; Di Santo, S.; Widmer, H.R. Antagonizing Nogo-receptor 1 promotes the number of cultured dopaminergic neurons and elongates their neurites. Neuroreport 2013, 24, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Schawkat, K.; Di Santo, S.; Seiler, S.; Ducray, A.D.; Widmer, H.R. Loss of Nogo-A-expressing neurons in a rat model of Parkinson’s disease. Neuroscience. 2015, 288, 59–72. [Google Scholar] [CrossRef]
- Kurowska, Z.; Brundin, P.; Schwab, M.E.; Li, J.Y. Intracellular Nogo-A facilitates initiation of neurite formation in mouse midbrain neurons in vitro. Neuroscience 2014, 256, 456–466. [Google Scholar] [CrossRef]
- Schnell, L.; Schwab, M.E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 1990, 343, 269–272. [Google Scholar] [CrossRef]
- Dupuis, L.; Gonzalez de Aguilar, J.L.; di Scala, F.; Rene, F.; de Tapia, M.; Pradat, P.F.; Lacomblez, L.; Seihlan, D.; Prinjha, R.; Walsh, F.S.; et al. Nogo provides a molecular marker for diagnosis of amyotrophic lateral sclerosis. Neurobiol. Dis. 2002, 10, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Nicolas, O.; Mingorance, A.; Ureña, J.M.; Tang, B.L.; Hirata, T.; Sáez-Valero, J.; Ferrer, I.; Soriano, E.; del Río, J.A. Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Kim, J.-E.; Sivula, M.; Strittmatter, S.M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004, 24, 6209–6217. [Google Scholar] [CrossRef] [PubMed]
- Willi, R.; Schwab, M.E. Nogo and Nogo receptor: Relevance to schizophrenia? Neurobiol. Dis. 2013, 54, 150–157. [Google Scholar] [CrossRef]
- Mohammed, R.; Opara, K.; Lall, R.; Ojha, U.; Xiang, J. Evaluating the effectiveness of anti-Nogo treatment in spinal cord injuries. Neural. Dev. 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Suter, G.; Perren, A.; Lugli, A. A next-generation tissue microarray (ngTMA) protocol for biomarker studies. J. Vis. Exp. 2014, 51893. [Google Scholar] [CrossRef]
- Zlobec, I.; Koelzer, V.H.; Dawson, H.; Perren, A.; Lugli, A. Next-generation tissue microarray (ngTMA) increases the quality of biomarker studies: An example using CD3, CD8, and CD45RO in the tumor microenvironment of six different solid tumor types. J. Transl Med. 2013, 11, 104. [Google Scholar] [CrossRef]
- Eriksen, N.; Stark, A.K.; Pakkenberg, B. Age and Parkinson’s disease-related neuronal death in the substantia nigra pars compacta. J. Neural. Transm. Suppl. 2009, 203–213. [Google Scholar] [CrossRef]
- Naskar, A.; Mahadevan, A.; Philip, M.; Alladi, P.A. Aging mildly affects dendritic arborisation and synaptic protein expression in human substantia nigra pars compacta. J. Chem. Neuroanat. 2019, 97, 57–65. [Google Scholar] [CrossRef]
- Smedfors, G.; Olson, L.; Karlsson, T.E. A Nogo-Like Signaling Perspective from Birth to Adulthood and in Old Age: Brain Expression Patterns of Ligands, Receptors and Modulators. Front. Mol. Neurosci. 2018, 11. [Google Scholar] [CrossRef]
- Buss, A.; Sellhaus, B.; Wolmsley, A.; Noth, J.; Schwab, M.E.; Brook, G.A. Expression pattern of NOGO-A protein in the human nervous system. Acta Neuropathol. 2005, 110, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Carvey, P.M.; Punati, A.; Newman, M.B. Progressive dopamine neuron loss in Parkinson’s disease: The multiple hit hypothesis. Cell. Transplant. 2006, 15, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Gertler, T.S.; Surmeier, D.J. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov. Disord. 2010, 25, S63–S70. [Google Scholar] [CrossRef]
- Kumari, A.; Thakur, M.K. Age-Dependent Decline of Nogo-A Protein in the Mouse Cerebrum. Cell. Mol. Neurobiol. 2014, 34, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Trifunovski, A.; Josephson, A.; Bickford, P.C.; Olson, L.; Brené, S. Selective decline of Nogo mRNA in the aging brain. Neuroreport 2006, 17, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Di Santo, S.; Widmer, H.R. Non-canonical actions of Nogo-A and its receptors. Biochem. Pharm. 2016, 100, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, L.K.; Das, S.K. The Regulatory Role of Reticulons in Neurodegeneration: Insights Underpinning Therapeutic Potential for Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2021, 41, 1157–1174. [Google Scholar] [CrossRef]
- Ineichen, B.V.; Kapitza, S.; Bleul, C.; Good, N.; Plattner, P.S.; Seyedsadr, M.S.; Kaiser, J.; Schneider, M.P.; Zörner, B.; Martin, R.; et al. Nogo-A antibodies enhance axonal repair and remyelination in neuro-inflammatory and demyelinating pathology. Acta. Neuropathol. 2017, 134, 423–440. [Google Scholar] [CrossRef]
- Kucher, K.; Johns, D.; Maier, D.; Abel, R.; Badke, A.; Baron, H.; Thietje, R.; Casha, S.; Meindl, R.; Gomez-Mancilla, B.; et al. First-in-Man Intrathecal Application of Neurite Growth-Promoting Anti-Nogo-A Antibodies in Acute Spinal Cord Injury. Neurorehabil. Neural. Repair 2018, 32, 578–589. [Google Scholar] [CrossRef]
- Xu, W.; Xiao, P.; Fan, S.; Chen, Y.; Huang, W.; Chen, X.; Liu, G.; Dang, C.; Zeng, J.; Xing, S. Blockade of Nogo-A/Nogo-66 receptor 1 (NgR1) Inhibits Autophagic Activation and Prevents Secondary Neuronal Damage in the Thalamus after Focal Cerebral Infarction in Hypertensive Rats. Neuroscience 2020, 431, 103–114. [Google Scholar] [CrossRef]
- Singh-Bains, M.K.; Mehrabi, N.F.; Tan, A.Y.S.; Faull, R.L.M.; Dragunow, M. Preparation, construction and high-throughput automated analysis of human brain tissue microarrays for neurodegenerative disease drug development. Nat. Protoc. 2021, 16, 2308–2343. [Google Scholar] [CrossRef]
- Twelves, D.; Perkins, K.S.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Disord. 2003, 18, 19–31. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Pecurariu, C.F.; Odin, P.; Van Hilten, J.J.; Antonini, A.; Rojo-Abuin, J.M.; Borges, V.; Trenkwalder, C.; Aarsland, D.; Brooks, D.J. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J. Neurol. 2012, 259, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Cholerton, B.; Johnson, C.O.; Fish, B.; Quinn, J.F.; Chung, K.A.; Peterson-Hiller, A.L.; Rosenthal, L.S.; Dawson, T.M.; Albert, M.S.; Hu, S.-C. Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat. Disord. 2018, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Murray, H.E.; Dexter, D.; McArthur, S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2004, 78, 513–522. [Google Scholar] [CrossRef]
- Buck, S.A.; Steinkellner, T.; Aslanoglou, D.; Villeneuve, M.; Bhatte, S.H.; Childers, V.C.; Rubin, S.A.; De Miranda, B.R.; O’Leary, E.I.; Neureiter, E.G.; et al. Vesicular glutamate transporter modulates sex differences in dopamine neuron vulnerability to age-related neurodegeneration. Aging Cell 2021, 20, e13365. [Google Scholar] [CrossRef]
- Shin, J.W.; Shim, E.S.; Hwang, G.H.; Jung, H.S.; Park, J.H.; Sohn, N.W. Cell size-dependent Nogo-A expression in layer V pyramidal neurons of the rat primary somatosensory cortex. Neurosci. Lett. 2006, 394, 117–120. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, F.; Han, J.; Wang, J. Patterns of Nogo-A, NgR, and RhoA expression in the brain tissues of rats with focal cerebral infarction. Transl. Res. 2009, 154, 40–48. [Google Scholar] [CrossRef]
- Nagamoto-Combs, K.; Morecraft, R.J.; Darling, W.G.; Combs, C.K. Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J. Neurotrauma. 2010, 27, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Theotokis, P.; Lourbopoulos, A.; Touloumi, O.; Lagoudaki, R.; Kofidou, E.; Nousiopoulou, E.; Poulatsidou, K.N.; Kesidou, E.; Tascos, N.; Spandou, E.; et al. Time course and spatial profile of Nogo-A expression in experimental autoimmune encephalomyelitis in C57BL/6 mice. J. Neuropathol. Exp. Neurol. 2012, 71, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Liechti, R.; Ducray, A.D.; Jensen, P.; Di Santo, S.; Seiler, S.; Jensen, C.H.; Meyer, M.; Widmer, H.R. Characterization of fetal antigen 1/delta-like 1 homologue expressing cells in the rat nigrostriatal system: Effects of a unilateral 6-hydroxydopamine lesion. PLoS ONE 2015, 10, e0116088. [Google Scholar] [CrossRef]
- Seiler, S.; Di Santo, S.; Andereggen, L.; Widmer, H.R. Antagonization of the Nogo-Receptor 1 Enhances Dopaminergic Fiber Outgrowth of Transplants in a Rat Model of Parkinson’s Disease. Front. Cell. Neurosci. 2017, 11, 151. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyer, G.-C.; Di Santo, S.; Hewer, E.; Andereggen, L.; Seiler, S.; Widmer, H.R. Co-Expression of Nogo-A in Dopaminergic Neurons of the Human Substantia Nigra Pars Compacta Is Reduced in Parkinson’s Disease. Cells 2021, 10, 3368. https://doi.org/10.3390/cells10123368
Eyer G-C, Di Santo S, Hewer E, Andereggen L, Seiler S, Widmer HR. Co-Expression of Nogo-A in Dopaminergic Neurons of the Human Substantia Nigra Pars Compacta Is Reduced in Parkinson’s Disease. Cells. 2021; 10(12):3368. https://doi.org/10.3390/cells10123368
Chicago/Turabian StyleEyer, Gian-Carlo, Stefano Di Santo, Ekkehard Hewer, Lukas Andereggen, Stefanie Seiler, and Hans Rudolf Widmer. 2021. "Co-Expression of Nogo-A in Dopaminergic Neurons of the Human Substantia Nigra Pars Compacta Is Reduced in Parkinson’s Disease" Cells 10, no. 12: 3368. https://doi.org/10.3390/cells10123368
APA StyleEyer, G.-C., Di Santo, S., Hewer, E., Andereggen, L., Seiler, S., & Widmer, H. R. (2021). Co-Expression of Nogo-A in Dopaminergic Neurons of the Human Substantia Nigra Pars Compacta Is Reduced in Parkinson’s Disease. Cells, 10(12), 3368. https://doi.org/10.3390/cells10123368






